Abstract
Purpose. We sought to evaluate our experience using yttrium-90 (90Y) resin microsphere hepatic radioembolization as salvage therapy for liver-dominant metastatic colorectal cancer (mCRC). Methods. A retrospective review of consecutive patients with unresectable mCRC who were treated with 90Y after failing first and second line systemic chemotherapy. Demographics, treatment dose, biochemical and radiographic response, toxicities, and survival were examined. Results. Fifty-one patients underwent 90Y treatments of which 69% were male. All patients had previously undergone extensive chemotherapy, 31% had undergone previous liver-directed therapy and 24% had a prior liver resection. Using RECIST criteria, either stable disease or a partial response was seen in 77% of patients. Overall median survival from the time of first 90Y treatment was 10.2 months (95% CI = 7.5–13.0). The absence of extrahepatic disease at the time of treatment with 90Y was associated with an improved survival, median survival of 17.0 months (95% CI = 6.4–27.6), compared to those with extrahepatic disease at the time of treatment with 90Y, 6.7 months (95% CI = 2.7–10.6 Conclusion: 90Y therapy is a safe locoregional therapy that provides an important therapeutic option to patients who have failed first and second line chemotherapy and have adequate liver function and performance status.
1. Introduction
Colorectal carcinoma, estimated to occur at an incidence of 148,810 cases in the USA in 2008 causing 49,960 deaths, is the major contributor of metastatic liver tumors [1]. Hepatic metastases are present in 15–25% of patients at presentation, and an additional 25–50% will develop liver metastases within 3 years following resection of the primary tumor [2, 3]. In approximately half of these patients, metastatic disease is confined to the liver, and 20% of all patients who die of metastatic colorectal cancer have metastases limited to the liver. Hepatic resection for colorectal liver metastases has become the standard of care, and currently remains the only potentially curative therapy. Unfortunately, curative resection is possible in less than 25% of those patients with metastases to the liver.
Currently, the armamentarium against unresectable liver tumors is composed of a number of liver-directed therapies aimed at reducing the hepatic tumor burden. Yttrium-90 (90Y) bound microspheres are an emerging tool for the treatment of primary and metastatic liver cancer that has had promising results. The use of whole-liver external beam radiation therapy for hepatic malignancies has been limited secondary to the relative intolerance of normal liver parenchyma to the dose of radiation necessary to have a response in neoplastic tissue. It has long been known that hepatic parenchyma is largely supplied by the portal system, but hepatic neoplasms are primarily supplied by the arterial system [4]. Therefore, therapy directed into the hepatic arterial system is preferentially targeted to the neoplasm with relative sparing of the normal parenchyma allowing for substantially higher doses of radiation or chemotherapeutic agents to be administered to the liver tumor tissue. Selective internal radiation therapy (SIRT), using 90Y microspheres delivered into the hepatic arterial system, takes advantage of the heterogeneity in blood supply between neoplastic and parenchymal tissue allowing for localized high-dose radiation therapy to be delivered to intrahepatic tumors. In addition to providing localized radiation therapy, the microspheres may also serve to provide an embolic component to the therapy leading to tumor ischemia. Pathologic examination of explants has shown that 90Y microspheres are dispersed preferentially to the periphery of neoplastic tissue [5].
90Y is a pure beta-emitting isotope with a maximal energy of 2.27 MeV and average energy of 0.94 MeV. The maximum range of emission in tissue is 11 mm with mean range of 2.5 mm, allowing tissue only in close proximity to the embolized microspheres to be treated. The half-life of 90Y is 64.1 hours with 94% of radiation delivered in 11 days [6]. Currently in the U.S. market, there are two commercially available microspheres that are irreversibly bound to 90Y; the microspheres are composed of either resin or glass. Both are biocompatible beads with an average diameter of 20–40 μm that are permanently implanted in the liver via embolization through the hepatic artery. The resin microspheres received U.S. Food and Drug Administration (FDA) approval in 2002 for unresectable liver metastases from primary colorectal cancer with adjuvant intrahepatic chemotherapy using floxuridine (FUDR). Glass microspheres were granted humanitarian device exemption (HDE) in 1999 for radiation treatment or as a neoadjuvant to surgery or transplantation in patients with unresectable hepatocellular carcinoma [6, 7].
Several studies have previously shown that 90Y microsphere radioembolization produce adequate response rates in colorectal cancer liver metastases with an acceptable toxicity profile [8–19]. However, many of these earlier experiences with 90Y occurred prior to widespread availability of the newer chemotherapeutic agents and regimens. With the addition of biological agents, such as bevacizumab and cetuximab, to chemotherapy regimens incorporating irinotecan and oxaliplatin the median survival rates and response rates improved in patients with mCRC [20, 21]. The effectiveness of 90Y in patients after failing the latest systemic chemotherapy has yet to be fully evaluated. We sought to retrospectively evaluate our single-institution experience treating patients with liver-dominant mCRC in the salvage setting with 90Y resin microsphere radioembolization.
2. Material and Methods
2.1. Patient Selection, Workup, and Treatment
All patients in this series were treated consecutively with 90Y radioembolization between August 2002 and May 2008 for liver-dominant mCRC at the University of Pittsburgh Medical Center. Patients eligible to receive 90Y were not candidates for hepatic resection or ablation, had progressive disease after first and second line chemotherapy, had Eastern Conference Oncology Group (ECOG) performance status of 0-1, had adequate hepatic function (serum total bilirubin <2.0), and had adequate renal and hematologic function. Progressive disease was defined as increase in tumor burden by radiographic volume or number of metastases. Both intra- and extrahepatic tumors were evaluated for progression, although intrahepatic progression was the determinate for 90Y treatment candidacy. Patients that were considered for 90Y treatment had either exhausted or refused standard chemotherapy regimens. Patients with extrahepatic metastases were treated only if the tumor burden outside the liver was <10% of total tumor burden and chemotherapy options were not available. Data was recorded via an Institutional Review Board approved protocol.
The technical details and dosimetry of the process have previously been described [22, 23]. However, we chose to use a modified partition model for the calculation of the 90Y microsphere activity to be administered to the patient, similar to the methodology used for 90Y glass microspheres. The prescribed activity was calculated to deliver 50 Gy to the targeted liver tissue, assuming a uniform distribution of microspheres in the normal liver parenchyma and tumors, with no correction for any activity shunted to the lungs:
| (1) |
The liver mass is determined from the target liver volume obtained from CT(cm3) × 0.00103 kg/cm3. This methodology was thought to more accurately estimate the radiation dose to the normal liver parenchyma for lobar and segmental treatments than the BSA or Empirical dosing methodologies. However, because of the nonuniform distribution of microspheres in the tumor and normal liver tissue, a proportionally larger radiation dose will be delivered to the tumor tissue and less to the normal liver [22, 23]. Only resin microspheres were used to treat patients in this study. Prior to 90Y administration, all patients had a selective visceral angiogram, technetium-99m-labeled macroaggregated albumin (99mTc-MAA) study, and a baseline CT or PET/CT.
The selective visceral angiogram allows for definitive assessment of the arterial anatomy and possible embolization of vessels that may lead to extrahepatic 90Y exposure. The 99mTc-MAA study allows pulmonary shunting to be evaluated. The use of 90Y radioembolization is avoided if there is any uncorrectable extrahepatic shunting to the gastrointestinal tract, or >0.6 GBq (corresponding to a lung dose of 30 Gy) is shunted to the lungs.
90Y was administered via unilobar treatments. When bilobar disease was present, the lobes were treated sequentially with approximately a one-month interval. Some patients had multiple treatments to the same lobe. The determination to treat the same lobe repetitively was made by evaluating the performance status, liver function, and extent of extrahepatic disease. However, the determination to retreat patients was based solely on progression of disease as assessed via CT. Early in our experience 17 patients had received floxuridine (FUDR) (5 mg/kg) infused via the hepatic artery just prior to instillation of 90Y microspheres. This was stopped due to concerns of FUDR-induced biliary sclerosis seen in prior hepatic artery infusion pump therapy cases, but not in these particular patients. After treatment, patients were observed in the hospital overnight and patients were discharged home the next day with oral narcotics and antiemetics.
2.2. Patient Followup and Evaluation
Toxicity data was reviewed from hospital records and laboratory data. Laboratory data was assessed using National Cancer Institute's Common Terminology Criteria for Adverse Events v3.0. Patients were followed with weekly laboratory data and routine office followup. A CEA response was defined as a ≥50% reduction in posttreatment value when compared to baseline measurements at the time of initial 90Y treatment. Followup imaging was performed every 3 months using either triphasic CTs or PET/CTs. The radiologic response was graded using the RECIST criteria [24]. In brief, the sum of the longest diameter of the five largest hepatic lesions was measured on baseline and follow-up imaging. A complete response was defined as disappearance of all target lesions, partial response was at least a 30% decrease in the overall diameter, and progressive disease was at least a 20% increase in diameter. Stable disease were those cases between partial response and progressive disease. Follow-up imaging from 30–180 days after the initial treatment were used for data analysis.
2.3. Statistics
Data were entered and verified using the Statistical Package for Social Sciences (SPSS version 16) for Windows (SPSS Inc., Chicago, IL). Kaplan-Meier survival analyses using log-rank methods were used to estimate overall survival of the entire sample as well as to test differences in survival between groups. Mean and median survival was reported (with 95% confidence intervals). Variables studied in the univariate analysis included gender, age (>50 and <50 years), presence of extrahepatic disease, prior liver-directed therapy, chemotherapy failure, number of treatments (<2 or ≥2), whether FUDR was given concurrently with90Y, CEA response, radiologic response, and total amount of radiation delivered prior to embolization (< or ≥80% of prescribed dose). Survival was calculated from the time of first 90Y treatment to the time of death.
3. Results
3.1. Demographics, Treatment Regimen, and Tumor Characteristics
Fifty-one patients were treated a total of 90 times with 90Y microspheres. The median age of patients treated was 64 years (range 37–83) (Table 1). All patients had undergone extensive chemotherapy with 33 (73%) patients receiving either bevacizumab or cetuximab, and 9 (20%) patients receiving both. Fourteen (31%) patients had received prior capecitabine. Previous liver-directed therapy was performed in 16 (31%) patients. Liver-directed treatment included radiofrequency ablation in 11 patients, and previous hepatic artery chemoinfusion in 5 patients. Liver resections were performed in 12 (23%) patients prior to 90Y therapy.
Table 1.
Patient characteristics of those treated with 90Y for liver-dominant metastatic colorectal cancer.
| n (%) | Median | Range | |
|---|---|---|---|
| Age (years) | 64 | 37–83 | |
| Male | 35 (68.6) | ||
| Female | 16 (31.4) | ||
| Time from diagnosis of metastases to 1st Rx (months) | 23.2 | 1.3–99.9 | |
| Extrahepatic disease present | 28 (58.3) | ||
| Pulmonary nodules present | 14 (28.5) | ||
| Previous liver-directed therapy | 16 (31.4) | ||
| Previous liver resection | 12 (23.5) | ||
| Previous RFA | 11 (21.5) | ||
| Previous hepatic artery chemoinfusion | 5 (9.8) | ||
| Failed either bevacizumab or cetuximab* | 33 (73.3) | ||
| Failed both bevacizumab or cetuximab | 9 (20.0) | ||
| Failed capecitabine | 14 (31.1) |
*Those receiving both bevacizumab and cetuximab also included.
Although all patients had liver-dominant metastatic disease, a substantial number had radiographically demonstrable extrahepatic metastases. The presence of extrahepatic disease was known in 28 (58%) patients at the time of 90Y treatment. The sites of extrahepatic disease included pulmonary nodules (n = 14), portocaval or retroperitoneal lymphadenopathy (n = 13), anastomotic recurrence or unresected primary colorectal tumor (n = 5), peritoneal disease (n = 4), bone metastases (n = 1), and adrenal metastases (n = 1).
The median time from the diagnosis of hepatic metastases to the first 90Y treatment was 23.2 months (range, 1.3–99.9 months). One treatment was administered to 20 (39%) patients, 2 treatments to 27 (53%) patients, and 4 treatments to 4 (8%) patients (Table 2). The median lung shunt was 3.3% (range, 0.4–11.5%). No patients were excluded from treatment due to either an unacceptable level of pulmonary shunting or uncorrectable shunting to the extrahepatic gastrointestinal tract. The median dose administered to the target lobe per treatment was 44.4 Gy. The median prescribed activity of 90Y administered was 1.10 GBq versus the median activity actually delivered of 0.89 GBq. FUDR was given prior to 90Y in 17 (33%) patients, all of which were treated at the start of the study period. In 67 (74%) treatments ≥80% of prescribed dose was administered. All of those with <80% of the prescribed dose received had the treatment terminated due to the stagnation of flow secondary to the embolic process. There was no survival differences found when the actual dose administered was analyzed. However, patients who had one or more of the treatments terminated before the target dose was reached had a trend (P = .12) towards improved survival. Those who had stagnation of flow prior to reaching the target dose had a median survival of 19.1 months (95% CI = 8.7–29.5) compared to 6.7 months (95% Cl = 2.3–11.0). The median percentage of the prescribed dose administered in all treatments was 92%. There was no relationship between premature embolization during treatment and concurrent administration of FUDR.
Table 2.
Treatment characteristics: Floxuridine (FUDR) was given with 90Y in some of our earlier patients. FUDR (5 mg/kg) was given just prior to administration of the 90Y microspheres.
| n (%) | Median | Range | |
|---|---|---|---|
| Total number of treatments | 90 | ||
| Patients with 1 treatment | 20 (39.2) | ||
| Patients with 2 treatments | 27 (52.9) | ||
| Patients with 4 treatments | 4 (7.8) | ||
| Radiation dose to target tissue per treatment (Gy) | 44.4 | 12.9–67.2 | |
| Radiation activity per treatment (GBq) | 0.89 | 0.16–2.20 | |
| FUDR with 90Y* | |||
| Yes | 17 (33.3) | ||
| No | 34 (66.7) | ||
| Lung shunt (%) | 3.3 | 0.4–11.5 | |
| Treatments terminated early due to embolic process | 40 (44.4) | ||
| Treatments with ≥80% of prescribed dose administered | 67 (74.4) |
*Those who had prior hepatic artery chemoinfusion separate from the time of 90Y administration are not included in this group.
3.2. CEA Response
In 41 patients, serial CEA levels were available for review. A CEA response (≥50% decreases in CEA from baseline) was seen in 17 (41%) patients (Table 3). For those who had a CEA response, the median value was 28.5% (range 1–42%) of the baseline before 90Y value. The CEA nadir was measured at a median of 62 (range 25–139) days after 90Y treatment.
Table 3.
Clinical endpoints: survival, biochemical, and radiologic response. Survival analyses to compare whether survival advantage was associated with studied variables was performed using log-rank analysis.
| n (%) | Median survival (months) | 95% CI1 | |
|---|---|---|---|
| Died during follow-up | 38 (74.5) | ||
| Overall survival (months) | 10.2 | 7.5–13.0 | |
| Gender | 51 (100) | P = .64 | |
| M | 35 (68.6) | 10.6 | 3.4–17.8 |
| F | 16 (31.4) | 10.2 | 0.0–22.0 |
| Age | 51 (100) | P = .18 | |
| <50 | 10 (20) | 5.3 | 0.8–9.8 |
| >50 | 41 (80) | 8.2 | 1.8–19.4 |
| FUDR given with 90Y | P = .72 | ||
| Yes | 17 (33.3) | 17.0 | 6.7–27.3 |
| No | 34 (66.7) | 8.2 | 4.2–12.3 |
| Extrahepatic disease present | 48 (100) | P = .07 | |
| Yes | 28 (58.3) | 6.7 | 2.7–10.6 |
| No | 20 (41.7) | 17.0 | 6.4–27.6 |
| CEA response2 | 41 (100) | P = .36 | |
| Yes | 17 (41.5) | 19.1 | 6.3–31.9 |
| No | 24 (42.9) | 9.3 | 6.0–12.7 |
| Radiographic Response3 | 31 (100) | P = .21 | |
| Progressive Disease | 7 (22.6) | 13.6 | 7.4–19.8 |
| Stable Disease | 20 (64.5) | 9.3 | 6.9–11.8 |
| Partial Response | 4 (12.9) | 21.5 | 13.7–29.1 |
195% Confidence interval.
2CEA response defined as a reduction in CEA ≥50% of pretreatment value.
3RECIST criteria used to compare baseline measurement just prior to 1st treatment with radiologic response during 1–6 months follow-up imaging.
3.3. Radiologic Response
Although all patients had baseline and follow-up imaging obtained, only 31 patients had imaging available for review. This is a reflection of our tertiary care center and many patients received scans closer to home, and these were not available for retrospective review. Extensive efforts were undertaken to obtain imaging that patients received outside institutions but many radiologic studies were not available for retrospective interpretation. Imaging was evaluated for a response to treatments using the RECIST criteria reviewing CT scans from 1–6 months after treatment. Although, many patients had PET/CT available towards the end of the series they were not obtained consistently enough for analysis in patients early in the series. No patients had a complete radiologic response. A partial response (PR) was observed in 4 (13%) patients, stable disease was seen in 20 (64%), and progressive disease (PD) in 7 (23%). Of the 7 patients that had PD during the 1–6 months follow-up period, 3 patients had met criteria within 60 days, 3 had developed PD from 61–120 days, and 1 after 121 days. Of the 4 patients with PR, 2 had met criteria within 60 days and 2 within 61–120 days.
3.4. Survival Analysis
Survival data was acquired from the electronic medical record and a search of the Social Security Death Index. Thirty-eight (74%) patients died during the follow-up period. The median survival with 95% confidence interval and P-values were calculated for several variables to identify differences in survival between groups. The variables for the univariate analyses included gender, age, presence of extrahepatic disease, prior liver-directed therapy, chemotherapeutic agents failed, number of treatments (< or ≥2), concurrent administration of FUDR, CEA response, radiologic response, and early termination due to embolic process. Of the tested variables, the only variable that showed a significant difference in survival was the use of cetuximab prior to 90Y treatments.
The overall median survival was 10.2 months (95% CI = 7.5–13.0) for the entire cohort. The overall mean survival was 14.4 months (95% CI = 10.6–18.1). The Kaplan-Meier survival curve including all treated patients is shown in Figure 2. When the presence or lack of extrahepatic disease was analyzed for an association with survival, there was a trend toward improved survival in the absence of extrahepatic disease. The median survival was 6.7 months with extrahepatic disease (95% CI = 2.7–10.6) compared to 17.0 months for those without extrahepatic disease (95% CI = 6.4–27.6) (Figure 1). Those who had received cetuximab had a significantly decreased median survival (P = .001) (5.1 months; 95% CI = 2.6–7.5) compared to patients that had not received cetuximab prior to 90Y (18.3 months; 95% CI = 6.5–30.0). A significant survival difference was also observed in those that had previously failed cetuximab and bevacizumab, but not those who failed only bevacizumab (Table 4). Although a larger proportion of patients received biological agents during the final years of the study period, analysis comparing the era of 2002–2004 versus 2005–2008 revealed no significant difference in survival between the two eras.
Figure 2.
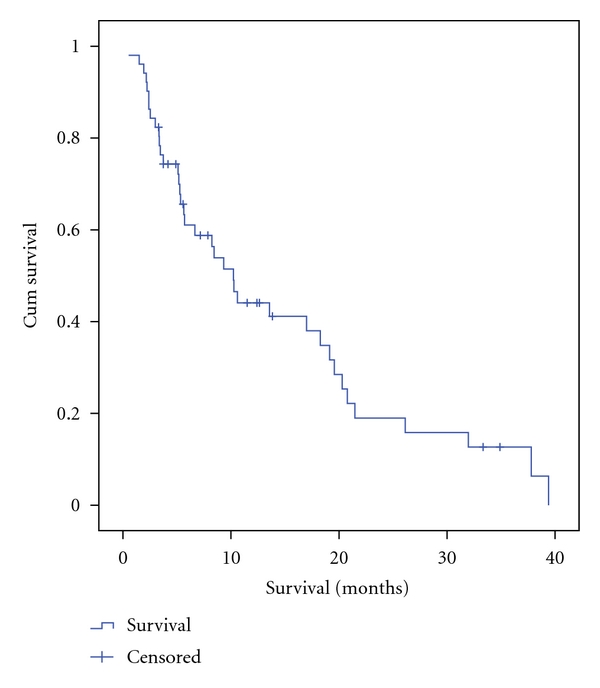
Kaplan-Meier survival curve for all patients (n = 51) treated with 90Y radioembolization. Survival was calculated from time of the first treatment with90Y. Median survival was estimated to be 10.2 months. The 95% confidence-interval is 7.5–13.0 months.
Figure 1.
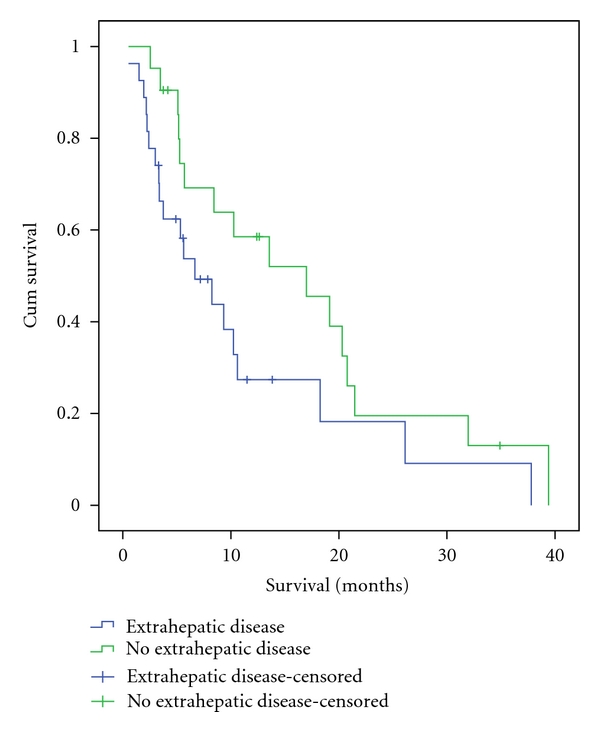
Kaplan-Meier survival curve comparing those with extrahepatic disease at the time of treatment versus those with disease localized to liver. The estimated median survival was 17.0 months for those without extrahepatic disease compared to 6.7 months. This difference was found not to be significant when using log-rank analysis, P-value of .07.
Table 4.
Survival analysis of those who had received bevacizumab and/or cetuximab prior to treatment with 90Y.
| n (%) | Median Survival (months) | 95% CI1 | |
|---|---|---|---|
| Failed cetuximab | 44 (100) | P = .001 | |
| Yes | 16 (36.4) | 5.1 | 2.6–7.5 |
| No | 28 (63.6) | 18.3 | 6.5–30.0 |
| Failed bevacizumab | 44 (100) | P = .36 | |
| Yes | 17 (38.6) | 8.2 | 5.0–11.4 |
| No | 27 (61.4) | 17.0 | 6.7–27.3 |
| Failed both bevacizumab and cetuximab | 44 (100) | P = .001 | |
| Yes | 9 (20) | 5.2 | 2.9–7.4 |
| No | 35 (80) | 13.6 | 4.9–22.2 |
195% Confidence interval.
CEA response was not associated with a significant improvement in survival (Figure 3). Those with a CEA decrease of ≥50% from baseline had a 19.1-month (95% CI = 6.4–31.9) median survival compared to 9.3 months (95% CI = 6.0–12.7) in those with less than a 50% decrease in CEA. When a radiologic response was analyzed for a survival advantage, there was not a significant association with survival observed (P = .22). Patients with a partial response had the longest median survival (21.5 months; 95% CI = 13.8–29.2) when compared to those who had progressive disease (13.6 months; 95% CI = 7.4–19.8) and those who had a stable radiological response (9.3 months; 95% CI = 6.9–11.8 months). The unexpected results of a longer median survival in those with progressive disease compared to stable disease is likely due to the small sample size and lack of events per group (i.e., 43% censored in progressive disease).
Figure 3.
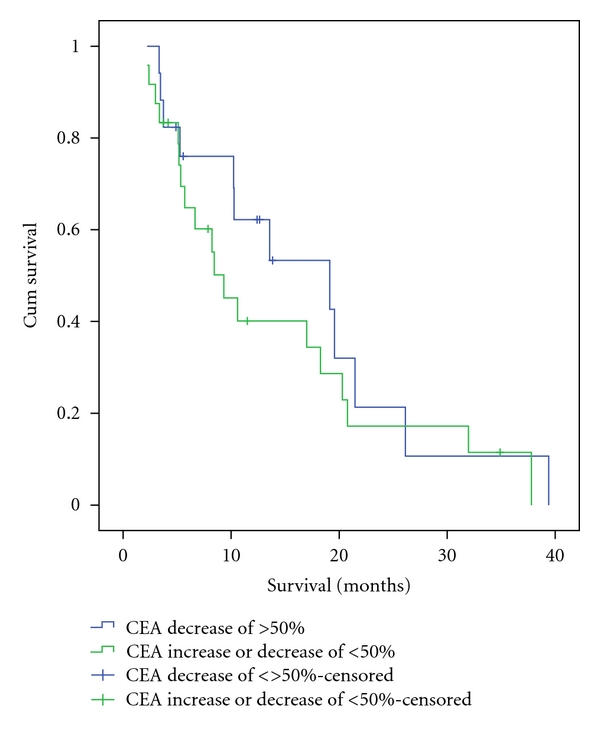
Kaplan-Meier survival curve comparing those with CEA response, as indicated by a decrease of ≥50% from baseline, to those who did not have a CEA response posttreatment. The estimated median survival was 19.1 months for those with a CEA response compared to 9.3 months. This difference was not found to be significant when using log-rank analysis P-value of .36.
3.5. Safety and Toxicity
Patient medical records were carefully reviewed to investigate patient complaints after treatment. The clinical toxicity profile was acceptable with fatigue, abdominal pain, and nausea being the most common subjective complaints documented; occurring in 22, 16, and 12% of patients, respectively. Complaints were minor, grade 1 or 2, and self-limiting generally resolving within one to two weeks after treatment. Three patients required hospital readmission within 30 days. Reasons for readmission included an upper GI bleed related to esophageal varices 4 days after treatment, unresolved abdominal pain and need for intravenous narcotics on postprocedure day 1, and the development of symptomatic brain metastases. One patient developed a complication at the time of the procedure developing ventricular tachycardia requiring ACLS and subsequent emergent cardiac catheterization. The posttreatment hepatic toxicity was assessed and found to be relatively mild. No patients had fulminant hepatic failure after treatment. Serial posttreatment bilirubin levels were available for review in 49 patients. Of the 47 patients who had a normal starting bilirubin levels, a grade 2 bilirubin toxicity was seen in 5 patients acutely (0–30 days) and 4 patients late (31–90 days). Late grade 3 or 4 toxicity was seen in two patients. The grade 4 toxicity was related to a biliary stricture and resolved with ERCP and stenting. No patients developed posttreatment gastric or duodenal ulceration, although all patients were placed on proton pump inhibitors prophylactically.
4. Discussion
Although the survival of patients with mCRC has been substantially extended with modern chemotherapy, the eventual progression of disease without a surgical cure is inevitable. Unfortunately, a surgical cure is only a viable option in the minority of patients. In this retrospective study we evaluated the efficacy and safety of using 90Y hepatic radioembolization in the salvage setting for advanced liver-dominant mCRC. Our cohort of patients consisted of a group that was highly pretreated with the current, most effective chemotherapeutic regimens. The median and mean survival of 10.2 and 14.4 months, respectively, after failure of all current treatment options is notable. The median survival is similar to that previously reported by others. Kennedy et al. had previously published their series of 208 patients treated with either whole liver or lobar 90Y for salvage therapy of unresectable mCRC. The median survival in this study was 10.5 months for those with treatment response and 4.5 months in those without a response to treatment [12]. Jakobs et al. had reported on a cohort of 41 patients that received mainly whole-liver 90Y that for liver-dominant mCRC with a median survival 10.5 months. One notable difference between the cohort reported here and that of Jakobs et al. is that in this cohort the presence of extrahepatic disease was 58.3% versus 17%, possibly signifying that our patients had more advanced disease. Sato et al. have also reported their two institution experiences of 137 patients with an assortment of primary malignancies treated with 90Y. Their cohort also contained 51 patients with mCRC, and they reported a median survival of 15.2 months in their subgroup analysis of colorectal cancer patients [15, 19].
There was significantly decreased survival observed in those who had previously failed cetuximab or both cetuximab and bevacizumab. A trend in decreased survival was seen in those who had previously failed bevacizumab. This result seems to suggest that benefit may be more limited in those who had previously failed biological chemotherapeutics. However, further validation of these findings is needed. Although unclear at this point, possible reasons for this difference could be that more aggressive tumor biology was selected or that patients were treated later in their disease course.
Surprisingly patients who had one or more of their treatments terminated with <80% of the prescribed dose administered had a trend towards improved survival. There is likely a range of effective dose of 90Y that elicits a tumor response, with patients who receive less than the prescribed dose also demonstrating a response to treatment most likely related to the completeness of the embolization. However, this observation requires further evaluation.
A trend towards improved survival was seen in those without extrahepatic disease at the time of treatment when compared to those with extrahepatic disease. The median survival was 17.0 months versus 6.7 months. Although this difference was not significant, our survival is comparable to the median survival of 17.5 months without extrahepatic disease and 6.9 months with extrahepatic disease that was previously reported [25]. The treatment of patients with widespread systemic disease has been an exclusion criteria for most treatments using locoregional therapies. In those who have already failed the first lines of the standard chemotherapeutic regimens, even in the face of a significant response of the liver metastases, they ultimately have progression of extrahepatic disease which will limit the survival. However, as seen in this study and others, the toxicity profile is acceptable in those with limited systemic disease. Although the benefit may not be substantial in those with extrahepatic disease, it is plausible to treat these patients since the liver is typically the primary life-limiting factor.
Even in this cohort of chemorefractory patients, an objective response to 90Y therapy was seen in a large proportion of those patients who had follow-up data available. A significant biochemical response, CEA decrease by ≥50%, was seen in 41% of patients. Although the median survival was improved in those with a CEA response, this was not a significant difference. Jakobs et al. had reported a significant difference in survival for those with any decrease in CEA with a median survival of 19.1 months in responders versus 5.4 months in nonresponders [19]. Kennedy et al. have also reported a significant difference in survival between those who had an objective response, either biochemically or radiographically [12]. The lack of significance seen in our study may be due to the larger number of patients with more advanced disease in our sample.
When an objective radiographic response using RECIST criteria was analyzed, 12.9% of patients had a partial response and 64.5% of patients had stable disease. In other similar retrospective studies the partial response was 17–35.5%, stable disease was 55–61%, and progressive disease was 9.8–10% [12, 19]. There was not a significant difference in survival associated with a radiographic response. However, it should be noted that follow-up imaging was available for only 31 of our patients and thus limits objective radiographic response analysis in this study.
The use of 90Y is shown to be well tolerated in patients with advanced disease. The observed toxicities in this report were similar to those noted by others with fatigue, abdominal pain, and nausea the most frequent subjective complaints. The incidence of these complaints was less than previously reported. Sato et al. had reported the incidence of fatigue, abdominal pain, and nausea to be 56%, 26%, and 23%, respectively, compared to 22%, 16%, and 12% observed in this cohort [15]. There were grade 2 bilirubin toxicities, either early or late, seen in only 9 patients, and grade 3 and 4 toxicities were observed in only one patient each. The pretreatment inclusion criteria of a total bilirubin level of <2.0 is likely adequate to select patients with adequate hepatic reserve to undergo treatment. Although there was one incidence of an upper gastrointestinal bleed from esophageal varices; soon after treatment we did not observe the known complications of treatment-related peptic ulcers and pneumonitis.
Our series has reinforced the findings of others. Mulcahy et al. reported their single-institution series of 72 patients using glass microspheres [26]. The treatment was well tolerated in these patients with self-limited treatment-related toxicities of fatigue, abdominal pain, and nausea, 61%, 25%, and 21%, respectively. Nine patients (12.6%) had grade 3 or 4 bilirubin toxicities. Using WHO criteria, a PR was noted in 40.3%, SD in 44.5%, and PD in 14.8%. The median time to PR was 4 months with a time to hepatic progression of 15.4 months. Survival analyses showed a median survival of 14.5 months from the time of initial treatment. Favorable predictors of survival were radiographic response, performance status, ≤25% tumor burden, and absence of extrahepatic disease. The median survival was not affected by the chemotherapy received prior to 90Y treatment.
Several others have looked at the efficacy and safety of 90Y radioembolization combined with a radiosensitizing chemotherapy regimen as salvage therapy [27–29]. In patients who had failed previous oxaliplatin- and irinotecan-based therapy, in a series of 46 evaluable patients radiographic response using RECIST criteria was CR in 2%, PR in 22%, SD in 24%, and PD in 44% [27]. In another recent publication, Ricke et al. published their phase I study analyzing the efficacy and safety of combining systemic chemotherapy, using irinotecan, and 90Y treatment for second-line therapy [28]. The combination therapy had an acceptable toxicity profile, not reaching the maximal tolerated dose using up to 100 mg/m2 of irinotecan on days 1 and 8 of a 3-week cycle. A large proportion of patients had extrahepatic disease, 48%, and the site of first disease progression after treatment was extrahepatic in 57%. The efficacy was promising with either radiographically PR or SD in 87% of patients. The median progression free survival was 6.0 months and progression free survival in the liver was 9.2 months. In yet another study, Van den Eynde et al. performed a multicenter randomized controlled phase III trial to assess the addition of 90Y resin microspheres to continuous infusion of 5FU in 46 patients [29]. The median time to liver progression was significantly longer in patients receiving RE compared with 5FU alone, 5.5, and 2.1 months, respectively. The median survival was 7.4 months in the 5FU-only arm and 9.9 months in patients receiving 5FU plus 90Y. This data leads us to question if patients with liver dominant mCRC should have multimodality treatment with 90Y and systemic chemotherapy earlier in the treatment algorithm.
It should be noted that the administration of 90Y for mCRC is a complex process that requires a multidisciplinary team. The cost of this process can be substantial. However, we feel that this cost compares favorably to a course of adjuvant therapy with biological agents such as bevacizumab. The treatments are generally well tolerated with the goal of maintaining a high quality of life during therapy.
In conclusion, our report represents one of the larger single-institution reports on the safety and efficacy of 90Y for chemorefractory patients with advanced liver-dominant mCRC. We provide data to support the use of 90Y in the salvage setting for patients after failing the latest chemotherapeutic regimens even in the presence of limited extrahepatic disease. The use of 90Y to halt or slow the progression of the hepatic tumor burden in patients with a terminal disease process in an attempt to extend survival seems to be achievable. The major limitations of this study is that it is a retrospective study and although based in the setting of a single-institution the patient population was heterogeneous with most patients being referred to our tertiary center from other institutions. We present evidence to suggest that 90Y is an effective and safe treatment option in the salvage setting; however, there is still further validation required in the form of prospective trials which are ongoing. In order to convincingly support the benefit gained with locoregional therapy, progression free survival needs to be determined. Overall survival is often suboptimal to determine benefit since progression of disease will often prompt alternative treatments. Further work also needs to be done to evaluate whether 90Y may have benefit earlier in the treatment algorithm prior to the situation in which most, if not all other options are limited.
Conflict of Interests
None of the authors have identified a conflict of interests.
Acknowledgment
This work is supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grants (K12 HD04910 and NCI-5K07CA118576) from the National Institute of Health.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer Journal for Clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Kemeny N, Paty P, Blumgart LH, Cohen AM. Treatment of colorectal cancer: hepatic metastasis. Seminars in Surgical Oncology. 1996;12(4):219–252. doi: 10.1002/(SICI)1098-2388(199607/08)12:4<219::AID-SSU3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Steele G, Ravikumar TS. Resection of hepatic metastases from colorectal cancer: biologic perspectives. Annals of Surgery. 1989;210(2):127–138. doi: 10.1097/00000658-198908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breedis C, Young G. The blood supply of neoplasms in the liver. The American journal of pathology. 1954;30(5):969–977. [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of 90Y microspheres in man: review of four explanted whole livers. International Journal of Radiation Oncology Biology Physics. 2004;60(5):1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6. SIR-Spheres® microspheres package insert. Sirtex Medical Inc., Wilmington, Mass, USA, 2009, http://www.sirtex.com/files/US20Package20Insert1.pdf.
- 7. TheraSphere® Yttrium-90 Glass Microspheres. MDS Nordion, Ottawa, Canada, 2009, http://www.mdsnordion.com/therasphere/physicians-package-insert/package-insert-us.pdf.
- 8.Gray BN, Burton MA, Kelleher DK, Anderson J, Klemp P. Selective internal radiation (SIR) therapy for treatment of liver metastases: measurement of response rate. Journal of Surgical Oncology. 1989;42(3):192–196. doi: 10.1002/jso.2930420313. [DOI] [PubMed] [Google Scholar]
- 9.Gray BN, Anderson JE, Burton MA, et al. Regression of liver metastases following treatment with yttrium-90 microspheres. Australian and New Zealand Journal of Surgery. 1992;62(2):105–110. doi: 10.1111/j.1445-2197.1992.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 10.Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres® plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Annals of Oncology. 2001;12(12):1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 11.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-spheres® plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. Journal of Surgical Oncology. 2004;88(2):78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. International Journal of Radiation Oncology Biology Physics. 2006;65(2):412–425. doi: 10.1016/j.ijrobp.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 13.Lim LC, Gibbs P, Yip D, et al. A prospective evaluation of treatment with selective internal radiation therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer. 2005;5, article 132 doi: 10.1186/1471-2407-5-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim L, Gibbs P, Yip D, et al. Prospective study of treatment with selective internal radiation therapy spheres in patients with unresectable primary or secondary hepatic malignancies. Internal Medicine Journal. 2005;35(4):222–227. doi: 10.1111/j.1445-5994.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 15.Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres—safety, efficacy, and survival. Radiology. 2008;247(2):507–515. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]
- 16.Mancini R, Carpanese L, Sciuto R, et al. A multicentric Phase II clinical trial on intra-arterial hepatic radiotherapy with 90Yttrium SIR-spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy: preliminary results on toxicology and response rates. In Vivo. 2006;20:711–714. [PubMed] [Google Scholar]
- 17.Stubbs RS, O’Brien I, Correia MM. Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. ANZ Journal of Surgery. 2006;76(8):696–703. doi: 10.1111/j.1445-2197.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- 18.Murthy R, Xiong H, Nunez R, et al. Yttrium 90 resin microspheres for the treatment of unresectable colorectal hepatic metastases after failure of multiple chemotherapy regimens: preliminary results. Journal of Vascular and Interventional Radiology. 2005;16(7):937–945. doi: 10.1097/01.RVI.0000161142.12822.66. [DOI] [PubMed] [Google Scholar]
- 19.Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic Yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. Journal of Vascular and Interventional Radiology. 2008;19(8):1187–1195. doi: 10.1016/j.jvir.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 21.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. Journal of Clinical Oncology. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 22.Salem R, Thurston KG. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies—part 1: technical and methodologic considerations. Journal of Vascular and Interventional Radiology. 2006;17(8):1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. International Journal of Radiation Oncology Biology Physics. 2007;68(1):13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Stubbs RS, Cannan RJ, Mitchell AW. Selective Internal Radiation Therapy with Yttrium Microspheres for Extensive Colorectal Liver Metastases. Journal of Gastrointestinal Surgery. 2001;5(3):294–302. doi: 10.1016/s1091-255x(01)80051-2. [DOI] [PubMed] [Google Scholar]
- 26.Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using Yttrium-90 microspheres. Cancer. 2009;115(9):1849–1858. doi: 10.1002/cncr.24224. [DOI] [PubMed] [Google Scholar]
- 27.Cosimelli M, Mancini R, Carpanese L. Phase II multicenter study of 90Yttrium resin microspheres for patients with unresectable colorectal liver metastases who had failed prior oxaliplatin- and irinotecan-based regimens. In: Proceedings of the ASCO Gastrointestinal Cancers Symposium; 2009; Abstract 452. [Google Scholar]
- 28.Ricke J, Rühl R, Seidensticker M, et al. Extensive liver-dominant Colorectal (CRC) Metastases failing multiple lines of systemic chemotherapy treated by 90Y Radioembolization: a matched-pair analysis. 11th World Congress of GI Cancer. Annals of Oncology. 2009;20(supplement 6) Abstract PD-002. [Google Scholar]
- 29.Van den Eynde M, Hendlisz A, Peeters M. Prospective randomized study comparing intraarterial injection of yttrium-90 resin microspheres with protracted IV 5FU continuous infusion versus IV 5FU continuous infusion alone for patients with liver-limited metastatic colorectal cancer refractory to standard chemotherapy. Journal of Clinical Oncology. 2009;27(supplement 7) ASCO Annual Meeting, abstract 4096. [Google Scholar]


