Abstract
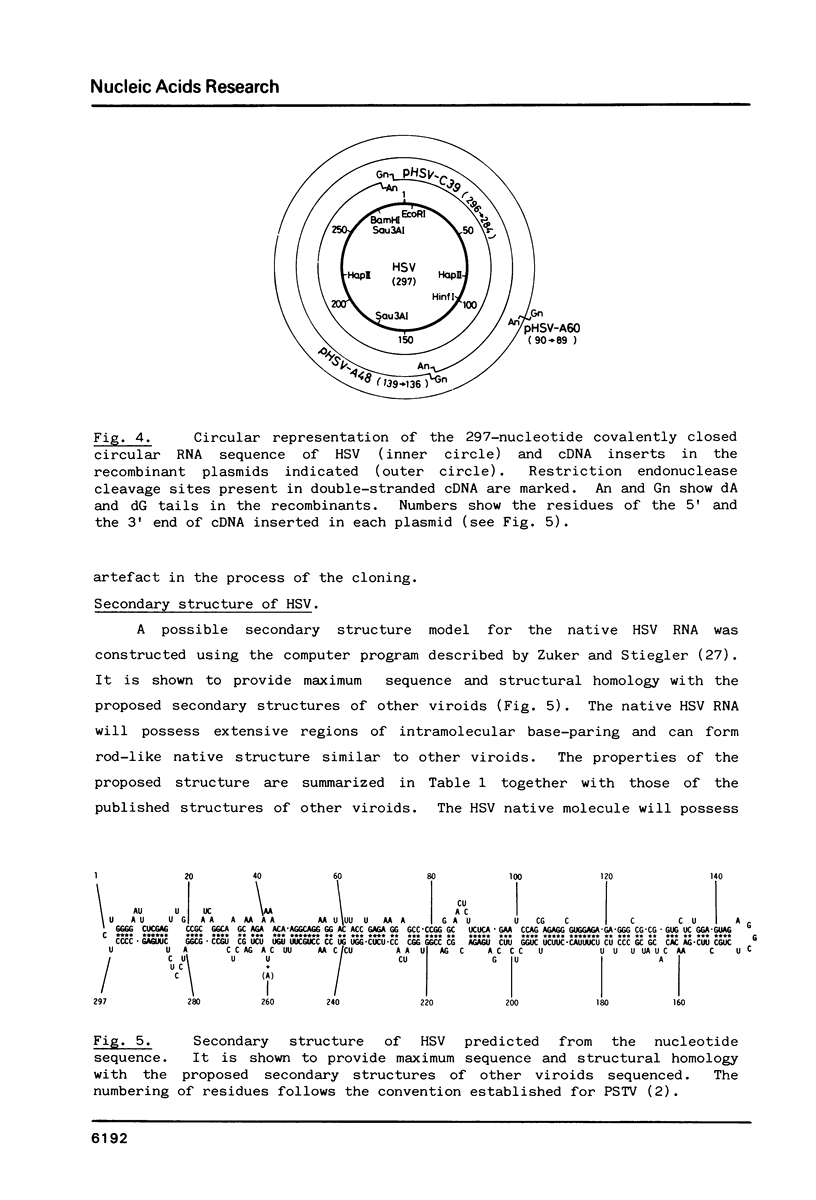
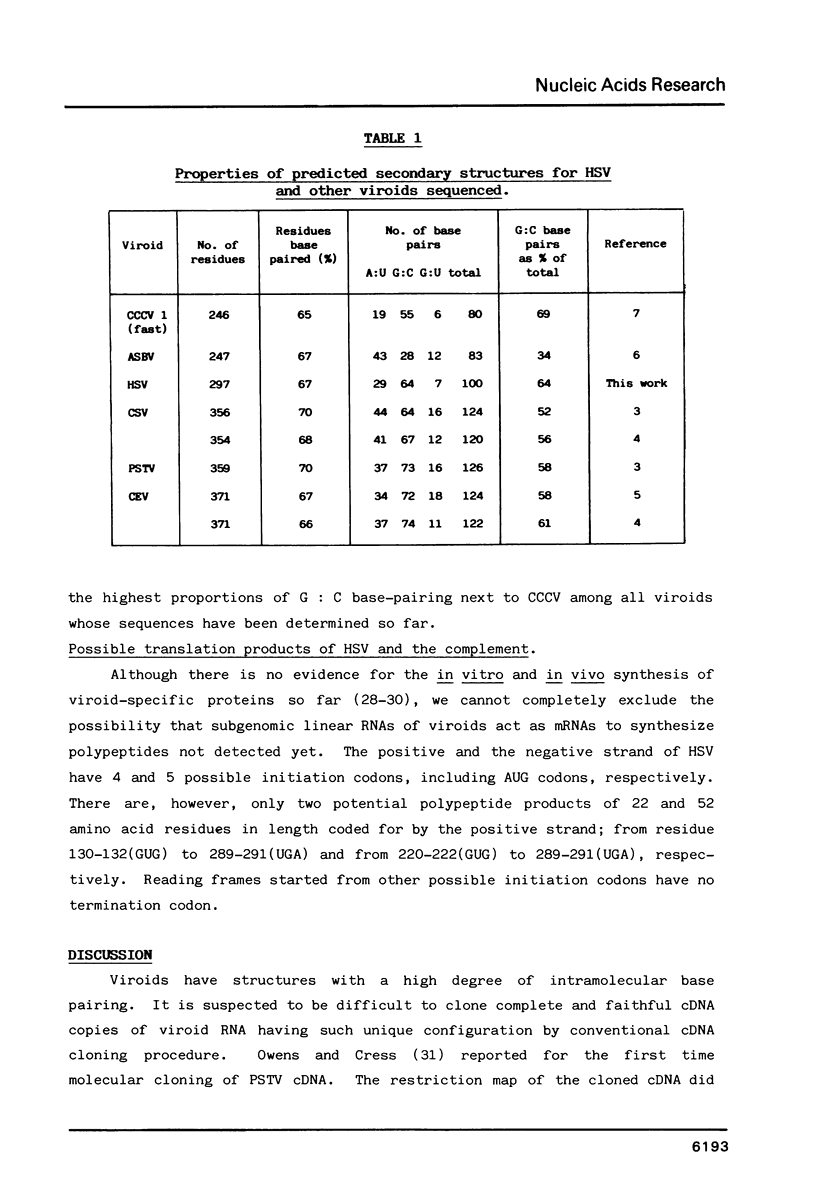
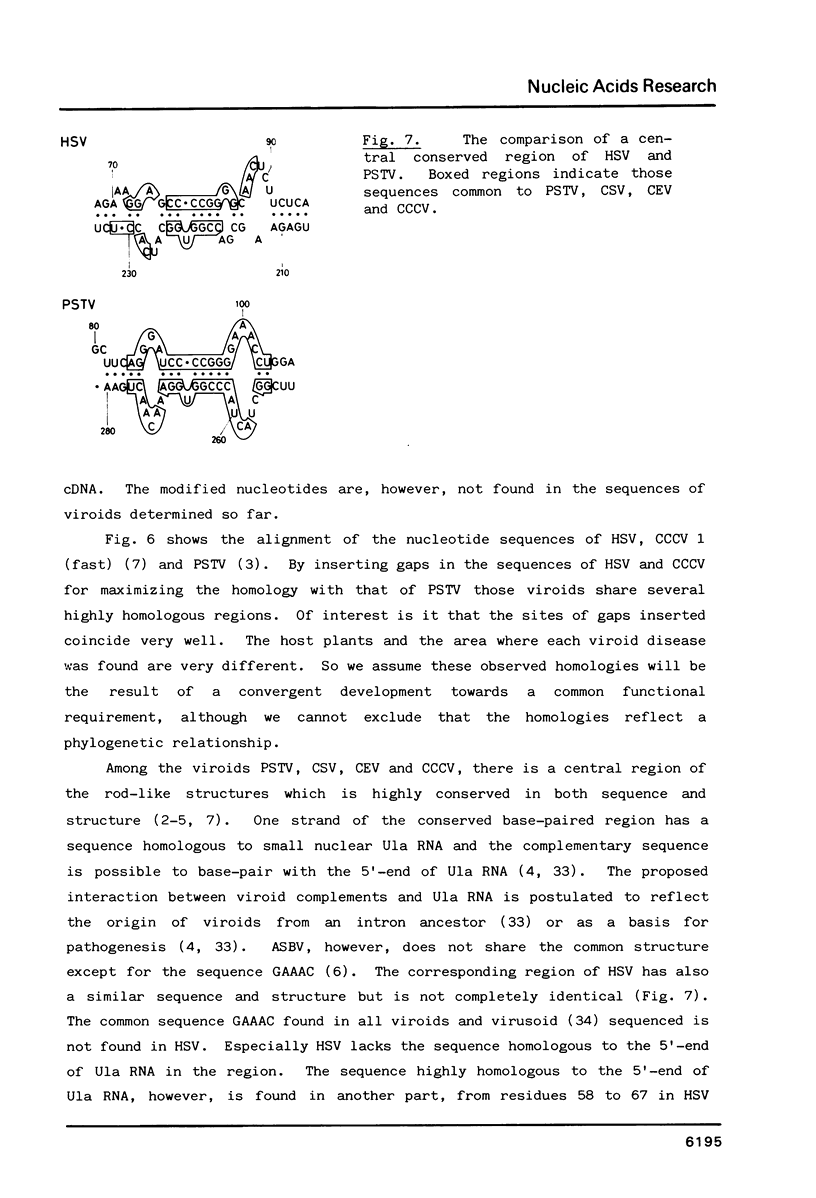
The complete cDNA of hop stunt viroid (HSV) has been cloned by the method of Okayama and Berg (Mol.Cell.Biol.2,161-170. (1982] and the complete nucleotide sequence has been established. The covalently closed circular single-stranded HSV RNA consists of 297 nucleotides. The secondary structure predicted for HSV contains 67% of its residues base-paired. The native HSV can possess an extended rod-like structure characteristic of viroids previously established. The central region of the native HSV has a similar structure to the conserved region found in all viroids sequenced so far except for avocado sunblotch viroid. The sequence homologous to the 5'-end of U1a RNA is also found in the sequence of HSV but not in the central conserved region.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Gough N. M. Nucleotide sequence of immunoglobulin heavy chain joining segments between translocated VH and mu constant regions genes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3630–3634. doi: 10.1073/pnas.77.6.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejero V., Semancik J. S. Exocortis viroid: alteration in the proteins of Gynura aurantiaca accompanying viroid infection. Virology. 1977 Mar;77(1):221–232. doi: 10.1016/0042-6822(77)90420-2. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P., Diener T. O. Potato spindle tuber viroid. XII. An investigation of viroid RNA as a messenger for protein synthesis. Virology. 1974 Sep;61(1):281–286. doi: 10.1016/0042-6822(74)90262-1. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Are viroids escaped introns? Proc Natl Acad Sci U S A. 1981 Aug;78(8):5014–5015. doi: 10.1073/pnas.78.8.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Viroids and their interactions with host cells. Annu Rev Microbiol. 1982;36:239–258. doi: 10.1146/annurev.mi.36.100182.001323. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Comparative sequence and structure of viroid-like RNAs of two plant viruses. Nucleic Acids Res. 1982 Jun 25;10(12):3681–3691. doi: 10.1093/nar/10.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oda K., Kato H., Saito I., Sugano S., Maruyama K., Masuda M., Shiroki K., Shimojo H. Expression of adenovirus type 12 E1A gene in monkey cells, using a simian virus 40 vector. J Virol. 1983 Jan;45(1):408–419. doi: 10.1128/jvi.45.1.408-419.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Cress D. E. Molecular cloning and characterization of potato spindle tuber viroid cDNA sequences. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5302–5306. doi: 10.1073/pnas.77.9.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. RNA intermediates in potato spindle tuber viroid replication. Proc Natl Acad Sci U S A. 1982 Jan;79(1):113–117. doi: 10.1073/pnas.79.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. Sensitive and rapid diagnosis of potato spindle tuber viroid disease by nucleic Acid hybridization. Science. 1981 Aug 7;213(4508):670–672. doi: 10.1126/science.213.4508.670. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Conejero V., Gerhart J. Citrus exocortis viroid: survey of protein synthesis in Xenopus laevis oocytes following addition of viroid RNA. Virology. 1977 Jul 1;80(1):218–221. doi: 10.1016/0042-6822(77)90395-6. [DOI] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu N., Ohno T., Meshi T., Okada Y. Molecular cloning and nucleotide sequence of the 30K and the coat protein cistron of TMV (tomato strain) genome. Nucleic Acids Res. 1983 Jun 11;11(11):3767–3778. doi: 10.1093/nar/11.11.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J. E., Gould A. R., Bruening G. E., Symons R. H. Citrus exocortis viroid: nucleotide sequence and secondary structure of an Australian isolate. FEBS Lett. 1982 Jan 25;137(2):288–292. doi: 10.1016/0014-5793(82)80369-4. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]