Abstract
Rationale
The ability to withhold reinforced responses—behavioral inhibition—is impaired in various psychiatric conditions including Attention Deficit Hyperactivity Disorder (ADHD). Methodological and analytical limitations have constrained our understanding of the effects of pharmacological and environmental factors on behavioral inhibition.
Objectives
To determine the effects of acute methylphenidate (MPH) administration and rearing conditions (isolated vs. pair-housed) on behavioral inhibition in adult rats.
Methods
Inhibitory capacity was evaluated using two response-withholding tasks, differential reinforcement of low rates (DRL) and fixed minimum interval (FMI) schedules of reinforcement. Both tasks made sugar pellets contingent on intervals longer than 6 s between consecutive responses. Inferences on inhibitory and timing capacities were drawn from the distribution of withholding times (interresponse times, or IRTs).
Results
MPH increased the number of intervals produced in both tasks. Estimates of behavioral inhibition increased with MPH dose in FMI and with social isolation in DRL. Nonetheless, burst responding in DRL and the divergence of DRL data relative to past studies, among other limitations, undermined the reliability of DRL data as the basis for inferences on behavioral inhibition.
Conclusions
Inhibitory capacity was more precisely estimated from FMI than from DRL performance. Based on FMI data, MPH, but not a socially enriched environment, appears to improve inhibitory capacity. The highest dose of MPH tested, 8 mg/kg, did not reduce inhibitory capacity but reduced the responsiveness to waiting contingencies. These results support the use of the FMI schedule, complemented with appropriate analytic techniques, for the assessment of behavioral inhibition in animal models.
Keywords: Impulsivity, Methylphenidate, Housing, Fixed minimum interval, Differential reinforcement of low rates, Temporal regulation, Model, Reinforcement, Stimulants, Timing
Introduction
Impulsivity is a critical component of several behavioral disorders including substance abuse (Bechara 2005; de Wit 2009; Perry and Carroll 2008), personality disorders (Chapman et al. 2008), and Attention Deficit Hyperactivity Disorder (ADHD; Barkley 1997). Behavioral disinhibition is the variety of impulsivity characterized by a chronic inability to withhold reinforced responses, which is a distinctive feature of ADHD (Barkley 1997). This variety of impulsivity is sensitive to pharmacological treatment (Aron et al. 2003; Boonstra et al. 2005; Broyd et al. 2005; Chamberlain et al. 2007; DeVito et al. 2009; Fillmore et al. 2003; Kratz et al. 2009; Nandam et al. 2011; O’Driscollet al. 2005; Potter and Newhouse 2004; Scheres et al. 2003; Snyder et al. 2008; Turner et al. 2004; Wilson et al. 2006) and environmental factors (Pressman et al. 2006; Young et al. 2009).
The preclinical evaluation of new pharmacological treatments for behavioral disinhibition requires the implementation of appropriate tests in animal models, typically rodents. Such tests are validated, in part, by demonstrating sensitivity to pharmacological effects analogous to those observed in humans. One such effect is the enhancement of behavioral inhibition induced by methylphenidate (MPH; Boonstra et al. 2005; Broyd et al. 2005; Kratz et al. 2009; Nandam et al. 2011; O’Driscoll et al. 2005; Trommer et al. 1991). Extant tests of behavioral inhibition do not reliably replicate this effect in rodents. Consider the 5-Choice Serial Reaction Time Task (5-CSRTT; Bari et al. 2008; Robbins 2002), in which impulsivity is measured as reports of the presence of a target stimulus before it is presented. Using the 5-CSRTT, some research has shown that 2-10 mg/kg MPH reduces impulsivity indices (Bizarro et al. 2004) but other studies suggest that 5 mg/kg MPH increases impulsivity (Navarra et al. 2008). In response-withholding tasks such as the differential reinforcement of low rates (DRL; Ferster and Skinner 1957) and lever holding (Sanabria and Killeen 2008), exposure to MPH yields deteriorated measures of inhibition (Emmett-Oglesby et al. 1980; Ferguson et al. 2007; Orduña et al. 2009; Pearl and Seiden 1976; Seiden et al. 1979) and timing (Mayorga et al. 2000). Evenden and Ko (2005) reported that 6 mg/kg MPH increased the number of extreme chains—i.e., very long and very short—produced using the fixed consecutive number (FCN) method with shock avoidance, which suggests disrupted counting.
A factor that may contribute to these inconsistent findings is the rearing conditions of subjects, which often varies between studies. Dalley et al. (2002) report reduced impulsivity in isolated rats in the 5-CSRTT, but these results were a nonsignificant trend. Contrary to the 5-CSRTT findings, isolated rats in DRL produced more premature responses (Ough et al. 1972). In an alternating 2-lever DRL, isolated rats made more lever presses than enriched rats, displaying impulsivity by earning fewer overall rewards (Morgan and Einon 1975). Because housing conditions have a substantial influence on impulsivity-related behavior such as the self-administration of drugs of abuse (e.g., Bardo et al. 2001; Thiel et al. 2009), it is likely that MPH effects on behavioral inhibition interact with rearing environment.
There are probably other confounding factors, aside from rearing environment, that contribute to the inconsistent effects of MPH on behavioral inhibition. Many of the conventional measures of inhibition are confounded by changes in motivation (Conrad et al. 1958; Doughty and Richards 2002), attentional processes (Hahn et al. 2002), and motor perseveration (Kramer and Rilling 1970; Roffman and Raskin 1997). Although many of these processes are likely to be related to impulsivity (e.g., Volkow et al. 2010), the precise assessment of the capacity to withhold reinforced responses requires their dissociation.
The present study sought to overcome the limitations of conventional research on animal impulsivity by (1) collecting data on the behavioral impact of MPH and rearing environment using the fixed minimum interval (FMI) schedule of reinforcement (Mechner and Guevrekian 1962), and (2) estimating inhibitory capacity from these data using the Temporal Regulation (TR) model of behavioral inhibition (Sanabria and Killeen 2008). Like DRL, the FMI paradigm requires animals to withhold a response for a fixed interval; unlike DRL, however, the interval-initiating response in FMI is different from the interval-terminating response. FMI empirically dissociates incentive motivation and perseverative motor responses, which are expressed in interval-initiating responses, from behavioral inhibition, which is expressed in the duration of the interval produced (Mechner and Guevrekian 1962). These measures are confounded in DRL (Doughty and Richards 2002; Richards et al. 1993). The primary goal of this study was to determine whether the detrimental effects of isolated housing and MPH on impulsivity, reported using DRL, change when measured in FMI using the TR model. Reversing the effect of MPH, in particular, would validate FMI as a behavioral inhibition paradigm, providing an essential tool for the preclinical evaluation of treatments of impulsivity.
The TR model posits that the distribution of response-withholding intervals is a mixture of two independent probability distributions. The first distribution comprises intervals that are sensitive to the waiting contingency, which are clustered around the target interval. The other distribution comprises intervals that are not sensitive to the waiting contingency, and generates what is often called the “DRL burst” (Richards et al. 1993). The TR model is intended to analytically dissociate aspects of response-withholding performance that are more informative of behavioral inhibition from those that are indicative of timing precision and other potentially confounding factors such as burst responses. More specifically, the TR model proposes the mean waiting interval as an estimate of inhibitory capacity.
Only one previous study has tested the effect of MPH on FMI performance (Mechner and Latranyi 1963). Their report, however, was limited to four rats tested at relatively high doses (6–48 mg/kg). They found that these high doses of MPH generally flattened the distribution of intervals produced. A secondary goal of this study was to extend Mechner and Latranyi’s (1963) research to a lower range of doses of MPH (0.5–8 mg/kg) and a larger number of rats.
Method
Subjects
Twenty-four male Wistar rats (Charles River, Laboratories, Hollister CA) served as subjects. Rats arrived at the laboratory on postnatal day (PND) 24. Rats had free access to food daily and were housed on a 12/12 h light-dark cycle (dawn at 6 am) in translucent polycarbonate cases (260 mm wide by 460 mm deep by 210 mm high) covered with Sanichip bedding and wire lids. Other specific housing conditions (rats per cage, objects in cage) were part of the experimental manipulation and are explained in the procedure section.
Apparatus
Experimental sessions were conducted in ten MED Associates® modular test chambers (four boxes were 305 mm long, 241 mm wide, and 210 mm high; six boxes were 305 mm long, 241 mm wide, and 292 mm high), each enclosed in a sound- and light-attenuating box equipped with a ventilating fan. The floor consisted of thin metal bars above a catch pan. The front and rear walls and the ceiling of the experimental chambers were made of clear plastic, with the front wall hinged and functioning as a door to the chamber. A square aperture (51-mm sides) located 15 mm above the floor and centered on an aluminum side panel on the right side of the chamber provided access to a receptacle (ENV-200-R2M) for 45 mg sugar flavored pellets (Dustless Precision pellets, product # F0042, Bio-Serv, Frenchtown, NJ) furnished with a head entry detector (ENV-254-CB). Each activation of the dispenser (ENV-203) delivered a single pellet. A retractable lever (ENV-112CM) was located on each side of the food hopper. Only the lever closest to the chamber door was operative; the other lever remained retracted and inoperative throughout the experiment. The center of the lever was 80 mm from the center of the food hopper, and 21 mm from the floor. Lever presses were recorded when a force of approximately 0.15 N was applied to the end of the lever. Three-color light stimuli (ENV 222M) were located directly above each retractable lever and could be illuminated yellow, green, and red. The ventilation fan mounted on the sound-attenuating chamber provided masking noise of approximately 60 dB. The test chambers could be dimly illuminated by a houselight located behind the left wall of the chamber. Experimental events were arranged via a Med-PC® interface connected to a PC controlled by Med-PC IV® software.
Procedure
Rearing environment
Rats were separated into two groups beginning postnatal day (PND) 25. Half of the rats were assigned to a mildly enriched environment (Group Paired, n=12) and half to an isolated environment (Group Isolated, n=12). The mildly enriched environment involved housing two rats per cage with a PVC pipe and a crumpled up sheet of paper towel. The PVC pipe was moved and a new paper towel was provided at least once a week, when cages were changed for cleaning. The isolated environment involved housing a single rat per cage with no objects. All rats had an experimental history with an autoshaping procedure from PND 35 to PND 86 using the same apparatus described in this report. The autoshaping procedure consisted of pairing either a 3-kHz tone or a lever insertion with the probabilistic delivery of a food pellet (p=0.1). Experience with one conditioning treatment or the other was counter-balanced across experimental groups.
Training
Training sessions were conducted once daily, 7 days per week for each rat, starting on PND 89. Sessions started with a 5-min habituation period, during which levers were retracted and the chamber was dark. Insertion of the lever and illumination of the houselight signaled the beginning of experimental conditions. The experimental procedure involved a 6-s waiting task with a conjunctive variable interval (VI) 60 schedule of reinforcement superimposed on the waiting schedule (Sagvolden and Berger 1996). The conjunctive VI schedule was included to maintain rates of reinforcement nearly constant across animals, thus minimizing the likelihood that performance was influenced by rate of reinforcement.
Initially, the target time and the VI requirement were set to 0.5 and 2 s, respectively. The target time increased with each reinforcer by 1.25% across sessions until reaching 6 s, where it remained constant. The 6-s target time was chosen to strengthen control by target time, which is weak at longer intervals (Doughty and Richards 2002; Sanabria and Killeen 2008). After reaching the 6-s target time, the VI requirement was increased to 9, 13, 19, 28, 42, and 60 s, in daily succession. The schedule of reinforcement of interresponse times (IRTs) greater than 6 s was then fixed at VI 60 s (intervals were drawn from a 12-item Fleshler-Hoffman distribution; Fleshler and Hoffman 1962).
The waiting task required rats to either press a lever (Group Lever, n=12) or remove their heads from the food hopper (Group Head, n=12) to start a clock. While the clock was running, the three-color light stimuli above both levers were illuminated and the houselight was turned off. A head entry into the food hopper stopped the clock and terminated the lights. The duration of the clock running constituted the interresponse time (IRT). If the IRT was longer than a programmed target time, it counted as a correct interval. Each correct interval was reinforced with a food pellet if the conjunctive VI had elapsed. Incorrect intervals were followed by a 2-s blackout. The difference in reinforcement contingencies between Groups Lever and Head are highlighted in Fig. 1.
Fig. 1.
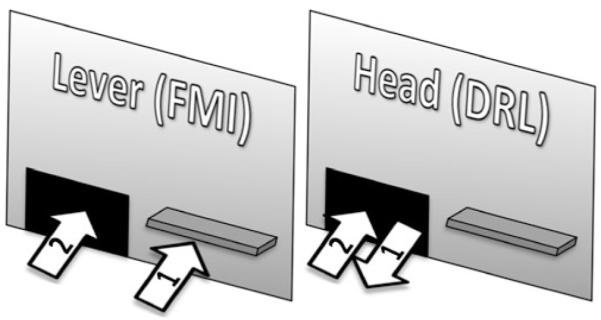
Illustration of reinforcement contingencies in Groups Lever (FMI) and Head (DRL). For Group Lever, intervals were initiated by a lever press (1) and terminated by a head entry into the food receptacle, detected by an infrared photocell (2); iterative lever presses had no programmed consequences. For Group Head, intervals were initiated by a head removal from the food receptacle (1) and terminated by a head entry (2); the lever was not functional. Intervals longer than 6 s were reinforced intermittedly according to a VI 60-s schedule
It is important to note that lever presses did not stop the clock. Thus, a rat in Group Lever could start the clock with a lever press and continue lever pressing while the clock was running. These subsequent lever presses had no programmed consequences. This is a potentially significant difference between the lever press-head entry sequence required from Group Lever and the conventional DRL arrangement, where each lever press restarts the clock. Whereas reinforcement was arranged in a (intermittent) FMI schedule for Group Lever, Group Head experienced contingencies more similar to DRL. For Group Head each clock-starting head exit involved a clock-stopping head entry, and thus repeated head exits were impossible. For this group, a lever was available but had no programmed consequences.
Each session ended after 75 min or after 40 food pellets were obtained, whichever happened first. Experimental sessions continued for a minimum of 15 days and until stable performance was attained, based on visual inspection.
MPH
Following ten sessions of stable performance, daily experimental sessions continued with MPH injections on Tuesdays and Fridays. Rats were injected with saline alone or methylphenidate hydrochloride (MPH; 0.5, 2, or 8 mg/ kg, i.p.; Hawkins Pharmaceutical Group, Minneapolis, MN) 15 min before each session. This dose range envelops the range of therapeutic doses for ADHD (Faraone et al. 2004), even when differences in bioavailability are taken into account given route of administration (i.p. vs. p.o.; Gerasimov et al. 2000), and includes a high dose (8 mg/kg) to allow for inverted U-shaped dose-dependent functions. Behavioral effects of MPH were likely elicited within the length of each session, because peak locomotor effects of 2-10 mg/kg i.p. MPH in rats are observed at 20-40 min, and decline to half 20-60 min later (Gerasimov et al. 2000). Two dose cycles were conducted, with order of dose injected counterbalanced across groups following a Latin square design.
Dependent measures
The mean number of intervals (correct and incorrect) produced, and the mean proportion of correct intervals were measured in each experimental session to determine performance stability. The stable measures of performance used for data analysis were total intervals (correct and incorrect) per hour, reinforcers collected per hour, proportion of correct intervals, and parameter estimates of the Temporal Regulation (TR) model. Behavioral inhibition parameters were estimated for each individual rat by fitting Sanabria and Killeen’s (2008) TR model to IRT distributions. According to the model, the distribution of IRTs is a mixture of a gamma and an exponential distribution (waiting and nonwaiting IRTs, respectively),
| (1) |
Equation 1 indicates that the probability of an IRT of duration t is a function of five parameters: p, N, c, k and δ. p is the proportion of waiting IRTs; 1-p, the proportion of nonwaiting IRTs. N and c are, respectively, the shape and scale parameters of gamma distribution Γ. k is the mean nonwaiting IRT. δ is the shortest interval possible (e.g., the time it takes a rat to move from the lever to the hopper in Group Lever). Parameter δ was estimated as the shortest IRT that the rat produced, and it was therefore not a free parameter (Brackney et al. 2011).
Temporal Regulation parameters were estimated using the method of maximum likelihood (Myung 2003). Two parameters of the distribution of waiting IRTs provided estimates of behavioral inhibition and timing precision. Response threshold θ was calculated as the mean waiting IRT divided by the target time, (N×c)/6, with c estimated in seconds. Higher response thresholds are indicative of longer waiting for the reward and, therefore, higher inhibitory capacity (Sanabria and Killeen 2008). The coefficient of variation of waiting IRTs, w, was computed as their standard deviation divided by their estimated mean, which reduces to √(1/N). Higher estimates of w are indicative of reduced timing precision. Sanabria and Killeen (2008) provide a more detailed discussion of the TR model.
Statistical analysis
Intervals per hour, reinforcers per hour, proportion of correct intervals, and estimates of p (proportion of waiting IRTs), k (mean nonwaiting IRT), θ (response threshold), and w (timing imprecision) were analyzed using two 2×4 mixed design ANOVAs, one for Group Lever and one for Group Head, with housing (Paired vs. Isolated) as between-subject factor and MPH dose as within-subject factor. When assumptions of sphericity were not met, a Huynh-Feldt correction was applied. Effects with p<0.05 were deemed significant; only significant effects are reported. Significant dose effects were further examined using repeated contrasts (i.e., comparing consecutive levels of MPH dose). Significant interaction effects were further examined using two-tail t-tests.
Results
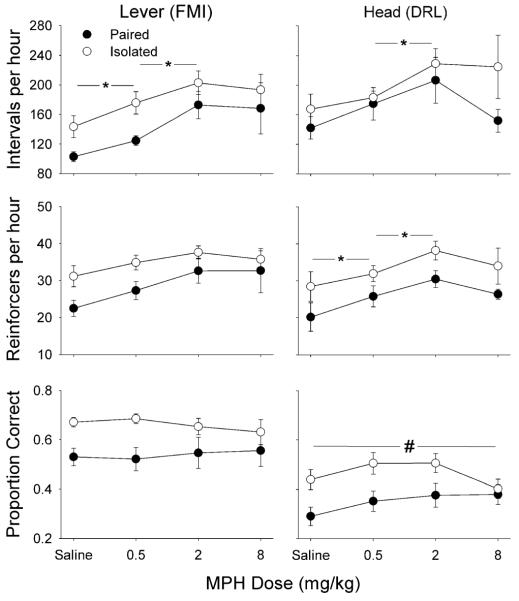
Figure 2 shows mean (±SEM) intervals per hour, reinforcers per hour, and proportion of correct intervals for each group of rats at each dose of MPH. Group Lever displayed a significant main effect of dose on intervals per hour (F2.2,22.2=4.65, p=0.018), which resulted from a 22% average increase in intervals per hour between saline and 0.5 mg/kg MPH (F1,10=9.42, p=0.012) and an additional 27% between 0.5 and 2 mg/kg (F1,10=7.23, p=0.023). Group Head also displayed a significant main effect of dose on intervals per hour (F3,30=3.95, p=0.017), which resulted from a 22% average increase in intervals per hour between 0.5 and 2 mg/kg MPH (F1,10=9.25, p=0.012).
Fig. 2.
Intervals produced per hour (top), reinforcers collected per hour (middle), and proportion of correct intervals produced (bottom) by Groups Lever (left) and Head (right), and Groups Paired (filled symbols) and Isolated (open symbols), at each dose of MPH. Asterisk significant effect of MPH dose, number sign significant effect of housing; α=0.05. Doses of 0.5 and 2 mg/kg MPH in Group Lever and of 2 mg/kg in Group Head significantly increased the number of intervals per hour relative to lower doses (p=0.012, 0.023 and 0.012, respectively). In Group Head, 0.5 and 2 mg/kg MPH significantly increased the number of reinforcers collected per hour (p=0.04, p=0.025), and isolated housing increased the proportion of correct intervals (p=0.035)
The number of reinforcers obtained per hour is shown in the middle panels of Fig. 2. No significant effect of housing or MPH was observed in Group Lever. Group Head displayed a significant main effect of MPH dose on reinforcers obtained per hour, (F3,30=4.47, p=0.01), which resulted from a 17% average increase between saline and 0.5 mg/kg MPH (F1,10=5.56, p=0.04) and an additional 19% between 0.5 and 2 mg/kg (F1,10=6.95, p=0.025).
The proportion of correct intervals in shown in the bottom panels of Fig. 2. No significant effect of housing or MPH was observed in Group Lever. Group Head displayed a significant main effect of housing (F1,10=5.98, p=0.035) on the proportion of correct intervals. Isolated rats produced IRTs longer than 6 s in nearly half of the trials, whereas Paired rats produced them in only about a third.
Figure 3 shows fits of the TR model to the mean distribution of IRTs for each dose of MPH in each group, plotted in 0.5-s bins. Curves were fit using the method of least squares. In general, the TR model provided a good description of the distributions of IRTs. MPH decreased the relative frequency of the shortest IRTs (<0.5 s) produced by Group Head. Close inspection of the graphs reveals that, in all groups, the 0.5 and 2 mg/kg doses of MPH shifted the IRT distributions slightly to the right, and the 8 mg/kg dose flattened the distribution of IRTs. Visible differences are also apparent between Groups Lever and Head. Group Head emitted a high proportion of very short IRTs, seen as the high, left tail of the distribution, a pattern not observed in the Lever group. Group Lever also had a higher proportion of IRTs tightly clustered around 6.
Fig. 3.
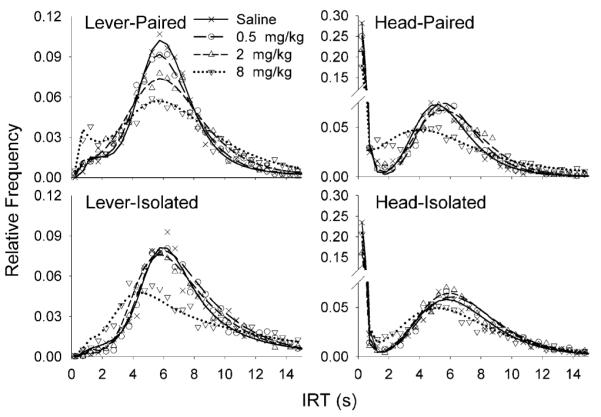
Distribution of IRTs shorter than 15 s in each task (columns)× housing (rows) group, for each MPH dose (symbols). Each data point is placed above the midpoint of the bin, so the first point on each graph shows the proportion of IRTs between 0 and 0.5 s, the next point between 0.5 and 1.0 s, and so on. Curves are fits of the Temporal Regulation (TR) model of behavioral inhibition (Eq. 1). DRL burst IRTs are visible on the left-end tail of the plots of Group Head; this pattern was absent in Group Lever. The highest dose, 8 mg/kg, of MPH flattened the IRT distributions of all groups. The TR model provided an adequate account of all the IRT distributions
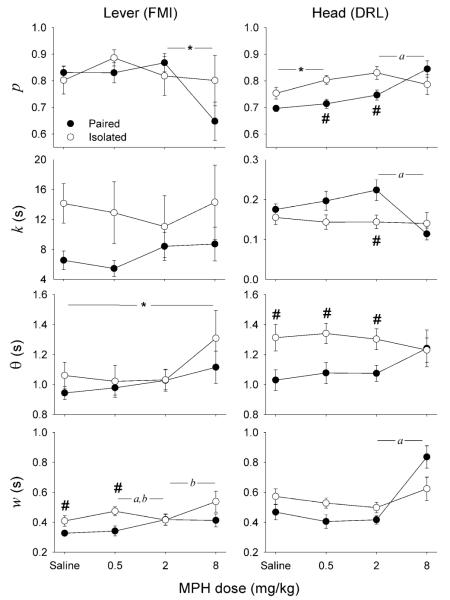
Figure 4 shows TR parameter estimates for each group at each MPH dose. Group Lever displayed a significant main effect of dose on p (F3,30=3.91, p=0.018), which resulted from an average reduction in p from 0.85 to 0.71 between 2 and 8 mg/kg MPH (F1,10=7.55, p=0.021).
Fig. 4.
Estimates of two Temporal Regulation (TR) parameters (p propotion of waiting intervals, k the mean nonwaiting IRT) and two waiting indices (θ response threshold, a measure of inhibitory capacity; w the coefficient of variation, a measure of timing imprecision), based on the perfomance of Groups Lever (left) and Head (right), Paired (filled symbols) and Isolated (open symbols), at each dose of MPH. Note the difference in y-axis scale between Lever and Head plots of k. Asterisk significant effect of MPH dose, number sign significant effect of housing, a=significant effect of MPH in Paired rats, b=significant effect of MPH in isolated rats, α=0.05. MPH of 8 mg/kg significantly reduced p in Group Lever (p=0.021), increased it in Group Head-Paired (p=0.016), reduced k in Group Head-Paired (p=0.004), and increased w in Groups Lever-Isolated (p =0.027) and Head-Paired (p=0.006). A significant and progressive increase of θ was observed over MPH doses in Group Lever (p= 0.016). Isolation increased θ at doses lower than or equal to 2 mg/kg in DRL (p=0.024-0.042)
Group Head displayed a significant main effect of dose and dose×housing interaction effect on p (F2.06,20.61=7.39, p=0.004; F2.06,20.61 =5.86, p=0.009, respectively). The main effect of dose was due to an increase in p from 0.73 to 0.77 between saline and 0.5 mg/kg MPH (F1,10=5.7, p=0.038). The interaction effect was restricted to the change in p between 2 and 8 mg/kg MPH (F1,10=17.97, p=0.002). At 0.5 and 2 mg/kg MPH, p was lower for Head-Paired than for Head-Isolated rats (t10=3.49, p=0.006, and t10=2.48, p= 0.032, respectively), but it increased in Head-Paired rats between 2 and 8 mg/kg (t4=4, p=0.016).
No significant effect of housing or MPH was observed on mean nonwaiting IRT, k, in Group Lever. Group Head displayed a significant dose×housing interaction effect on k (F3,30=3.83, p=0.02). This effect was restricted to the change in k between 2 and 8 mg/kg MPH (F1,10=12.93, p=0.005). At 2 mg/kg MPH, k was longer for Paired relative to Isolated rats (t10=2.65, p=0.024), but it declined by about half in Paired rats between 2 and 8 mg/kg (t4=5.88, p=0.004). Note that the difference in estimates of k between Groups Lever and Head is roughly two orders of magnitude.
Group Lever displayed a significant main effect of dose on the response threshold, θ (F3,30=4.01, p=0.016). Repeated contrasts were not significant suggesting that the effect was not due to any particular dose but that, instead, mean θ increased progressively with MPH dose. Group Head also displayed significant dose×housing interaction on θ (F3,30=3.33, p=0.033). This effect was restricted to the change in θ between 2 and 8 mg/kg MPH (F1,10=8.06, p=0.018), which resulted from Isolated rats waiting longer than Paired rats at saline, 0.5, and 2 mg/kg (t10=2.33, p=0.042, t10=2.65, p=0.024, and t10=2.4, p=0.037, respectively), but not at 8 mg/kg MPH.
Both Groups Lever and Head displayed a significant dose×housing interaction effect on w (F3,30=3.01, p=0.046 and F2.27,22.7=6.48, p=0.005, respectively). For Group Lever, this effect was observed between 0.5 and 2 mg/kg (F1,10=18.23, p=0.002) and between 2 and 8 mg/kg MPH (F1,10=7.14, p=0.023). At saline and 0.5 mg/kg, w was higher for Isolated relative to Paired rats (t10=2.32, p=0.043 and t10=2.81, p=0.018, respectively); w decreased for Isolated rats (t4=8.26, p=0.001) and increased for Paired rats (t6=2.89, p=0.028) at 2 mg/kg, reaching virtually identical estimates (0.42); w increased again for Isolated rats at 8 mg/kg (t4=3.39, p=0.027). For Group Head, the interaction effect was restricted to the change in w between 2 and 8 mg/kg MPH (F1,10=7.16, p=0.023), which resulted from w doubling for Paired rats (t4=5.39, p=0.006), but not Isolated rats, between these doses.
Discussion
Behavioral inhibition estimates from FMI
The main purpose of this study was to explore the effects of rearing environment and MPH on behavioral inhibition. To draw inferences on inhibition, a group of rats (Lever) was exposed to an FMI schedule of reinforcement. To estimate the index of inhibitory capacity θ (Sanabria and Killeen 2008), FMI performance was analyzed using the TR model of behavioral inhibition. As shown in Fig. 4, MPH, but not pair-rearing, enhanced inhibitory capacity in Lever rats. The effect of MPH is consistent with evidence from human research (Boonstra et al. 2005; Broyd et al. 2005; Kratz et al. 2009; Nandam et al. 2011; O’Driscoll et al. 2005; Trommer et al. 1991). Unlike estimates of θ from FMI performance, the proportion of correct intervals was not sensitive to the inhibitory-enhancing effects of MPH (Fig. 2, bottom-right panel). Response-withholding performance statistics such as “efficiency” or “accuracy” do not appear to be sensitive to stimulant-induced inhibitory enhancement (cf., Ferguson et al. 2007; Mayorga et al. 2000; van den Bergh et al. 2006).
An alternative explanation to the effect of MPH on θ is that it reduced the motivation for rewards, thus depressing the overall rate of responding (Brackney et al. 2011). This explanation, however, is inconsistent with the dose-dependent increase in the number of intervals per hour (Fig. 2, top-right panel). The MPH-induced increase in intervals produced is consistent with the increase in response rate systematically observed in rats exposed to MPH in other response-withholding preparations (Emmett-Oglesby et al. 1980; Ferguson et al. 2007; Orduña et al. 2009; Pearl and Seiden 1976; Seiden et al. 1979), and even when response-withholding contingencies are not in place (Heyman 1992; Ts’o et al. 1976). It may be that MPH enhances incentive motivation (Mechner and Guevrekian 1962), reduces aversive properties of delayed reward (Brown and Flory 1972; Shiels et al. 2009; Solanto et al. 2001) or lengthens the delay-of-reinforcement gradient (Johansen et al. 2007; Sagvolden et al. 1988). Future research should further explore the motivational and learning effects of MPH, separately from its inhibitory-enhancing effects.
It is possible that a further enhancement of the rearing environment of Paired rats (e.g., with more objects, rats, space, etc.) would have raised estimates of behavioral inhibition in FMI. It is also possible that the reduction in space per rat in the Paired condition countered any inhibitory-enhancing potential that social rearing might have had. Nonetheless, the manipulation of rearing environment was strong enough to influence temporal precision in all rats, and various other measures obtained from Head rats. Relative to other behavioral effects of rearing environment, its effect on behavioral inhibition was very weak.
Behavioral inhibition estimates from DRL
As expected, the distribution of IRTs in the Head condition was similar to those observed in more conventional DRL paradigms using lever pressing as the target response (e.g., Doughty and Richards 2002). The left-end tail of the Head IRT distributions (Fig. 3) and estimates of TR parameter p (Fig. 4) indicate that 25-30% of Head IRTs were short response bursts. Despite delaying reinforcement, burst responding is a signature property of DRL performance (Kramer and Rilling 1970; Richards et al. 1993). They are also an indication that factors unrelated to inhibition may influence DRL performance.
Estimates of θ and the proportion of correct sequences obtained from Head rats suggest that Isolated rats were less impulsive than Paired rats when MPH dose was 0-2 mg/kg. Such inference is inconsistent with prior research on rat DRL performance (Ough et al. 1972; Morgan and Einon 1975) that suggest that impoverished rearing environments promote impulsivity. Various differences in procedural parameters may have contributed to the inconsistency across DRL studies, e.g., the use of head pokes vs. lever pressing as the target response, and waiting requirements of 6 vs. 20 (Ough et al. 1972) vs. 30 s (Morgan and Einon 1975). This divergence in results suggests that inferences on behavioral inhibition drawn from DRL data are highly dependent on procedural parameters, and are therefore not reliable.
Nonwaiting IRTs
In FMI, short response bursts are captured in repeated lever presses, which do not delay reinforcement, separately from IRTs. This allows for a more straightforward observation of the distribution of waiting IRTs, compared to DRL. It does not mean, however, that nonwaiting IRTs are absent in FMI. Based on estimates of p (Fig. 4), the prevalence of non-waiting intervals in FMI is reduced by nearly 10% relative to DRL. The long durations of those IRTs (k=5-15 s) suggest that they are qualitatively different from DRL bursts (k= 0.1-0.2 s). These long nonwaiting IRTs may reflect lapses in attention, embodied in counter failures (Bizo et al. 2006) or other stop/reset mechanism (Buhusi and Meck 2006). These lapses are probably also present in DRL data, but confound-ed with θ. Such confound would explain why the difference in k between Groups Lever-Paired and Lever-Isolated, although statistically nonsignificant, is somewhat reflected in the difference in θ between Groups Head-Paired and Head-Isolated (Fig. 4). Longer periods of task delinquency, not enhanced inhibitory capacity, may explain the superior performance of Isolated rats in DRL.
Although FMI data shows that MPH dose-dependently improved inhibitory capacity, it also shows that the highest dose of MPH, 8 mg/kg, reduces the proportion of waiting IRTs (p). Because of the long duration of nonwaiting FMI IRTs, their prevalence yields flatter IRT distributions similar to those observed when timing is disrupted. These flatter distributions are observed with increasing doses of MPH in Fig. 3 and in Mechner and Latranyi’s (1963) report.
Conclusions
A detailed examination of IRTs in FMI uncovered the inhibition-enhancement effect of MPH in adult male rats. This effect is well demonstrated in humans, but conventional inhibition paradigms have been unable to replicate it systematically. Furthermore, the analysis of FMI IRTs dissociated the inhibitory effects of MPH from the non-inhibitory effects of rearing conditions. These results support the use of FMI as a behavioral inhibition paradigm. FMI is uniquely fit for the preclinical assessment of pharmacological treatments of impulsivity.
Acknowledgements
This study was supported by startup funds from the College of Liberal Arts and Sciences, Arizona State University, to Federico Sanabria. Pablo Covarrubias spent a stay at ASU supported by an award from the National Council of Science and Technology, Conacyt-Mexico. The authors wish to acknowledge Peter Killeen’s guidance and Janet Neisewander’s support with resources and advice, and recognize their helpful comments on earlier versions of the manuscript. Jonathan Schiro helped in data analysis and figure editing. Gabriel Mazur assisted with data analysis. The data for this paper were gathered as partial fulfillment of the requirements for the degree of Master of Arts in Psychology, for the first author, in Arizona State University.
Contributor Information
Jade C. Hill, Arizona State University, Tempe, AZ, USA
Pablo Covarrubias, University of Guadalajara-CUCI, Ocotlan, Mexico.
Joel Terry, University of Nevada, Las Vegas, NV, USA.
Federico Sanabria, Arizona State University, Tempe, AZ, USA; Department of Psychology, P.O. Box 871104, Tempe, AZ 85287-1104, USA.
References
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous amphetamine in female and male rats. Psychopharmacology. 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Bizo LA, Chu JY, Sanabria F, Killeen PR. The failure of Weber’s law in time perception and production. Behav Process. 2006;71:201–210. doi: 10.1016/j.beproc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Kooij JJS, Oosterlaan J, Sergeant JA, Buitelaar JK. Does methylphenidate improve inhibition and other cognitive abilities in adults with childhood-onset ADHD? J Clin Exp Neuropsychol. 2005;27:278–298. doi: 10.1080/13803390490515757. [DOI] [PubMed] [Google Scholar]
- Brackney RJ, Cheung THC, Neisewander JL, Sanabria F. The isolation of motivational, motoric, and schedule effects on operant performance: a modeling approach. J Exp Anal Behav. 2011;96:17–38. doi: 10.1901/jeab.2011.96-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG, Flory RK. Schedule-induced escape from fixed-interval reinforcement. J Exp Anal Behav. 1972;17:395–403. doi: 10.1901/jeab.1972.17-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Johnstone SJ, Barry RJ, et al. The effect of methylphenidate on response inhibition and the event-related potential of children with attention deficit/hyperactivity disorder. Int J Psychophysiol. 2005;58:47–58. doi: 10.1016/j.ijpsycho.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Interval timing with gaps and distracters: evaluation of the ambiguity, switch, and time-sharing hypotheses. J Exp Psychol Anim Behav Process. 2006;32:329–338. doi: 10.1037/0097-7403.32.3.329. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, del Campo N, Dowson J, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Chapman AL, Leung DW, Lynch TR. Impulsivity and emotion dysregulation in borderline personality disorder. J Pers Disord. 2008;22:148–164. doi: 10.1521/pedi.2008.22.2.148. [DOI] [PubMed] [Google Scholar]
- Conrad DG, Sidman M, Herrnstein RJ. The effects of deprivation upon temporally spaced responding. J Exp Anal Behav. 1958;1:59–65. doi: 10.1901/jeab.1958.1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Pereira EA, Li PM, Robbins TW. Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology. 2002;164:329–340. doi: 10.1007/s00213-002-1215-y. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Clark L, et al. Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD) Psychopharmacology. 2009;202:531–539. doi: 10.1007/s00213-008-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty AH, Richards JB. Effects of reinforcer magnitude on responding under differential-reinforcement-of-low-rate schedules of rats and pigeons. J Exp Anal Behav. 2002;78:17–30. doi: 10.1901/jeab.2002.78-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Taylor KE, Dafter RE. Differential effects of methylphenidate on signalled and non-signalled reinforcement. Pharmacol Biochem Behav. 1980;13:467–470. doi: 10.1016/0091-3057(80)90257-9. [DOI] [PubMed] [Google Scholar]
- Evenden J, Ko T. The psychopharmacology of impulsive behaviour in rats VIII: effects of amphetamine, methylphenidate, and other drugs on responding maintained by a fixed consecutive number avoidance schedule. Psychopharmacology. 2005;180:294–305. doi: 10.1007/s00213-005-2163-0. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Spencer T, Aleardi M, Pagano C, Biederman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperacticity disorder. J Clin Psychopharmacol. 2004;24:24–29. doi: 10.1097/01.jcp.0000108984.11879.95. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Paule MG, Cada A, Fogle CM, Gray EP, Berry KJ. Baseline behavior, but not sensitivity to stimulant drugs, differs among spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rat strains. Neurotoxicol Teratol. 2007;29:547–561. doi: 10.1016/j.ntt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of reinforcement. Appleton; East Norwalk, CT: 1957. [Google Scholar]
- Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–152. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. J Exp Anal Behav. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, et al. Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295:51–57. [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology. 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Effects of methylphenidate on response rate and measures of motor performance and reinforcement efficacy. Psychopharmacology. 1992;109:145–152. doi: 10.1007/BF02245492. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Killeen PR, Sagvolden T. Behavioral variability, elimination of responses, and delay-of-reinforcement gradients in SHR and WKY rats. Behav Brain Funct. 2007;3:60. doi: 10.1186/1744-9081-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer TJ, Rilling M. Differential reinforcement of low rates: a selective critique. Psychol Bull. 1970;74:225–254. [Google Scholar]
- Kratz O, Diruf MS, Studer P, et al. Effects of methylphenidate on motor system excitability in a response inhibition task. Behav Brain Funct. 2009;5:12. doi: 10.1186/1744-9081-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga AJ, Popke EJ, Fogle CM, Paule MG. Similar effects of amphetamine and methylphenidate on the performance of complex operant tasks in rats. Behav Brain Res. 2000;109:59–68. doi: 10.1016/s0166-4328(99)00165-5. [DOI] [PubMed] [Google Scholar]
- Mechner F, Guevrekian L. Effects of deprivation upon counting and timing in rats. J Exp Anal Behav. 1962;5:463–466. doi: 10.1901/jeab.1962.5-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechner F, Latranyi M. Behavioral effects of caffeine, methamphetamine, and methylphenidate in a rat. J Exp Anal Behav. 1963;6:331–342. doi: 10.1901/jeab.1963.6-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Einon D. Incentive motivation and behavioral inhibition in socially-isolated rats. Physiol Behav. 1975;15:405–409. doi: 10.1016/0031-9384(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Myung IJ. Tutorial on maximum likelihood estimation. J Math Psychol. 2003;47:90–100. [Google Scholar]
- Nandam LS, Hester R, Wagner J, et al. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry. 2011;69:902–904. doi: 10.1016/j.biopsych.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, et al. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Dépatie L, Holahan ALV, et al. Executive functions and methylphenidate response in subtypes of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1452–1460. doi: 10.1016/j.biopsych.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Orduña V, Valencia-Torres L, Bouzas A. DRL performance of spontaneously hypertensive rats: dissociation of timing and inhibition of responses. Behav Brain Res. 2009;201:158–165. doi: 10.1016/j.bbr.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Ough BR, Beatty WW, Khalili J. Effects of isolated and enriched rearing on response inhibition. Psychon Sci. 1972;27:293–294. [Google Scholar]
- Pearl RG, Seiden LS. The existence of tolerance to and cross-tolerance between d-amphetamine and methylphenidate for their effects on milk consumption and on differential-reinforcement-of-low-rate performance in the rat. J Pharmacol Exp Ther. 1976;198:635–647. [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology. 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Pressman LJ, Loo SK, Carpenter EM, et al. Relationship of family environment and parental psychiatric diagnosis to impairment in ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:346–354. doi: 10.1097/01.chi.0000192248.61271.c8. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, Seiden LS. DRL interresponse-time distributions: quantification by peak deviation analysis. J Exp Anal Behav. 1993;60:361–385. doi: 10.1901/jeab.1993.60-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Raskin LA. Stereotyped behavior: effects of d-amphetamine and methylphenidate in the young rat. Pharmacol Biochem Behav. 1997;58:1095–1102. doi: 10.1016/s0091-3057(97)00321-3. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Berger DF. An animal model of attention deficit disorder: the female shows more behavioral problems and is more impulsive than the male. Eur Psychol. 1996;1:113–122. [Google Scholar]
- Sagvolden T, Slatta K, Arntzen E. Low doses of methylphenidate (ritalin) may alter the delay-of-reinforcement gradient. Psychopharmacology. 1988;95:303–312. doi: 10.1007/BF00181938. [DOI] [PubMed] [Google Scholar]
- Sanabria F, Killeen PR. Evidence for impulsivity in the spontaneously hypertensive rat drawn from complementary response-withholding tasks. Behav Brain Funct. 2008;4:7. doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Swanson J, et al. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol. 2003;31:105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Andresen J, MacPhail RC. Methylphenidate and d-amphetamine: effects and interactions with alphamethyltyrosine and tetrabenazine on DRL performance in rats. Pharmacol Biochem Behav. 1979;10:577–584. doi: 10.1016/0091-3057(79)90236-3. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Jr, Reynolds B, et al. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2009;17:291–301. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AM, Maruff P, Pietrzak RH, Cromer JR, Snyder PJ. Effect of treatment with stimulant medication on nonverbal executive function and visuomotor speed in children with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2008;14:221–226. doi: 10.1080/09297040701220005. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer BL, Hoeppner JAB, Zecker SG. The go-no go test in attention deficit disorder is sensitive to methylphenidate. J Child Neurol. 1991;6:S128–S131. doi: 10.1177/0883073891006001s13. [DOI] [PubMed] [Google Scholar]
- Ts’o TO, Hance AJ, Killam KF. Performance enhancement effects of d-amphetamine, methylphenidate, pipradrol and phenindamine in rats. Psychopharmacologia. 1976;46:65–72. doi: 10.1007/BF00421551. [DOI] [PubMed] [Google Scholar]
- Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adultattention-deficit/hyperactivity disorder. Biol Psychiatry. 2004;55:1031–1040. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneous hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.97. doi:10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HK, Cox DJ, Merkel RL, Moore M, Coghill D. Effect of extended release stimulant-based medications on neuropsychological functioning among adolescents with attention-deficit/hyperactivity disorder. Arch Clin Neuropsychol. 2006;21:797–807. doi: 10.1016/j.acn.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, et al. Behavioral disinhibition: liability for externalizing sprectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]