Abstract
Study Objective
To describe (1) the treatment interval for adolescent females with Chlamydia trachomatis (CT), Neisseria gonorrhoeae (GC), or Trichomonas vaginalis (TV); (2) the proportion treated in ≤7 days; and (3) factors influencing the treatment interval.
Design and Participants
Charts of sexually active females from an urban teen health center who participated in a larger study and were positive for CT, GC or TV (N = 58) were retrospectively reviewed for dates of treatment, and compared to demographic and symptom data. The treatment interval was defined as days from visit to treatment. CT and/or GC were analyzed together (CT/GC) because presumptive treatment covered both infections, and the diagnostic test (nucleic acid amplification) differed from that of TV (wet mount or culture).
Results
The median treatment interval was 0 days for TV, 5 days for CT/GC, and 3 days for any STI. Overall, 39 (69%) were treated within 7 days of their visit. Those with TV were more likely than those with CT/GC to receive treatment at their initial visit (58 vs. 6%). Genitourinary symptoms increased the odds of treatment in ≤7 days. The treatment interval was significantly shorter for subjects who had their prescriptions phoned to a pharmacy than for those who returned to clinic for treatment (median 2.5 vs. 8 days).
Conclusions
Where presumptive treatment is uncommon, providers were more likely to prescribe same day therapy to symptomatic patients or those with TV on wet mount. Additional strategies are needed to improve the proportion of adolescent females treated in ≤7 days.
Keywords: Adolescent, Chlamydia Infections/*diagnosis/*therapy/*prevention & control, Female, Health Services Accessibility/*standards, Outcome Assessment (Health Care), Retrospective Studies, Time Factors
INTRODUCTION
It is well known that adolescent females are at risk for sexually transmitted infections (STIs). A public health approach to this epidemic requires three steps: adequate screening, timely treatment, and effective prevention of new infections. The Institute of Medicine, the US Preventive Services Task Force (USPSTF), and the Center for Disease Control (CDC) recommend annual Chlamydia trachomatis (CT) screening for all sexually active women under the age of 25, regardless of race/ethnicity or symptoms.1, 2 Other experts advocate screening every six months for sexually active adolescent females.3
However, once screened, the next important step is providing timely treatment for infected patients. Patients infected with CT or Neisseria gonorrhea (GC) who are untreated may develop pelvic inflammatory disease (PID), which can cause infertility and pelvic pain.4, 5 Some experts recommend that the interval to treatment for STIs should be less than seven days, due in part to the increased risk of PID. In addition, patients left untreated contribute to the overall public health burden by further transmitting their STIs. Moreover, evidence shows that individuals affected with STIs such as CT or Trichomonas vaginalis (TV) are at increased risk of acquiring HIV,6 herpes simplex virus,7 and of having poorer clearance of human papillomavirus.8
While several studies have evaluated adherence to and effectiveness of screening guidelines,9 few studies have assessed clinicians’ ability to provide timely treatment to adolescent females. In adult women with positive STI tests in the Emergency Department/walk-in clinic, 32% were not treated, and four percent of these developed PID before returning to the medical center for their second visit.10 Another study shows that of 157 women positive for CT in an Emergency Department, 20% of the patients were untreated for 14–60 days, and 25% were lost to follow up.11 One retrospective study examined the treatment interval for CT or GC positive subjects seen in one of several STI clinics. In this study, the median treatment interval, defined as days from specimen collection to treatment, was 7 to 18 days, and they found no association between delayed treatment and race, age, or history of STI.12 These studies all indicate the need for improving the timeliness of STI treatment.
Timely follow up may be more difficult to achieve for teen patients due to barriers such as transportation, finances, and the need for confidential services. One retrospective cohort analysis found a median interval to treatment for CT of 14 days in private adolescent clinics.13 However, these authors did not evaluate predictors of delayed treatment or the effect of different medication delivery approaches, such as medications dispensed on-site versus written or phoned-in prescriptions. In addition, no studies have evaluated the interval to treatment for trichomoniasis.
Therefore, this study aims to add three things to the existing literature: (1) To perform a comparison of the interval to treatment for CT, GC & TV, in a setting where many teens have established relationships with their health care providers; (2) To describe the proportion of patients treated in less than seven days; and (3) To explore any factors influencing the delay to treatment such as age, race, symptoms, condom use, and method of medication delivery.
MATERIALS AND METHODS
A retrospective analysis was performed on a subset of patients participating in a separate study of STIs and urinary tract infections (UTIs). The STI/UTI study was a cross sectional study of sexually active females presenting to a hospital-based teen health center between May 2003 and June 2005 (N = 200). All subjects completed prospective interviews on symptoms, sexual history, race, and contraceptive use, and were tested for STIs. For CT and GC, a urine nucleic acid amplification test (NAAT) was performed, and for TV, a vaginal swab for wet mount and culture was obtained. The clinical findings, STI test results, and presumptive provider diagnosis were recorded.
All STI results became part of the subjects’ clinical medical record. Therefore, the routine clinical protocol was followed for any positive STI. First, the clinical laboratory faxed positive test results to the clinic triage nurses. The fax was retrieved (Monday-Friday), the patient’s chart was pulled, and registration information was retrieved from the hospital computer database. Patients were contacted by phone, using a cell number if one was recorded on the chart. If no contact was made after three attempts, the patient’s chart was flagged and a certified letter was sent. When contacted, patients were offered two options for treatment: they could come in for an appointment where medication could be dispensed directly from the center’s stock, or the nurse could phone in a prescription to a pharmacy of the patient’s choice.
One year after the completion of enrollment, the charts of those with a positive STI were retrospectively reviewed to ascertain the clinical care and treatment provided by their health care providers. This included the date and type of treatment given (specific antibiotic), as well as the method of medication delivery (whether the patient returned to the clinic for care or requested a prescription called to a pharmacy). These data were entered into an Excel spreadsheet and appended to the research database. The original STI study and the retrospective chart review were approved by the local Institutional Review Board.
The outcome variable was interval to treatment, defined as the days between the date of visit where the STI sample was obtained to the date of documented treatment, via either a return to office visit or prescription called to a pharmacy. Positive tests for CT and/or CG (CT/GC) were evaluated together because their diagnostic tests were similar (NAAT), and both test results were available 24–48 hours after specimen collection. In addition, presumptive treatment covered both infections. This differed from TV, which was diagnosed via wet mount at office visit or by culture within 1–5 days. Therefore, treatment intervals were examined separately for CT/GC, TV, and any STI. Finally, we dichotomized the treatment interval at a cut point of ≤7 days. The independent variables examined included symptoms (vaginal discharge, itching or odor, dysuria, and abdominal or pelvic pain), race, condom use at last sexual intercourse, interval since last sexual intercourse, and treatment delivery. Mann-Whitney tests were used to compare continuous, non-normally distributed variables and chi-square testing was used for dichotomous variables. In addition, a logistic model was used to predict which patients were treated in less than seven days.
RESULTS
Of the 200 subjects tested for TV, GC, or CT, 58 (29%) were found to have positive results and comprise the study sample. Of these, 23 (40%) were positive for TV only, 25 (43%) were positive for CT/ GC only, and 17% (10 of 58) had a combination of CT/GC and TV. Subjects were aged 15–20, with a mean of 18 years (Table 1). Fifty-one subjects (88%) self-identified their race as black, two (3%) as white, and five (9%) as mixed. Vaginal symptoms were reported by 28 (48%) of patients; abdominal pain was reported by eighteen (31%) of patients; and multiple symptoms, such as pelvic pain, vaginal symptoms, and urinary symptoms, were identified by 44 (76%) of patients. Unprotected sex, defined as lack of condom use at last sexual intercourse, was reported in 23 (40%) of patients.
Table 1. Selected subject characteristics for those with a positive STI, for the whole group and stratified by treatment interval ≤7 days.
(Number and column percentages are shown.)
| Total N (%) | Treated in >7 days N (%) | Treated in ≤ 7 days N (%) | P value (chi-square test) | |
|---|---|---|---|---|
| Black | 51 (88) | 16 (84) | 35 (88) | .54 |
|
| ||||
| Condom at last contact | 35 (60) | 11 (58) | 24 (61) | .79 |
|
| ||||
| Age >8 | 41 (71) | 13 (68) | 29 (72) | .79 |
|
| ||||
| Wet mount positive for TV | 14 (33) | 1 (9) | 13 (42) | .11 |
|
| ||||
| Any genitourinary symptoms | 44 (75) | 10 (53) | 34 (87) | .004 |
|
| ||||
| Interval to treatment in days: | ||||
| Median | 3 | 13 | 1 | |
| Mean | 9 | 24 | 1.7 | |
| Range | 0–90 | 8–90 | 0–6 | |
|
| ||||
| Total N | 58 | 19 | 39 | |
Interval to treatment
At the initial study visit, 18 (31%) were treated with an antibiotic that was appropriate for their infection, 24 (41%) returned to clinic for treatment, 12 (20%) had their prescriptions called to a pharmacy, and 4 (7%) were lost to follow up for >90 days. Nineteen out of 33 TV positive patients were treated on the same day of their visit, versus 2 of 35 CT/GC positive subjects (58 vs 6%, chi2 = 21.2, p < 0.001). TV patients were treated with metronidazole based on a positive wet mount for TV (13/19), or according to a clinical diagnosis of bacterial vaginosis; CT/GC patients were treated with effective antibiotics covering clinical diagnoses of cervicitis or PID.
For the complete sample, the median interval to treatment was 0 days for TV, 5 days for CT/GC, and 3 days for any STI. Overall, 39 (69%) of subjects with a positive STI were treated within seven days of their visit (Table 1). On bi-variate testing, the only variable associated with treatment in ≤7 days was the presence of any genitourinary symptoms, which had an unadjusted odds ratio of 8.1 (95% CI 2.0–30) for earlier treatment. There were no differences in treatment in ≤7 days by other factors such as race, age, interval since last sexual intercourse, or reported history of condom use. When all variables were entered into a logistic regression model, no new predictors were identified.
When patients treated on the same day of their visit were excluded, Figure 1 illustrates the Kaplan-Meier curve of the cumulative proportion of subjects treated by the interval to treatment for the TV positive subjects (N = 14), the CT/GC positive subjects (N = 35), and those positive for any STI (N = 40 due to overlapping infections). It required 27 days to treat 90% of those infected with any STI. The median time to treatment for TV not diagnosed at the initial visit was eleven days, compared to five days for CT/GC; however, due to small numbers, this did not reach statistical significance. Four patients (one with CT/GC and three with TV) did not have documented treatment within 90 days of a positive test result and were considered lost to follow-up; therefore, the survival curve is censored at the last treatment date (65 days).
Figure 1.
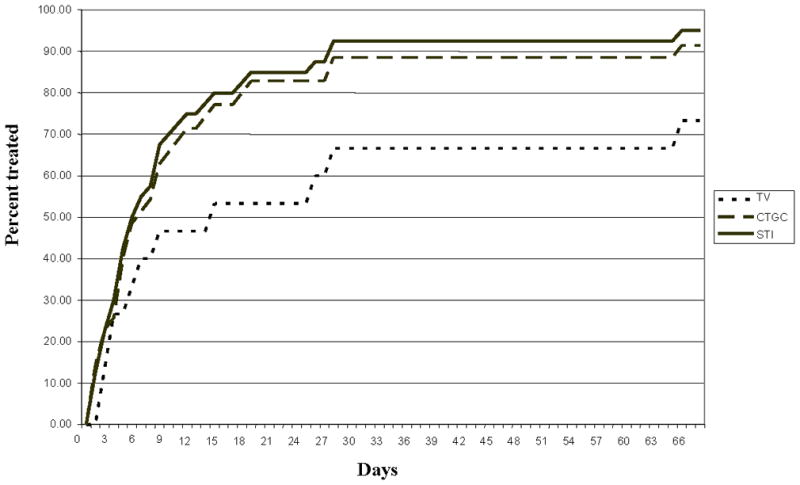
Distribution of the cumulative proportion of STI positive subjects treated, by type of infection and days after initial visit, for the 40 subjects who were not treated at the visit.
For the 40 STI positive subjects who were not treated on the day of visit, it was examined whether the method of medication delivery affected the interval to treatment (Table 2). The majority of subjects (60%) returned to clinic for treatment rather than having a prescription phoned to the pharmacy. Those who returned did not differ from those who chose call in by age, race, reported condom, use or symptoms. However, the treatment interval was significantly shorter for those who chose to have their prescription phoned in to the pharmacy compared to those who returned to clinic for treatment (median 2.5 vs. 8 days), and this difference was significant (p<.01 by Mann-Whitney test). In addition, a higher proportion of those with a phone-in prescription received treatment in less than seven days compared to those who returned to clinic (91 vs. 41%, p = .016). However, because our clinical protocol does not require confirmation that phoned in prescriptions were received by the patient, this shorter interval truly reflects only an interval to notification and assumption of treatment.
Table 2.
Differences between subjects according to treatment delivery. Number and column percentages are shown for the 40 subjects who were not treated on the day of visit.
| Phone- in | Return to clinic | Lost to follow-up | P value (chi-square test except as noted) | |
|---|---|---|---|---|
| Black | 10 (83) | 20 (83) | 4 (100) | .67 |
|
| ||||
| Condoms at last contact | 8 (67) | 16 (67) | 1 (25) | .26 |
|
| ||||
| Age ≥8 | 8 (67) | 18 (69) | 2 (50) | .5 |
|
| ||||
| Any genitourinary symptoms | 7 (58) | 16 (67) | 4 (100) | .5 |
|
| ||||
| Treatment in ≤7 days | 11 (91) | 10 (41) | 0 (0) | .016 |
|
| ||||
| Interval in days: | ||||
| Median | 2.5a | 8 b | <.01M | |
| Mean | 3 | 12.5 | ||
| Range | 1–8 | 2–65 | ||
|
| ||||
| Total: Column N (row %) |
12 (30) | 24 (60) | 4 (10) | 40 (100) |
Mann-whitney and equality of medians tests
days to notification
days to observed treatment
DISCUSSION
Clinicians who test patients for STIs are often faced with the dilemma of whether to treat empirically or await test results. In this study, we demonstrated that in a setting where routine testing is the norm and presumptive treatment rates are low, the median interval to treatment for CT/GC was five days and two thirds of patients were treated within seven days of their visit. This interval is much shorter than prior reports of a median 14-day interval in adolescents and 8- to 26-day intervals in adults.12,13 Providers were more likely to offer same day therapy to symptomatic patients over asymptomatic patients. In addition, there appeared to be no demographic or historical findings, such as reported condom use or other risky behaviors, which influenced provider practice.
The disparity found in same day treatment between TV and CT/GC can be attributed to the rapid wet mount test for TV, which allows same day treatment for the 60% of infections that were detected in wet mount. However, no comparable rapid test exists for CT/GC, and the NAAT can take up to two days to obtain results.
This study demonstrates that even in a teen health center where patients knew their providers and where both patients and providers were aware of their participation in an STI study, there remained a significant delay to treatment, with one third of patients being treated in greater than seven days. In our population, 25% of girls with TV and 40% of girls with CT/GC received treatment more than seven days after their date of testing, and it required up to four weeks to reach 90% of those infected with an STI. This long interval increases the potential for the development of pelvic inflammatory disease, pelvic pain, infertility, and further spread of infections.
The results also indicate that most patients returned to the office for treatment, rather than having their prescriptions phoned to a pharmacy. While a return to clinic has the advantage of ensuring that the medication was received correctly, it may contribute further to the delay, as patient or provider schedules may be busy.
This study was limited by several factors. First, although demographics and symptoms were recorded prospectively as part of the STI/UTI study, this is a retrospective study, so four cases lacked documentation of treatment and follow up within 90 days of positive test result. Secondly, in keeping with the standard of care, this study assumes that a prescription phoned to the pharmacy constituted documentation of adequate treatment, while in reality it is unknown when (or if) patients actually picked up their medications. Additionally, other factors that may influence an adolescent’s choice of how to receive her medication such as cost, confidentiality, and nurse’s encouragement to return for a visit were not explored. We also could not examine whether provider recommendations for partner treatment were followed. The retrospective design did not allow us to assess for consequences such as PID or the need for repeat testing when the interval was prolonged. Finally, this study was limited by its rather small sample size of 58 positive patients. A larger population study would allow for further analysis. However, these findings led to the development of a quality improvement process in our clinic, which is ongoing, and we hope to publish those results in the future.
It is recognized that adolescents present challenges for follow-up and timely treatment. Therefore, future studies should explore unknown factors that prevent untreated teens from returning to care in a timely fashion. We should also evaluate system strategies to reduce the interval between visit and treatment, such as a dedicated results hotline, printed material, or electronic access to results. In addition, we may need to assess whether patients who have their prescriptions phoned to the pharmacy truly receive their medications and how this factor impacts both the delay and the accuracy of STI treatment.
Acknowledgments
Financial support:
Dr. Huppert was supported by a K-23 career development award (NIH/NIAID 5K23AI63182). Ms. Malik was supported by the Medical Student Summer Research Program at University of Cincinnati College of Medicine (NIH/NIDDK T35 Training grant #DK 60444-05).
Footnotes
This work was presented as oral platform at the annual meeting of the North American Society of Pediatrics and Adolescent Gynecology, May 19, 2006, Orlando, FL.
Authorship: Ms. Malik contributed to the design of the study, performed the chart reviews, data entry, initial analyses, and manuscript draft. Dr. Huppert designed the original and retrospective studies, supervised chart review and data entry, completed the analyses, revised the manuscript, and approved the final version to be published.
Contributor Information
Amina I Malik, University of Cincinnati College of Medicine, Cincinnati, OH
Jill S. Huppert, Department of Pediatrics, Division of Adolescent Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
References
- 1.Centers for Disease Control and Prevention. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections. MMWR. 2002;51(RR-15) [PubMed] [Google Scholar]
- 2.U.S. Preventive Services Task Force. Screening for Clamydial Infection. Vol. 29. Washington, DC: US Preventive Services Task Force; 1996. Guide to Clinical Preventive Services; pp. 347–359. [Google Scholar]
- 3.Burstein G, Waterfield G, Joffe A, Zenilman J, Quinn T, Gaydos C. Screening for gonorrhea and chlamydia by DNA amplification in adolescents attending middle school health centers. Opportunity for early intervention. Sex Transm Dis. 1998;25(8):395–402. doi: 10.1097/00007435-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hillis SD, Joesoef R, Marchbanks PA, Wasserheit JN, Cates W, Jr, Westrom L. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993 May;168(5):1503–1509. doi: 10.1016/s0002-9378(11)90790-x. [DOI] [PubMed] [Google Scholar]
- 5.Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med. 1996 May 23;334(21):1362–1366. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 6.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992 Mar-Apr;19(2):61–77. [PubMed] [Google Scholar]
- 7.Gottlieb SL, Douglas JM, Jr, Foster M, et al. Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk-reduction counseling. J Infect Dis. 2004 Sep 15;190(6):1059–1067. doi: 10.1086/423323. [DOI] [PubMed] [Google Scholar]
- 8.Shew M, Fortenberry JD, Tu W, et al. Association of condom use, sexual behaviors and sexually transmitted infections with the duration of genital HPV infection. J Adolesc Health. 2005 February;36(2):102–103. doi: 10.1001/archpedi.160.2.151. [DOI] [PubMed] [Google Scholar]
- 9.St Lawrence JS, Montano DE, Kasprzyk D, Phillips WR, Armstrong K, Leichliter JS. STD screening, testing, case reporting, and clinical and partner notification practices: a national survey of US physicians. Am J Public Health. 2002 Nov;92(11):1784–1788. doi: 10.2105/ajph.92.11.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmann LH, Richey CM, Waites K, Schwebke JR, Hook EW., 3rd Patterns of Chlamydia trachomatis testing and follow-up at a University Hospital Medical Center. Sex Transm Dis. 1999 Oct;26(9):496–499. doi: 10.1097/00007435-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Yealy DM, Greene TJ, Hobbs GD. Underrecognition of cervical Neisseria gonorrhoeae and Chlamydia trachomatis infections in the emergency department. Acad Emerg Med. 1997 Oct;4(10):962–967. doi: 10.1111/j.1553-2712.1997.tb03659.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong D, Berman SM, Furness BW, Gunn RA, Taylor M, Peterman TA. Time to treatment for women with chlamydial or gonococcal infections: a comparative evaluation of sexually transmitted disease clinics in 3 US cities. Sex Transm Dis. 2005 Mar;32(3):194–198. doi: 10.1097/01.olq.0000154494.95138.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foglia G, Rhodes P, Goldberg M, St Louis ME. Completeness of and duration of time before treatment after screening women for Chlamydia trachomatis infections. Sex Transm Dis. 1999 Sep;26(8):421–425. doi: 10.1097/00007435-199909000-00001. [DOI] [PubMed] [Google Scholar]


