Abstract
Background
The purpose of this study was to determine if orthotropic heart transplantation (OHT) performed within 90-days of an initial heart transplant (RETX) should be a contraindication to re-transplantation based on inferior outcomes when compared to primary OHT recipients (control).
Methods
De-identified data were obtained from the United Network for Organ Sharing. The study population included all adult heart transplant recipients > 18 years old from 1995–2008 (n=26,804). Multivariable regression was performed in order to assess the simultaneous effect of multiple risk factors on post-transplant graft failure at 90 days (PTGF). Secondary outcomes of interest included infection, stroke, and dialysis during the transplant hospitalization as well as primary non-function of the graft at 90 days.
Results
Among the study cohort, there were 90 (0.34%) RETX patients. Median survival in this group was 1.6 years compared to 10.5 years for controls. Unadjusted PTGF, infection, dialysis, and primary non-function was significantly higher (p<0.001) in the RETX group. After risk-adjustment, however, PTGF (p=0.545), infection (p=0.696), dialysis (p=0.664), stroke (p=0.115), and primary non-function (p =0.531), did not differ significantly between the two groups.
Conclusions
When controlling for pre-transplant recipient characteristics, re-transplantation within 90 days of a previous transplant is not associated with increased morbidity or mortality. However, unadjusted overall survival was significantly worse in the RETX group. This suggests that although re-transplantation at 90 days alone is not a risk factor for inferior outcomes, given the significant comorbidities of these patients, the indications for re-transplantation within 90 days must be critically examined.
Keywords: transplantation, cardiac, graft failure
INTRODUCTION
Primary graft failure (PGF) remains the most common cause of morbidity and mortality in the early post-transplant period [1]. PGF is broadly defined as severe dysfunction of the cardiac allograft characterized by low cardiac output, hypotension, and high filling pressures in the absence of secondary causes of graft failure such as hyperacute rejection, unresponsive pulmonary hypertension, or technical surgical problems [2]. According to the International Society of Heart and Lung Transplantation Registry, from January 1992 to June 2006, the most common cause of death within the first 30-days after heart transplantation was PGF, which accounted for nearly one-third of deaths [3].
To support recipients with graft failure, a number of new therapies have been applied in clinical practice, most notably, temporary mechanical circulatory support (MCS) [4–6]. Recent studies of temporary MCS have found only moderate success with this indication [7–9]. Less than 50% of patients with early graft failure treated with MCS recovered or survived until re-transplantation [10]. In the absence of graft recovery, re-transplantation remains the only definitive treatment for PGF. However, the outcomes of re-transplantation after early graft failure have not been well studied in a large series due, in part, to the low prevalence of this complication.
The goal of this study was to assess whether a previous orthotopic heart transplant within the past 90 days should be a contraindication to re-transplantation. Using national data from the United Network for Organ Sharing, this study compares morbidity and mortality in heart transplant recipients re-transplanted within 90 days, to primary heart transplant recipients (controls). We hypothesized that unadjusted and risk-adjusted survival among recipients re-transplanted at less than 90 days would be inferior compared to controls.
MATERIAL AND METHODS
Data Collection
Approval for this study was granted by Columbia University’s Institutional Review Board, and use of this data is consistent with the United Network for Organ Sharing (UNOS) Data Use Agreement. The Standard Transplant Analysis and Research Dataset were provided by UNOS (data source #061809-6). The dataset contains de-identified information collected from the UNetSM database forms, including the Transplant Candidate Registration form, the Transplant Recipient Registration form, and the Transplant Recipient Follow-up form. These data are the basis for the UNOS Thoracic Registry.
Study population
Between January 1, 1995 and December 31, 2008, there were 26,804 heart transplant recipients aged 18 years and older. Patients were divided into two groups based on re-transplant status. The control group (n=26,696) consisted of primary transplant recipients (i.e. patients who received only one heart transplant during the study period). The re-transplant group (RETX) consisted of patients (n=90) who were re-transplanted within 90 days of a previous heart transplant during the study period (there were an additional 18 patients re-transplanted beyond 90 days who were not included in the risk-factor analysis). Follow-up data was provided through June 18, 2009, with a mean follow-up time of 4.72 ± 3.80 (range: 0 – 14.2) years. Patients were followed from the date of transplant until death, cardiac re-transplantation, or date of last known follow-up which was the last day of follow-up data provided by UNOS. The analysis included 126,356 graft-years at risk.
Outcomes and definitions
Our primary outcome measure was post-transplant graft failure at 90 days (PTGF) which was defined as patient death or re-transplantation. Secondary outcomes of interest were: in-hospital morbidity, including incidence of infection, stroke, and need for dialysis, as well as primary non-function of the graft at 90 days. In this analysis, primary non-function is defined as death or re-transplantation within 90 days of the index cardiac transplant due to graft failure not related to rejection, infection, or technical surgical issues.
Data analysis
Continuous variables were reported as mean ± standard deviation and compared using the Student’s t-test. To compare categorical variables, the chi-squared test was used. Multivariable logistic regression was performed (backward, remove p>0.15) to assess the simultaneous effect of multiple variables on the primary and secondary outcome measures. Patients with missing data were excluded from regression analysis; no imputation methods were employed. The odds ratio (OR) and 95% confidence interval (95% CI) were reported for each variable. To analyze unadjusted long-term survival, Kaplan-Meier analysis was used with log-rank test. The conventional p-value of 0.05 or less was used to determine the level of statistical significance. All reported p-values are two-sided. All data were analyzed using the statistical software package, Stata 9 (Stata Corp, College Station, TX).
RESULTS
Study population
There were a total of 26,804 heart transplant recipients considered in the analysis. There were 26,696 patients who received a single transplant during the study period and 108 patients who received a second transplant within one year of their index transplant. Of these re-transplant patients, 50% (n=54) were re-transplanted in five days or less, and 83.3% (n=90) were re-transplanted within 90 days. The 18 patients re-transplanted beyond 90 days were not included in the risk-factor analysis. The baseline characteristics of the RETX group at the time of re-transplantation and the control group at the time of initial transplantation are shown in Table 1. There were several significant differences between groups. While the control group was older, the RETX group had laboratory markers of renal and hepatobiliary impairment that were higher than the control group. Also, the RETX group had a higher proportion of patients that were intubated, on inotropes, and requiring mechanical circulatory support prior to re-transplantation.
TABLE 1.
Recipient and Donor Demographics and Characteristics
Note that data for the re-transplant group is based on clinical characteristics at the time of re-transplant rather than the initial transplant.
| Controls | Re-transplant < 90 days |
p-value | |
|---|---|---|---|
| N | 26,696 | 90 | |
| Recipient age (years) | 51.9 ± 11.9 | 48.6 ± 12.8 | 0.004 |
| Donor age (years) | 31.2 ± 12.6 | 33.5 ± 13.1 | 0.96 |
| Diabetic recipient | 5,339 (20.0%) | 14 (15.6%) | 0.23 |
| Underweight (BMI < 18.5) | 952 (3.6%) | 6 (6.7%) | 0.10 |
| Severely/Morbidly obese (BMI > 35) | 1,009 (3.8%) | 7 (7.8%) | 0.04 |
| Recipient eGFR (mL/min/m2) | 52.5 ± 13.3 | 28.6 ± 25.3 | <0.001 |
| Total bilirubin >2 dl/mg | 2,955 (11.1%) | 33 (36.7%) | < 0.001 |
| Intubated | 727 (2.7%) | 53 (58.9%) | < 0.001 |
| Inotropic support | 13,121 (49.1%) | 71 (78.9%) | < 0.001 |
| Donor : Recipient weight <0.7 | 1,183 (4.4%) | 4 (4.4%) | 0.88 |
| Transplant year | 2001.3 ± 4.1 | 2001.1 ± 3.7 | 0.59 |
| IABP | 1402 (5.2%) | 27 (30.0%) | <0.001 |
| ECMO | 84 (0.3%) | 14 (15.6%) | < 0.001 |
| LVAD | 4,352 (16.3%) | 33 (36.7%) | < 0.001 |
| RVAD | 33 (0.1%) | 2 (2.2%) | <0.001 |
| BiVAD | 378 (1.4%) | 5 (5.6%) | 0.001 |
BiVAD: biventricular assist device, BMI: body mass index, ECMO: extracorporeal membrane oxygenation, eGFR: estimated glomerular filtration rate, IABP: intra-aortic balloon pump, LVAD: left ventricular assist device, MCS: mechanical circulatory support, RVAD: right ventricular assist device
Post-transplant graft survival
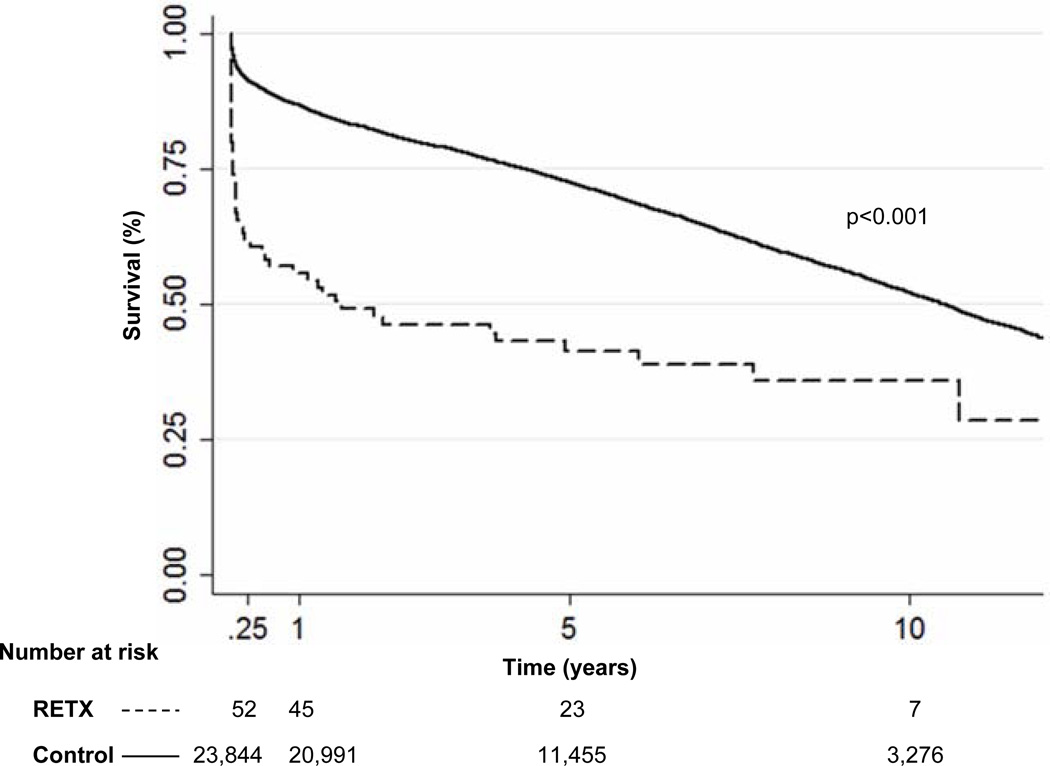
Compared to controls, unadjusted PTGF rates were lower for RETX patients across all time points, including 90 days. Consequently, overall actuarial survival was significantly worse in the RETX patients compared to the control group (p<0.001) [Figure 1]. The median survival in the RETX cohort was 1.6 years compared to 10.5 years for controls. When all patients re-transplanted were stratified by length of time from the index heart transplant (0 – 90 days, 90 days – 1 year, and > 1 year), there was a significant stepwise increase in mortality as time from index transplant decreased (p<0.001). Considering all patients re-transplanted during the study period, median survival was 2.7 years for patients re-transplanted at 90 days – 1 year (n=18), and 8.4 years among patients re-transplanted at >1 year (n=670).
Figure 1.
Kaplan Meier analysis of patients re-transplanted within 90 days of an initial transplant (RETX) versus primary heart transplant recipients (control)
Secondary Outcomes
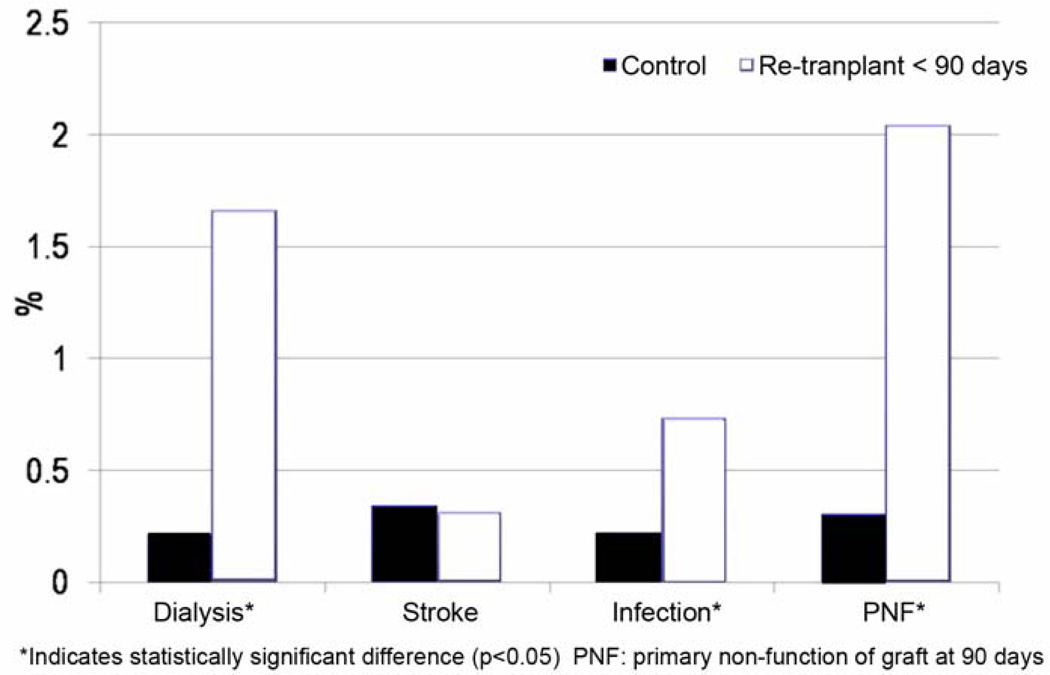
In the RETX cohort, unadjusted rates of in-hospital infection (p<0.001), need for dialysis (p<0.001), and primary graft non-function (p<0.001) were significantly higher than in the control group [Figure 2]. However, the rate of stroke was similar between groups (p=0.993).
Figure 2.
Unadjusted secondary outcome measures
Risk adjusted outcomes
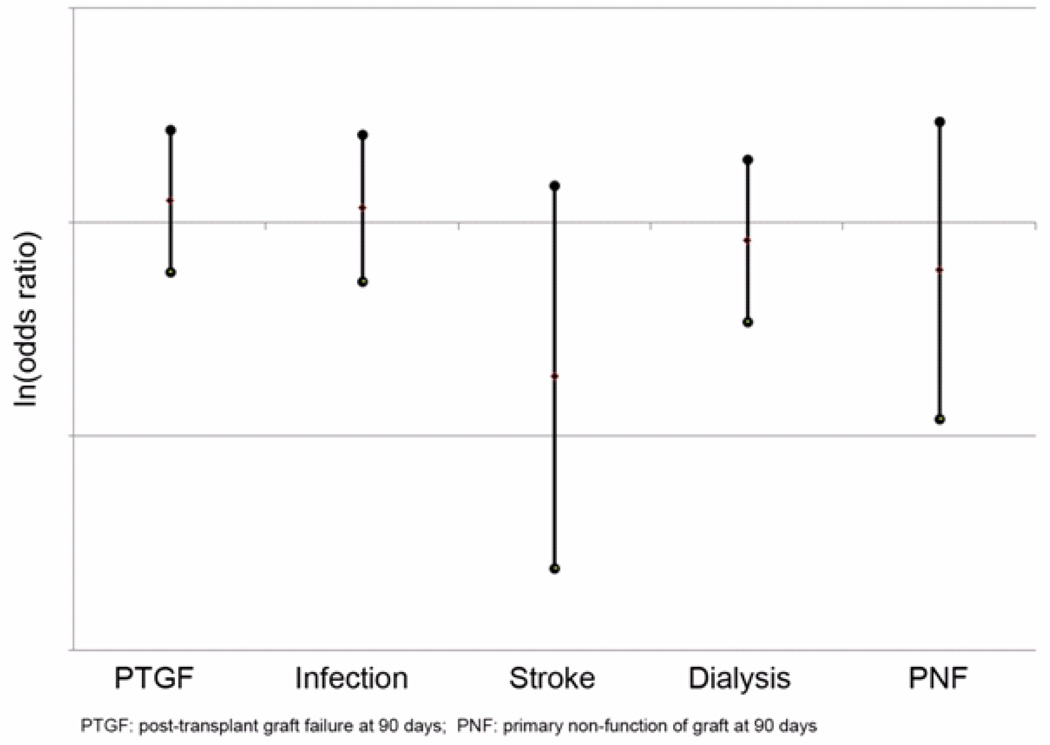
The primary and secondary outcomes were analyzed again after using multivariable logistic regression to adjust for differences in baseline risk between groups [Figure 3]. After risk-adjustment, there was no significant difference in the odds of PTGF for RETX patients when compared to controls (OR=1.264, 0.592–2.702, p=0.545). In addition, there was no significant difference in risk of in-hospital stroke (OR=0.191, CI=0.0242 – 1.501, p=0.115), infection (OR=1.171, CI=0.531–2.581, p=0.696), dialysis (OR=0.824, CI=0.343–1.974, p=0.664), or primary graft non-function (OR=0.600, CI=0.121 – 2.975, p = 0.531) between groups.
Figure 3.
Risk-adjusted primary and secondary outcome measures
Post-transplant Graft Survival in the RETX group
Additional multivariable logistic regression was performed to determine risk factors for worse outcome after re-transplant in the RETX group. Risk factors for PTGF in the RETX group are listed in Table 2. The risk factors with the highest odds ratios included: extra-corporeal membrane oxygenation, BMI < 18.5, biventricular assist device at the time of transplant, and total bilirubin > 2 dl/mg.
TABLE 2.
Risk Factors for Post-Transplant Graft Failure at 90 Days in RETX group
| Odds Ratio | 95% CI Lower Limit |
95% CI Upper Limit |
p-value | |
|---|---|---|---|---|
| ECMO | 40.20 | 2.40 | 673.27 | 0.010 |
| Underweight (BMI <18.5) | 30.39 | 1.35 | 686.60 | 0.032 |
| BiVAD | 15.16 | 1.27 | 180.51 | 0.031 |
| Total bilirubin > 2 dl/mg | 13.58 | 2.51 | 73.44 | 0.002 |
| Severely/Morbidly obese (BMI > 35) | 6.98 | 0.89 | 54.81 | 0.065 |
| IABP | 6.32 | 1.26 | 31.76 | 0.025 |
| Diabetic recipient | 4.48 | 0.79 | 25.57 | 0.091 |
| Intubated | 3.46 | 0.80 | 15.08 | 0.098 |
| Recipient eGFR (mL/min/m2) | 0.94 | 0.90 | 0.97 | 0.000 |
| Transplant year (per yr) | 0.68 | 0.56 | 0.84 | 0.000 |
| Donor age (years) | 1.03 | 0.97 | 1.09 | 0.312 |
| LVAD | 2.00 | 0.45 | 8.80 | 0.361 |
| RVAD | 3.05 | 0.20 | 46.76 | 0.423 |
| Donor : Recipient weight <0.7 | 30.35 | 0.01 | 159988.80 | 0.435 |
| Recipient age (years) | 1.02 | 0.97 | 1.06 | 0.523 |
| Inotropic support | 0.81 | 0.05 | 13.66 | 0.883 |
BiVAD: biventricular assist device, BMI: body mass index, ECMO: extracorporeal membrane oxygenation, eGFR: estimated glomerular filtration rate, IABP: intra-aortic balloon pump, LVAD: left ventricular assist device, MCS: mechanical circulatory support, RVAD: right ventricular assist device
COMMENT
Given the high morbidity associated with early graft failure and the critical scarcity of organs available for heart transplantation, use of donor organs as a salvage strategy for recipients with early graft failure must be critically reviewed. Overall, re-transplantation within one year of the index heart transplant was rare. During the study period of 14 years, less than one percent of the original cohort underwent re-transplantation within one year. However, the vast majority of re-transplants within one-year were performed within 90-days of the index transplant, and thus this group of patients was chosen for analysis.
As expected, unadjusted survival was significantly worse among this group. The median survival in patients re-transplanted within 90 days was only 1.6 years, compared with 10.5 years in primary heart transplant recipients. Likewise, RETX patients had significantly higher rates of post-operative complications including renal failure requiring dialysis, infection, and primary non-function of the graft within 90 days. Interestingly, however, when differences in baseline characteristics were adjusted for in multivariable analysis, the observed differences in survival and in-hospital complications between the RETX and control groups were no longer significant. These findings suggest that the poor outcomes achieved by recipients re-transplanted within 90 days is not a function of re-transplantation itself, but rather a result of intrinsic patient morbidity at the time of re-transplantation. In general, RETX patients were much sicker than controls and demonstrated significant markers of end organ dysfunction. RETX patients were more likely to have lower estimated glomerular filtration rates and serum bilirubin levels > 2 dl/mg. In addition, RETX patients were also more likely to be intubated, require inotropic support, and mechanical circulatory assistance (90%), including extracorporeal membrane oxygenation (ECMO) and a ventricular assist device (VAD).
Previous studies have demonstrated that cardiac transplantation may be an efficacious strategy among patients with primary graft failure [10–13]. However, studies have also shown that the relative survival benefit of repeat transplantation is significantly dependent on the duration of time from the index transplant, as well as patient characteristics prior to re-transplantation [14, 15]. Studies demonstrating a benefit of re-transplantation have generally focused on transplants occurring within one year after the index transplant. Furthermore, reports have demonstrated that over the past decade, survival after repeat transplantation has improved [16]. This analysis focused on the early graft failure population, specifically patients receiving a second transplant within 90 days of the index transplant, because this represented the majority of patients being re-transplanted within 1 year. Although this analysis supports the findings that early re-transplant improves survival, the quantity of life years gained remains low (1.6 years) when compared to that of a primary heart transplant. Likewise, even when re-transplantation is delayed to 90 days - 1 year after transplant, unadjusted median survival, though improved over the 0–90 day group, was still only 2.7 years.
In the current setting of limited organ donations, these findings of decreased survival raise important ethical considerations regarding the decision to proceed with repeat transplantation after early graft failure. Further adding to the complexity of the decision-making process is the growing role of temporary mechanical circulatory support.
Commonly, recipients with early graft dysfunction or graft failure are supported with mechanical circulatory support (MCS) including temporary VADs. However, recent studies of temporary MCS have found only moderate success with this approach. Furthermore, our recent study of a non-transplant population suggests temporary mechanical support in “acute insult” patients (e.g. cardiogenic shock, acute myocardial infarction, post-cardiotomy, myocarditis) is extremely costly at more than $10,000 per day (17). In multivariate regression analysis the need for circulatory support with extra-corporeal membrane oxygenation (ECMO), bi-ventricular assist device (BiVAD), and intra-aortic balloon pump (IABP) were among the highest risk factors for poor outcome following re-transplantation. Specifically, three-quarters of RETX recipients requiring ECMO support at re-transplant and two-thirds of RETX recipients supported by BiVAD at re-transplantation were dead within 1 year, implying that bridging early graft failure recipients with ECMO or BiVAD is unlikely to significantly extend patient survival. Interestingly, neither LVAD nor RVAD were associated with PTGF in the RETX group. This suggests that there is potentially a subset of patients who may benefit from bridge-to-transplantation therapies that enable optimization of end-organ function prior to cardiac re-transplantation, although further research is necessary to define this patient population and the ideal timing and type of mechanical circulatory support.
Limitations
There are several limitations to this analysis. First, large patient registries often have incomplete data entry. Fields contained within the UNOS database used for this analysis, however, were well populated with a 90–99% data entry rate for the majority of variables. Second, although the UNOS reporting system provides definitions for variables in data guidelines, there could be inaccuracies in individual center reporting to UNOS. Third, data is not provided by UNOS on the specific causes of mortality following transplantation. Although our regression model demonstrated moderate discrimination, significant variability remains unexplained. We speculate that some of the variability stems from differences in patient functional status, severity of illness, and technical aspects of the implant procedures that were not captured by the UNOS dataset. Hemodynamic parameters were not included, as these values are dynamic and often confounded by other factors, including use of inotropes, vasoactive medications, and sedation, that are not well characterized in the UNOS registry. Moreover, we attempted to limit variables in our regression model to those that could be generalizable to transplant centers across the US and would not be subject to variability in interpretation. Use of additional center-specific variables in the future would likely improve model discrimination. In addition, our analysis of re-transplant patients considered only pre re-transplants risk factors in an attempt to create a parsimonious regression model. Both pre index transplant and pre re-transplant risk factors, however, may impact clinical outcomes in this population although there would likely be overlap of similar risk factors adding to modeling complexity. Finally, some variables in our multivariable regression were statistically significant but had wide confidence intervals. These risk factors will require further study to more clearly characterize the magnitude of their role in PGF.
Conclusion
When controlling for pre-transplant recipient characteristics, re-transplantation within 90 days of a previous transplant is not associated with increased morbidity or mortality. However, unadjusted overall survival was significantly worse in the RETX group. This suggests that though re-transplantation at 90 days alone is not a risk factor for diminished outcomes, given the significant comorbidities of these patients, indications for re-transplantation within 90 days must be critically reviewed through continued research. In multivariate regression analysis the need for circulatory support with extra-corporeal membrane oxygenation, bi-ventricular assist devices, and an intra-aortic balloon pump were among the highest risk factors for poor outcome following re-transplantation. In summary, clinicians, patients, and their families must recognize that re-transplantation is often unlikely to provide substantial benefits, and clear treatment goals must be set prior to considering re-transplantation in recipients with early graft failure.
ACKNOWLEDGMENTS
We thank UNOS for supplying this data, especially Jennifer L. Wainright, PhD, and Katarina Linden, PhD, for their assistance with our analysis. This work was supported in part by Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This work was supported in part by NIH Training Grant 5T32HL007854-13 (Dr. Iribarne).
Abbreviations and Acronyms
- 95% CI
95% confidence interval
- eGFR
Estimated Glomerular Filtration Rate
- MCS
Mechanical Circulatory Support
- OR
Odds ratio
- OHT
Orthotopic heart transplant
- PGF
Primary graft failure (at any time)
- PTGF
Post-transplant graft failure at 90 days
- RETX
Re-transplanted within 90 days of previous transplant
- UNOS
United Network for Organ Sharing
- VAD
Ventricular Assist Device
Discussion
90. Should Graft Failure Recipients be Retransplanted Within 90 Days of a Previous Transplant? Paper presented by Mark J. Russo, MD, New York, NY. mr2143@columbia.edu
Discussion by Francis D. Pagani, MD, Michigan
fpagani@umich.edu
Dr. F. Pagani (Ann Arbor, MI):
Given this data, you would advocate for retransplantation in this setting?
90. Should Graft Failure Recipients be Retransplanted Within 90 Days of a Previous Transplant? Response by Mark J. Russo, MD, New York, NY.
DR. RUSSO: No. It is not that retransplantation within 90 days is necessarily a risk factor for worse outcome, but because the overall morbidity burden of these patients at the time that they would be retransplanted is so high, their outcomes are likely to be poor, and I would say, again, there is a rarely a good indication to do this. This also raises the second question, and it was one of the things that motivated our interest in looking at this question, of when you have patients who have primary graft failure dysfunction coming out of the OR and you have put them on a device, then what is the end game and what are the factors that we should be looking at in terms of triaging patients? And I think that most of us, and certainly at our center, if a patient has severe graft dysfunction where they aren't otherwise going to leave the OR, then they are universally put on devices and supported in hopes that the graft function will recover. But that is a difficult thing to predict and certainly a problem I think in terms of quality of life issues, cost issues, and systems issues.
DR. PAGANI: Importantly, it would have been informative to have more data on the PRA status of these patients at the time of retransplantation.
DR. RUSSO: Sure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting: Society of Thoracic Surgeons Annual Meeting, 2010, Fort Lauderdale, FL
REFERENCES
- 1.Kirklin JK, Naftel DC, Bourge RC, et al. Evolving trends in risk profiles and causes of death after heart transplantation: a ten-year multi-institutional study. J Thorac Cardiovasc Surg. 2003;125(4):881–890. doi: 10.1067/mtc.2003.168. [DOI] [PubMed] [Google Scholar]
- 2.Jahania MS, Mullett TW, Sanchez JA, Narayan P, Lasley RD, Mentzer RM., Jr. Acute allograft failure in thoracic organ transplantation. J Card Surg. 2000;15(2):122–128. [PubMed] [Google Scholar]
- 3.Taylor DO, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report--2008. J Heart Lung Transplant. 2008;27(9):943–956. doi: 10.1016/j.healun.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Marasco SF, Esmore DS, Negri J, et al. Early institution of mechanical support improves outcomes in primary cardiac allograft failure. J Heart Lung Transplant. 2005;24(12):2037–2042. doi: 10.1016/j.healun.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Santise G, Petrou M, Pepper JR, Dreyfus G, Khaghani A, Birks EJ. Levitronix as a short-term salvage treatment for primary graft failure after heart transplantation. J Heart Lung Transplant. 2006;25(5):495–498. doi: 10.1016/j.healun.2005.11.458. [DOI] [PubMed] [Google Scholar]
- 6.Marasco SF, Esmore DS, Negri J, et al. Early institution of mechanical support improves outcomes in primary cardiac allograft failure. J Heart Lung Transplant. 2005;24(12):2037–2042. doi: 10.1016/j.healun.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Stepanenko A, Potapov EV, Jurmann B, et al. Outcomes of elective versus emergent permanent mechanical circulatory support in the elderly: A single-center experience. J Heart Lung Transplant. 2010;29(1):61–65. doi: 10.1016/j.healun.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Minev PA, El-Banayosy A, Minami K, Körtke H, Kizner L, Körfer R. Differential indication for mechanical circulatory support following heart transplantation. Intensive Care Med. 2001;27(8):1321–1327. doi: 10.1007/s001340101006. [DOI] [PubMed] [Google Scholar]
- 9.John R, Liao K, Lietz K, et al. Experience with the Levitronix CentriMag circulatory support system as a bridge to decision in patients with refractory acute cardiogenic shock and multisystem organ failure. J Thorac Cardiovasc Surg. 2007;134(2):351–358. doi: 10.1016/j.jtcvs.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 10.Morgan JA, Naseem TM, Cheema FH, et al. Experience With Levitronix CentriMag Mechanical Assistance For Primary Graft Failure After Cardiac Transplantation. Under review. [Google Scholar]
- 11.Goerler H, Simon A, Gohrbandt B, et al. Cardiac re-transplantation: is it justified in times of critical donor organ shortage? Long-term single-center experience. Eur J Cardiothorac Surg. 2008;34(6):1185–1190. doi: 10.1016/j.ejcts.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Atluri P, Hiesinger W, Gorman RC, et al. Cardiac re-transplantation is an efficacious therapy for primary cardiac allograft failure. J Cardiothorac Surg. 2008;3:26. doi: 10.1186/1749-8090-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topkara VK, Dang NC, John R, et al. A decade experience of cardiac re-transplantation in adult recipients. J Heart Lung Transplant. 2005;24(11):1745–1750. doi: 10.1016/j.healun.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Magee JC, Barr ML, Basadonna GP, et al. Repeat organ transplantation in the United States, 1996–2005. Am J Transplant. 2007;7(5 Pt 2):1424–1433. doi: 10.1111/j.1600-6143.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 15.Shuhaiber JH, Kim JB, Hur K, et al. Comparison of survival in primary and repeat heart transplantation from 1987 through 2004 in the United States. Ann Thorac Surg. 2007 Jun;83(6):2135–2141. doi: 10.1016/j.athoracsur.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Radovancevic B, McGiffin DC, Kobashigawa JA, et al. Re-transplantation in 7,290 primary transplant patients: a 10-year multi-institutional study. J Heart Lung Transplant. 2003;22(8):862–868. doi: 10.1016/s1053-2498(02)00803-3. [DOI] [PubMed] [Google Scholar]
- 17.Hong KN, Russo MJ, Ascheim DD, et al. American Heart Association Scientific Sessions. Orlando, FL: 2009. The outcomes and costs of intracorporeal and extracorporeal left ventricular assist devices: an analysis of the Centers for Medicare and Medicaid Services Database. [Google Scholar]