Abstract
Background
Aprotinin was used frequently in children undergoing congenital heart surgery with the aim of reducing bleeding, until it was taken off the market following adult studies reporting increased mortality and renal failure. We evaluated the safety of aprotinin in a large multi-institutional cohort of children undergoing congenital heart surgery.
Methods
The Pediatric Health Information Systems Database was used to evaluate aprotinin in children (0–18y) undergoing congenital heart surgery at 35 US children’s hospitals from 2003–2007. Propensity scores were constructed to account for potential confounders including age, sex, race, prematurity, genetic syndrome, type of surgery [Risk Adjustment in Congenital Heart Surgery (RACHS-1) category], center, and center volume. Multivariable analysis, adjusting for propensity score and individual covariates was performed to evaluate in-hospital mortality, post-operative renal failure requiring dialysis, and length of stay (LOS). Sub-analysis was performed in the high-risk cohort undergoing re-operation.
Results
There were 30,372 patients included: 56% male, median age 7m (interquartile range 36d–3.2y). Overall, 44% received aprotinin. In multivariable analysis, there was no difference in post-operative mortality (OR 1.00, 95%CI 0.99,1.01), dialysis (OR 1.00, 95%CI 0.99,1.01), or LOS [least square mean difference (LSM) −0.44d, 95%CI −1.01,0.13] between aprotinin recipients and non-recipients. In patients undergoing re-operation, there was also no difference in mortality or dialysis. Aprotinin recipients had significantly reduced LOS (LSM difference −2.05d, 95%CI −3.29,−0.81),
Conclusions
These data suggest aprotinin is not associated with increased mortality or dialysis in children undergoing congenital heart surgery, and that further evaluation of aprotinin in this population could be undertaken without undue risk.
Keywords: congenital heart disease, outcomes
Introduction
Aprotinin, an anti-fibrinolytic agent, was often utilized in children undergoing congenital heart surgery with the aim of reducing intra- and post-operative bleeding (1,2). Aprotinin also has anti-inflammatory properties which may be beneficial in attenuating the inflammatory response associated with cardiopulmonary bypass (3–5). However, in 2007 aprotinin was taken off the market following studies which reported increased mortality and renal failure in adults (5,6). As a result, aprotinin was also no longer available to children undergoing congenital heart surgery, who are more prone to bleeding during and after cardiac surgery compared with adults (7). Small single-center studies have suggested that aprotinin may not be associated with adverse outcomes in children (8,9). Guzzetta evaluated 200 neonates undergoing congenital heart surgery and found no difference in mortality or renal failure in aprotinin recipients and non-recipients (8).
Thus, given the potential benefits and perhaps more favorable safety profile compared with adults, there is interest in further study of aprotinin in children. As studies to date have been limited in scope and size, a more comprehensive analysis of safety outcomes associated with aprotinin is necessary in order to determine whether further prospective study could be undertaken without undue risk.
The primary objective of this study was to evaluate safety outcomes associated with aprotinin in a large multi-institutional cohort of children undergoing congenital heart surgery. Sub-analysis by Risk Adjustment in Congenital Heart Surgery, version 1 (RACHS-1) category, and in those undergoing re-operation was also performed.
Methods
Data Source
Data for this multi-center retrospective cohort study were obtained from the Pediatric Health Information System (PHIS) Database, which contains resource utilization data from 39 freestanding, tertiary care children’s hospitals affiliated with the Child Health Corporation of America (Shawnee Mission, KS). PHIS-participating hospitals, which account for 20% of all tertiary care children’s hospitals, provide discharge data including patient demographics, diagnoses, and procedures. Billing data are also available detailing medications, imaging studies, laboratory tests, and supplies charged to each patient. Data quality and reliability are assured through a joint effort between Child Health Corporation of America and participating hospitals, including bimonthly coding consensus meetings, coding consistency reviews, and quarterly data quality reports. The Children’s Hospital of Philadelphia Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Population
Centers with an average annual congenital heart surgery volume of <50 cases per year (n=2) and those with wide deviation from other centers in their practice pattern regarding post-operative dialysis, a key outcome measure, (n=2, likely related to routine use of peritoneal dialysis in post-operative management of fluid status in certain patient groups) were excluded. Thus, 35 centers were included in the analysis. Children ≤ 18 years of age discharged from these centers between January 1, 2003 and December 31, 2007 who underwent congenital heart surgery classified in any of the RACHS-1 categories were included (10). The RACHS-1 method has been used extensively in risk stratification of patients undergoing congenital heart surgery as previously described (10,11). Patients who did not require cardiopulmonary bypass [International Classification of Diseases, Ninth Revision (ICD-9) procedure code 39.61] in conjunction with their surgery, and those who received any other anti-fibrinolytic therapy (aminocaproic acid or tranexamic acid) were excluded.
Data Collection and Study Definitions
Aprotinin recipients were defined as those who received aprotinin on the day of surgery. Other variables collected included patient age, sex, race/ethnicity, weight (for neonates), prematurity (ICD-9 codes 765.0x–765.3x) or any type of genetic syndrome/chromosomal abnormality (ICD-9 diagnosis codes 758.0–758.9, 553.3, 756.6–756.7, 759.7–759.9), and primary payer (private insurance, government insurance, other). Center characteristics collected included region and average annual congenital heart surgery volume (only surgeries included in the RACHS-1 categories) during the study period.
Outcome Measures
The primary outcome was in-hospital mortality. Secondary outcomes included total and intensive care unit length of stay, and post-operative renal failure requiring peritoneal or hemodialysis (ICD-9 codes 39.95 or 54.98).
Statistical Analysis
Study variables were summarized using frequencies and percentages for categorical variables, and median and interquartile range (IQR) for continuous variables. Patient and center characteristics and unadjusted outcomes were compared between aprotinin recipients and non-recipients using Chi-square and Wilcoxon Rank-Sum tests. Similar to previous studies, we combined RACHS-1 categories 5 and 6 for analysis due to the small number of patients in RACHS-1 category 5 (11). Stratified analysis was performed to evaluate whether the impact of aprotinin on outcome varied by RACHS-1 risk category. The distribution of aprotinin use by center was evaluated, and compared with center average annual volume of RACHS-1 classified surgeries, using Spearman’s correlation.
Propensity scores were then constructed using multivariable logistic regression to estimate the likelihood of receiving aprotinin given an observed set of baseline confounders (12). Variables entered into the model included age, sex, race/ethnicity, prematurity, genetic syndrome, RACHS-1 category, center, and average annual center volume. Patient weight (for neonates) was initially considered, but not included in the final model as it was highly collinear with prematurity. The model’s c-statistic was 0.78.
Multivariable analysis was performed to evaluate outcomes independently associated with aprotinin, adjusting for propensity score, each individual covariate, and within-center clustering. Adjusting for both the propensity score and individual covariates in modeling has been termed “doubly robust” in that if either the propensity score adjustment or the confounding variable adjustment is correct, the final estimate of the effect should be correct (13). For dichotomous outcome variables, modeling consisted of logistic regression using the method of generalized estimating equations (GEE). For continuous outcome variables, mixed effect models were used treating center as a random effect. Analyses were performed both for the overall cohort and stratified by RACHS-1 category. Odds ratios and 95% confidence intervals are reported for the GEE models, and difference in least square means and 95% confidence intervals comparing aprotinin recipients and non-recipients from the mixed effects models.
Finally, a sub-analysis was performed in the subset of the cohort undergoing re-operation. This analysis was restricted to 24 centers who have submitted data annually to the PHIS database since 1999. Patients undergoing re-operation were defined as those who had undergone previous RACHS-1 classified congenital heart surgery from 1999–2007 at the same institution. In this sub-population, unadjusted and adjusted analyses were performed as outlined above.
All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). We used the conservative Bonferroni correction to account for multiple comparisons. Thus, a p-value <0.01 was considered statistically significant.
Results
Patient Characteristics and Aprotinin Use
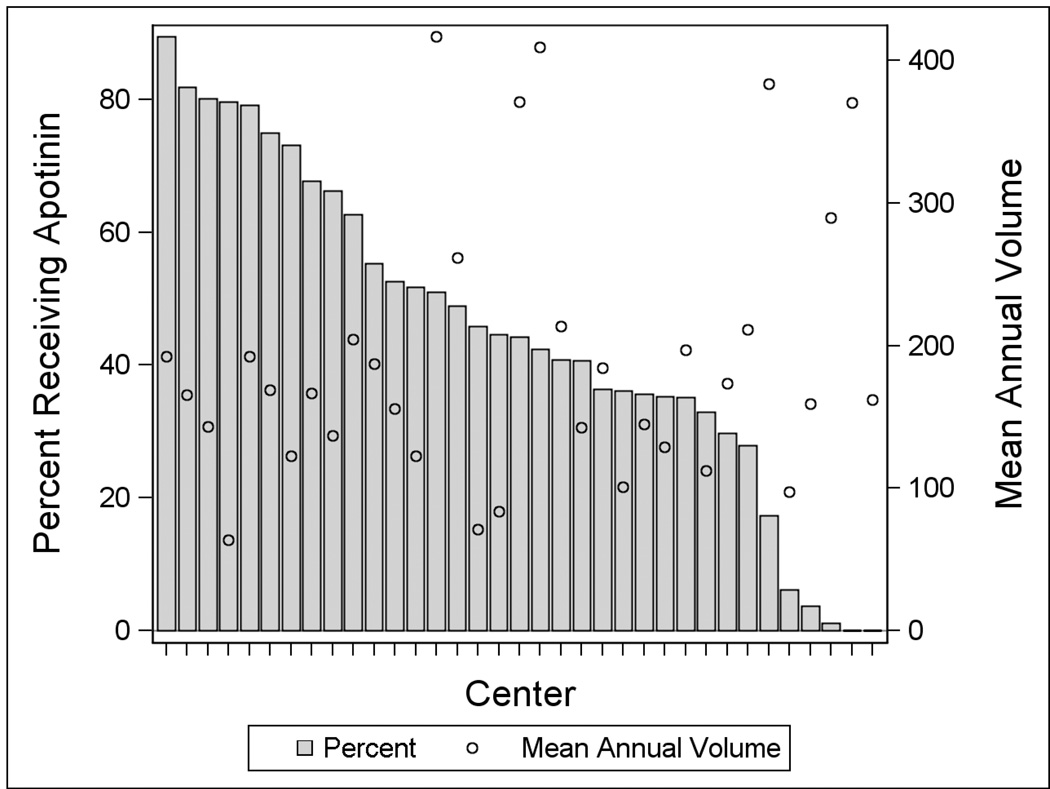
A total of 30,372 patients from 35 centers were included (Table 1). Overall, 56% were male and median age was 7 months (IQR 36 days–3.2 years). Forty-four percent (n=13,331) of patients received aprotinin. Receipt of aprotinin was associated with older age, higher RACHS-1 category, government insurance, and being treated at a center in the North Central US. Those with a genetic syndrome/abnormality were less likely to receive aprotinin. Aprotinin use by center varied with a range of 0–89% of patients per center receiving aprotinin (Figure 1). There was not a significant linear correlation between center volume and use of aprotinin (p=0.2).
Table 1.
Patient and Center Characteristics
| Overall | No Aprotinin | Aprotinin | p | |
|---|---|---|---|---|
| 30372 | 17041 (56.1) | 13331 (43.9) | ||
| Sex | ||||
| Male | 16918 (55.7) | 9347 (54.85) | 7571 (56.8) | <0.001 |
| Age | ||||
| ≤ 30d | 6944 (22.9) | 3985 (23.4) | 2959 (22.2) | <0.001 |
| 31d-1y | 10919 (36.0) | 6401 (37.6) | 4518 (33.9) | |
| 2y–5y | 7861 (25.9) | 4329 (25.4) | 3532 (26.5) | |
| 6y–12y | 2684 (8.8) | 1390 (8.2) | 1294 (9.7) | |
| >12 y | 1964 (6.5) | 936 (5.5) | 1028 (7.8) | |
| Median [IQR], years | 0.6 [0.1, 3.2] | 0.5 [0.1, 3] | 0.7 [0.2, 3.6] | <0.001 |
| Race | ||||
| Non-Hispanic White | 15539 (53.7) | 8568 (53.3) | 6971 (54.3) | <0.001 |
| Non-Hispanic Black | 3761 (13.0) | 2165 (13.5) | 1596 (12.4) | |
| Hispanic | 5469 (18.9) | 2866 (17.8) | 2603 (20.3) | |
| Asian | 925 (3.2) | 551 (3.4) | 374 (2.9) | |
| Other | 3226 (11.2) | 1940 (12.1) | 1286 (10.0) | |
| RACHS Level | ||||
| 1 | 2575 (8.5) | 1878 (11.0) | 697 (5.2) | <0.001 |
| 2 | 11059 (36.4) | 6330 (37.2) | 4729 (35.5) | |
| 3 | 11945 (39.3) | 6360 (37.3) | 5585 (41.9) | |
| 4 | 3211 (10.6) | 1650 (9.7) | 1561 (11.7) | |
| 5/6 | 1582 (5.2) | 823 (4.8) | 759 (5.7) | |
| Genetic abnormality | ||||
| Yes | 4594 (15.1) | 2713 (15.9) | 1881 (14.1) | <0.001 |
| Prematurity | ||||
| Yes | 1321 (4.4) | 786 (4.6) | 535 (4.0) | 0.03 |
| No | 5789 (19.1) | 3258 (19.1) | 2531 (19.0) | |
| NA | 23262 (76.6) | 12997 (76.3) | 10265 (77.0) | |
| Payor | ||||
| Government | 12423 (40.9) | 6711 (39.4) | 5712 (42.9) | <0.001 |
| Private | 11267 (37.1) | 6412 (37.6) | 4855 (36.4) | |
| Other | 6674 (22.0) | 3915 (23.0) | 2759 (20.7) | |
| Center Census Group | ||||
| Northeast | 4255 (14.0) | 3067 (18.0) | 1188 (8.9) | <0.001 |
| South | 9662 (31.8) | 5419 (31.8) | 4243 (31.8) | |
| North Central | 8750 (28.8) | 4161 (24.4) | 4589 (34.4) | |
| West | 7705 (25.4) | 4394 (25.8) | 3311 (24.8) | |
| Center Volume | ||||
| <150 | 1947 (6.4) | 1282 (7.5) | 665 (5.0) | <0.001 |
| 150–250 | 14503 (47.8) | 7306 (42.9) | 7197 (54.0) | |
| 251–350 | 4797 (15.8) | 2353 (13.8) | 2444 (18.3) | |
| >350 | 9125 (30.0) | 6100 (35.8) | 3025 (22.7) | |
Figure 1.
Distribution of Aprotinin Use by Center (bar). The average annual congenital heart surgery volume is also shown (circle).
Outcomes
Unadjusted outcomes are displayed in Table 2 (overall) and Supplementary Table 1 (stratified by RACHS-1 category). Adjusted outcomes are displayed in Table 3. In multivariable analysis, there was no difference in mortality, length of stay, or dialysis among aprotinin recipients and non-recipients (Table 3a). Results were similar in stratified analysis by RACHS-1 category (Table 3b).
Table 2.
Unadjusted Outcomes
| Overall | No Aprotinin | Aprotinin | p | |
|---|---|---|---|---|
| Mortality | 964 (3.2) | 532 (3.1) | 432 (3.2) | 0.558 |
| Dialysis | 331 (1.1) | 161 (0.9) | 170 (1.3) | 0.006 |
| Total length of stay (days) | 7 [4, 16] | 7 [4, 16] | 8 [4, 16] | <0.001 |
| ICU length of stay (days) | 3 [1, 7] | 3 [1, 7] | 3 [1, 8] | <0.001 |
Data are displayed as n (%) for dichotomous variables and median (IQR) for continuous variables.
Table 3.
| a. Adjusted Outcomes | ||
|---|---|---|
| OR (95% CI) | p | |
| Mortality | 1.00 (0.99, 1.01) | 0.447 |
| Dialysis | 1.00 (0.99, 1.01) | 0.975 |
|
LSM Difference Aprotinin - No Aprotinin |
||
| Total length of Stay (days) | −0.44 (−1.01, 0.13) | 0.127 |
| ICU length of Stay (days) | 0.18 (−0.15, 0.51) | 0.291 |
| b. Adjusted Outcomes Stratified by RACHS-1 Category | ||
|---|---|---|
| RACHS-1 Category 1 | ||
| OR (95% CI) | p | |
| Mortality | 1.01 (0.99, 1.02) | 0.094 |
| Dialysis | 1.00 (0.99, 1.01) | 0.868 |
|
LSM Difference Aprotinin - No Aprotinin |
||
| Total length of Stay (days) | −1.09 (−3.30, 1.13) | 0.337 |
| ICU length of Stay (days) | −0.24 (−1.15, 0.67) | 0.602 |
| RACHS-1 Category 2 | ||
| OR (95% CI) | ||
| Mortality | 1.00 (0.99, 1.01) | 0.471 |
| Dialysis | 1.00 (0.99, 1.01 | 0.243 |
|
LSM Difference Aprotinin - No Aprotinin |
||
| Total length of Stay (days) | −0.23 (−1.02, 0.57) | 0.575 |
| ICU length of Stay (days) | 0.25 (−0.14, 0.63) | 0.213 |
| RACHS-1 Category 3 | ||
| OR (95% CI) | ||
| Mortality | 1.00 (0.99, 1.01) | 0.465 |
| Dialysis | 1.00 (0.99, 1.01) | 0.299 |
|
LSM Difference Aprotinin - No Aprotinin |
||
| Total length of Stay (days) | −0.40 (−1.28, 0.49) | 0.377 |
| ICU length of Stay (days) | 0.18 (−0.35, 0.71) | 0.505 |
| RACHS-1 Category 4 | ||
| OR (95% CI) | ||
| Mortality | 0.99 (0.97, 1.01) | 0.353 |
| Dialysis | 0.99 (0.97, 1.01) | 0.335 |
|
LSM Difference Aprotinin - No Aprotinin |
||
| Total length of Stay (days) | −0.66 (−3.08, 1.76) | 0.593 |
| ICU length of Stay (days) | 0.23 (−1.29, 1.75) | 0.769 |
| RACHS-1 Category 5/6 | ||
| OR (95% CI) | ||
| Mortality | 0.98 (0.93, 1.03) | 0.476 |
| Dialysis | 1.00 (0.97, 1.03) | 0.953 |
|
LSM Difference Aprotinin - No Aprotinin |
||
| Total length of Stay (days) | −1.18 (−4.87, 1.24) | 0.244 |
| ICU length of Stay (days) | −1.78 (−4.03, 0.47) | 0.121 |
LSM = least square means
In sub-analysis of 4,234 patients undergoing re-operation, there was no difference in mortality or post-operative dialysis (Table 4). Aprotinin recipients had significantly reduced length of stay.
Table 4.
Adjusted Outcomes in Sub-group Undergoing Re-operation
| OR (95% CI) | p | |
|---|---|---|
| Mortality | 0.99 (0.98, 1.01) | 0.113 |
| Dialysis | 1.00 (0.99, 1.01) | 0.929 |
|
LSM Difference Aprotinin - No Aprotinin |
||
| Total length of Stay (days) | −2.05 (−3.29, −0.81) | 0.001 |
| ICU length of Stay (days) | −0.82 (−1.59, −0.05) | 0.037 |
LSM = least square means
Discussion
In the largest study of aprotinin in the pediatric population to date, we evaluated the safety of aprotinin in children undergoing congenital heart surgery. Contrary to the adverse events associated with aprotinin in adults undergoing cardiac surgery, aprotinin was not associated with increased mortality or dialysis in this large multi-institutional cohort of children undergoing congenital heart surgery.
Historically, most medications used in children have not been adequately studied and treatment decisions are instead based on extrapolation from adult data, clinical experience, or small observational studies (14). Inadequate information on drug safety and efficacy may deny children potential therapeutic benefits, place them at risk for adverse events, or result in treatment with ineffective therapies. A growing body of evidence has highlighted some of the potential pitfalls of this approach. For example, many children with heart failure are treated with carvedilol due to the beneficial effects demonstrated in multiple adult heart failure studies (15). However, in a recent randomized trial, carvedilol did not improve heart failure outcomes in children and adolescents with symptomatic heart failure (16). In another study, the optimal dose of clopidogrel in children with a cardiac condition at risk for arterial thrombosis was only one fifth of what would be given if extrapolating from adult data (17).
In the case of aprotinin, this antifibrinolytic agent was used frequently in children undergoing cardiac surgery. Children are more prone to bleeding during and after cardiac surgery compared with adults as a result of greater dilutional effects associated with the cardiopulmonary bypass circuit due to smaller blood volumes, immaturity of the coagulation system, and association of congenital heart disease with coagulation abnormalities (18,19). Intra-operative and post-operative bleeding can be associated with prolonged operative times, hemodynamic instability, re-operation, and need for transfusion of blood products, all of which may be associated with prolonged length of stay and increased mortality risk (20). Prolonged operative times and length of hospital stay have also been shown to be associated with poor neurodevelopmental outcomes (21,22). Although the risk of viral transmission with blood product transfusion is low, there can be other adverse effects such as allergic reaction, augmentation of the pro-inflammatory state that exists after cardiopulmonary bypass, and allo-sensitization (7).
Results from our study confirm that aprotinin was used commonly, with nearly half of children undergoing congenital heart surgery at 35 US hospitals receiving aprotinin from 2003–2007. In 2007, aprotinin was taken off the market following the BART trial in which aprotinin was associated with increased mortality in a randomized study of 2331 adults undergoing cardiac surgery (5). This was primarily related to an increased risk of death from cardiovascular causes. In addition, observational data in adults undergoing cardiac surgery suggested that aprotinin was associated with an increased risk of renal failure requiring dialysis (6). The mechanism for these adverse events is thought to be related to the thrombotic potential of aprotinin, and other renal effects including interference in normal functioning of the proximal tubule, bradykinin system, and renal artery vasoreactivity (23,24).
In contrast, recent small single-center studies suggest that the adverse outcomes in adults treated with aprotinin may not occur in pediatric patients. Guzzetta evaluated 200 neonates undergoing congenital heart surgery in an observational study and found that aprotinin was not significantly associated with post-operative serum creatinine levels or need for dialysis (8). Backer et al. evaluated a more heterogeneous group of 1251 children and adolescents undergoing congenital heart surgery, and found that aprotinin was not associated with post-operative renal failure, dialysis, or mortality compared with historical controls (9). However, these previous studies were limited by small sample size, narrow scope, and the use of historical controls. Our study confirms these results in a large, multi-institutional, diverse group of patients undergoing congenital heart surgery. Interestingly, in addition to these observational studies, there have been several randomized trials of aprotinin in children undergoing congenital heart surgery. However, evaluation of safety was limited in these small trials, and in a recent meta-analysis combining results from 12 trials, mortality and renal failure could not be assessed (1).
While the focus of this study was to assess safety outcomes associated with aprotinin, multiple other analyses have evaluated efficacy. Several adult trials have shown that aprotinin is associated with decreased post-operative bleeding and need for transfusion compared with placebo, and importantly, may be more effective than other antifibrinolytic agents (2,5). A meta-analysis of 12 pediatric trials found a significant reduction in blood product transfusion associated with aprotinin (1). However, it should be noted that these trials have been limited by important methodologic concerns including small sample size, heterogeneous patient population, lack of standardized transfusion protocols, and varying doses of aprotinin used (1). In addition, not all pediatric trials have demonstrated a beneficial effect of aprotinin, and some studies have suggested that aprotinin may be of benefit in only certain subgroups (25,26). Costello found that only the subgroup undergoing re-operation had a significant decrease in transfusions associated with aprotinin in a small observational study of patients undergoing congenital heart surgery (26). While it was not the objective of this study to evaluate efficacy, we did observe that the subgroup undergoing re-operation, who are likely at highest risk for bleeding, had significantly reduced length of stay associated with aprotinin. This effect was not seen in other subgroups evaluated. As opposed to other antifibrinolytic agents, aprotinin has also been shown to have anti-inflammatory properties (3,4). Previous single-center pediatric series have reported a reduction in inflammatory markers in patients who receive aprotinin, as well as a reduction in post-operative myocardial dysfunction and ionotropic support (27,28). These properties, along with the potential impact of aprotinin on bleeding, may have contributed to the decreased length of stay we observed in the subgroup undergoing re-operation in our study.
Limitations
This study is subject to the limitations of any observational investigation including selection bias and the potential impact of confounders. We performed a propensity analysis to account for patient confounders and adjusted for propensity score as well as individual covariates in our models. It is possible that there may be other measured or unmeasured confounders impacting our analysis. In addition, our study is subject to the limitations of the database. The database does not contain information regarding the dose of aprotinin, volume of blood product transfusion, or chest tube output/bleeding following surgery. We were also unable to assess re-operation due to bleeding. However, these factors are unlikely to impact our evaluation of safety, the primary objective of this study. We were also not able to specifically assess outcomes associated with aprotinin in the subgroup requiring deep hypothermic circulatory arrest. Previous adult studies have suggested that the use of aprotinin in patients where circulatory arrest is required during surgery may exacerbate any renal toxicity, while other studies have not (29,30). The use of circulatory arrest is not captured in the PHIS database; however, many of the surgeries in RACHS-1 categories 5/6 require circulatory arrest, and we did not find any difference in dialysis or post-operative mortality in this subgroup in stratified analysis. Regarding our analysis of renal failure, the database does not contain laboratory information such as creatinine levels. Thus, it is possible we may have underestimated the impact of aprotinin on milder degrees of renal dysfunction not requiring dialysis. In addition, in our sub-analysis of patients undergoing re-operation we were limited to evaluating patients undergoing re-operation at the same institution; therefore we may not have captured all patients undergoing re-operation. Finally, there are limited data regarding the accuracy of coding of congenital heart surgery in administrative datasets. Random coding errors may be mitigated by the use of large datasets and groupings of procedures, as in this study. The similarities between overall outcomes of patients undergoing congenital heart surgery in our dataset compared with other large clinical datasets support the validity of our data (31).
Conclusions
This study represents the largest analysis of aprotinin in the pediatric population to date. Contrary to studies of adult patients undergoing cardiac surgery, aprotinin was not associated with increased mortality or renal failure requiring dialysis in children undergoing congenital heart surgery. These data suggest further evaluation of aprotinin in this population could be undertaken without undue risk.
Supplementary Material
Acknowledgments
Dr. Pasquali receives support (KL2 RR024127-02) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and from the American Heart Association (AHA) Mid-Atlantic Affiliate Clinical Research Program. Dr. Shah receives support from the National Institute of Allergy and Infectious Diseases (K01 AI73729) and the Robert Wood Johnson (RWJ) Foundation Physician Faculty Scholar Program.
Footnotes
Disclosures
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NIH, AHA, or RWJ Foundation. The authors had full control of the design of the study, methods used, outcome parameters, analysis of data and production of the written report.
References
- 1.Arnold DM, Fergusson DA, Chan AK, et al. Avoiding transfusions in children undergoing cardiac surgery: A meta-analysis of randomized trials of aprotinin. Anesth Analg. 2006;102:731–737. doi: 10.1213/01.ane.0000194954.64293.61. [DOI] [PubMed] [Google Scholar]
- 2.Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128:442–448. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 3.Tassani P, Augustin N, Barankay A, Braun SL, Zaccaria F, Richter JA. High dose aprotinin modulates the balance between proinflammatory and anti-inflammatory response during coronary artery bypass graft surgery. J Cardiovasc Thorac Surg. 2000;14:682–686. doi: 10.1053/jcan.2000.18328. [DOI] [PubMed] [Google Scholar]
- 4.Hill GE, Alonso A, Spurzem JR, et al. Aprotinin and methylprednisolone equally blunt cardiopulmonary bypass-induced inflammation in humans. J Thorac Cardiovasc Surg. 1995;110:1658–1662. doi: 10.1016/S0022-5223(95)70027-7. [DOI] [PubMed] [Google Scholar]
- 5.Fergusson DA, Hebert PC, Mazer CD, et al. A comparison of aprotinin and lysine analgues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–2331. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 6.Mangano D, Tudor JC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 7.Petaja J, Lundstrom U, Leijala M, Peltola K, Siimes MA. Bleeding and use of blood products after heart operations in infants. J Thorac and Cardiovas Surg. 1995;109:524–529. doi: 10.1016/S0022-5223(95)70284-9. [DOI] [PubMed] [Google Scholar]
- 8.Guzzetta NA, Evans FM, Rosenberg ES, et al. The impact of aprotinin on postoperative renal dysfunction in neonates undergoing cardiopulmonary bypass. Anesth Analg. 2009;108:448–455. doi: 10.1213/ane.0b013e318194007a. [DOI] [PubMed] [Google Scholar]
- 9.Backer CL, Kelle AM, Stewart RD, et al. Aprotinin is safe in pediatric patients undergoing cardiac surgery. J Thorac and Cardiovas Surg. 2007;134:1421–1426. doi: 10.1016/j.jtcvs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 11.Welke KF, Diggs BS, Karamlou T, Ungerleider RM. Comparison of pediatric cardiac surgical mortality rates from national administrative data to contemporary clinical standards. Ann Thorac Surg. 2009;87:216–223. doi: 10.1016/j.athoracsur.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:841–853. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 13.Imbens GW. Nonparametric estimation of average treatment effects under exogeneity: a review. Rev Econ Stat. 2004;86:4–29. [Google Scholar]
- 14.Sanders SP. Conducting pediatric cardiovascular trials. Am Heart J. 2001;142:218–223. doi: 10.1067/mhj.2001.117064. [DOI] [PubMed] [Google Scholar]
- 15.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 16.Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 17.Li JS, Yow E, Berezny KY, et al. Dosing of clopidogrel for platelet inhibition in infants and young children: primary results of the PICOLO trial. Circulation. 2008;117:553–559. doi: 10.1161/CIRCULATIONAHA.107.715821. [DOI] [PubMed] [Google Scholar]
- 18.Kern FH, Morana NJ, Sears JJ, Hickey PR. Coagulation defects in neonates during cardiopulmonary bypass. Ann Thorac Surg. 1992;54:541–567. doi: 10.1016/0003-4975(92)90451-9. [DOI] [PubMed] [Google Scholar]
- 19.Goldschmidt B, Sarkadi B, Gardos G, Matlary A. Platelet production and survival in cyanotic congenital heart disease. Scand J Haematol. 1974;13:110–115. doi: 10.1111/j.1600-0609.1974.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 20.Spiess BD. Transfusion of blood products affects outcome in cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8:267–281. doi: 10.1177/108925320400800402. [DOI] [PubMed] [Google Scholar]
- 21.Mahle WT, Visconti KJ, Freier C, et al. Relationship of surgical approach to neurodevelopmental outcomes in hypoplastic left heart syndrome. Pediatrics. 2006;117:e90–e97. doi: 10.1542/peds.2005-0575. [DOI] [PubMed] [Google Scholar]
- 22.Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 23.Vio CP, Oestreicher E, Olavarria V, Velarde V, Mayfield RK, Jaffa AA. Cellular distribution of exogenous aprotinin in the rat kidney. Biol Chem. 1998;379:1271–1277. doi: 10.1515/bchm.1998.379.10.1271. [DOI] [PubMed] [Google Scholar]
- 24.Maier M, Starlinger M, Zhegu Z, Rana H, Binder BR. Effect of the protease inhibitor aprotinin on renal hemodynamics in the pig. Hypertension. 1985;7:32–38. doi: 10.1161/01.hyp.7.1.32. [DOI] [PubMed] [Google Scholar]
- 25.Eaton MP. Antifibrinolytic therapy in surgery for congenital heart disease. Anesth Analog. 2008;106:1087–1100. doi: 10.1213/ane.0b013e3181679555. [DOI] [PubMed] [Google Scholar]
- 26.Costello JM, Backer CL, de Hoyos A, Binns HJ, Mavroudis C. Aprotinin reduces operative closure time and blood product use after pediatric bypass. Ann Thorac Surg. 2003;75:1261–1266. doi: 10.1016/s0003-4975(02)04667-2. [DOI] [PubMed] [Google Scholar]
- 27.Wippermann CF, Schmid FX, Eberle B, et al. Reduced inotropic support after aprotinin therapy during pediatric cardiac operations. Ann Thorac Surg. 1999;67:173–176. doi: 10.1016/s0003-4975(98)00974-6. [DOI] [PubMed] [Google Scholar]
- 28.Tweddell J, Berger S, Frommelt CP, et al. Aprotinin improves outcome of single ventricle palliation. Ann Thorac Surg. 1996;62:1329–1336. doi: 10.1016/0003-4975(96)00670-4. [DOI] [PubMed] [Google Scholar]
- 29.Mora Mangano CT, Neville MJ, Hsu PH, Mignea I, King J, Miller C. Aprotinin, blood loss, and renal dysfunction in deep hypothermic circulatory arrest. Circulation. 2001;104:I-276–I-281. doi: 10.1161/hc37t1.094702. [DOI] [PubMed] [Google Scholar]
- 30.Sundt TM, III, Kouchoukos NT, Saffitz JE, et al. Renal dysfunction and intravascular coagulation with aprotinin and hypothermic circulatory arrest. Ann Thorac Surg. 1993;55:1418–1424. doi: 10.1016/0003-4975(93)91082-x. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs JP, Jacobs ML, Lacour-Gayet FG, et al. Stratification of complexity improves the utility and accuracy of outcomes analysis in a multi-institutional congenital heart surgery database. Pediatr Cardiol. 2009;30:1117–1130. doi: 10.1007/s00246-009-9496-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.