Abstract
The ETS family of transcription factors plays an essential role in controlling endothelial gene expression. Multiple members of the ETS family are expressed in the developing endothelium and evidence suggests that the proteins function, to some extent, redundantly. However, recent studies have demonstrated a crucial non-redundant role for ETV2, as a primary player in specification and differentiation of the endothelial lineage. Here, we review the contribution of ETS factors, and their partner proteins, to the regulation of embryonic vascular development.
Keywords: ETS, Endothelial, Gene expression, Development
1. Introduction
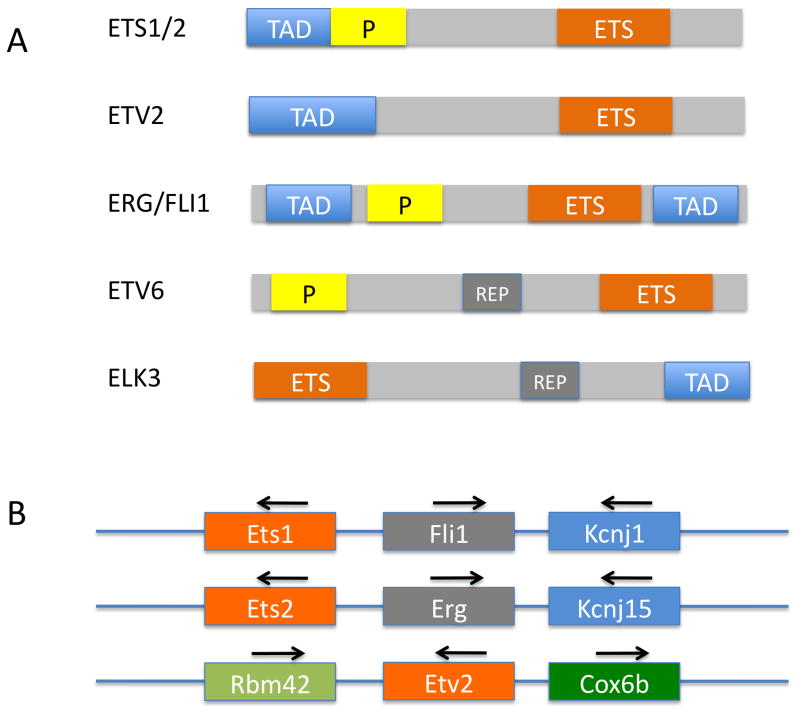
The first member of the Ets gene family, Ets1, was identified as a viral oncogene in the E26 avian leukemia retrovirus (E26 transformation specific-1) [1]. Since this initial discovery, approximately 30 Ets family genes have been identified in various vertebrate species with 27 and 26 Ets genes identified in the human and mouse genomes respectively [2,3]. The majority of ETS factors activate gene transcription however, some ETS proteins can act as transcriptional repressors, while others may perform dual functions either as a repressor or activator depending on the target or post-translational modifications [4,5]. ETS proteins are characterized by a highly conserved DNA binding domain referred to as the ETS domain (Fig. 1A), which is a winged helix-turn-helix that binds to the core DNA sequence 5’-GGA(A/T)-3’. The winged helix-turn-helix motif is approximately 85 amino acids long and consists of three alpha-helices and four anti-parallel beta-sheets. The third helix is primarily responsible for DNA-binding specificity [6,7]. A subset of ETS factors contains a ‘Pointed’ domain thought to function in protein-protein interactions (Fig. 1A).
Fig. 1.
ETS proteins expressed in the endothelium. A. Domain structure of ETS factors. The conserved ETS domain (ETS), which includes the sequence specific binding domain, is present in all ETS proteins. The transcription activating domain (TAD) is present in all proteins except ETV6, which only exhibits repressor function. The pointed domain (P) is involved in protein-protein interactions and is present in ETS proteins that can undergo dimerization. ETV6 and ELK3 contain a transcriptional repressor domain (REP). B. Genomic organization Ets1/Ets2/Etv2 subfamily of ETS factors. Note that the Ets1 and Ets2 genes are located adjacent to Fli1 and Erg respectively and that the genomic region shows clear signs of duplication. Although Etv2 is closely related to Ets1 and Ets2 within the ETS domain, the genomic organization surrounding Etv2 is clearly distinct.
ETS proteins regulate multiple biological processes including cell proliferation, apoptosis and differentiation of multiple cell lineages [4,8] (Table 1). The deregulation of ETS family proteins is associated with many human cancers, particularly leukemias [9–11]. While ETS factors are involved in many different aspects of development and disease, evidence accumulated over the past few decades has shown that ETS transcription factors are particularly important for development of the hematopoietic and vascular lineages. In this review we will focus specifically on the role of ETS factors during embryonic development of the vascular endothelium. For consistency, we will use the formal ETS nomenclature adopted for human and mouse genes (see Table 1 for a list of endothelial ETS genes and their synonyms).
Table 1.
Ets Nomenclature
| Subfamily | Official Symbol | Aliases | Name |
|---|---|---|---|
|
| |||
| Ets | Ets1 | E26 avian leukemia oncogene 1 | |
| Ets2 | E26 avian leukemia oncogene 2 | ||
|
| |||
| Erg | Erg | E-26 oncogene related | |
| Fli1 | EWSR2, Sic1 | Friend leukemia integration 1 | |
|
| |||
| ER71 | Etv2 | ER71, Etsrp, Etsrp71 | Ets variant 2 |
|
| |||
| TCF | Elk3 | Net, SAP2, Erp, Etrp | ELK3 |
|
| |||
| TEL | Etv6 | Tel | Ets variant 6 (TEL oncogene) |
|
| |||
Vascular development is a coordinated process during which endothelial precursor cells arise from the mesoderm, proliferate, migrate, aggregate to form vascular cords, and then lumenate to form hollow tubes. These processes require changes in cell proliferation, adhesion, motility, and cell spreading. ETS factors directly activate genes encoding the cellular machinery required for such processes. ETS targets include matrix degrading proteases, cell adhesion molecules such as integrins, cadherins and intercellular adhesion molecules, and receptor tyrosine kinases for the Angiopoietin and VEGF-mediated signaling pathways, all of which have essential roles in the vasculature. Numerous studies, some of which will be discussed later in detail, demonstrate an essential role of ETS factors in regulating endothelial gene expression throughout development.
2. ETS proteins are key transcriptional regulators of endothelial gene expression
Virtually every characterized endothelial promoter and/or enhancer contains essential ETS binding sites [12], and it is thought that ETS proteins directly regulate expression of most, if not all, endothelial genes [11]. For example, studies using mouse and Xenopus transgenics have shown that ETS binding motifs are required for transcriptional activation of the Tie1, Tek, Cdn5, Eng, Mef2c and Kdr genes in endothelial [13–19]. Survey of the ETS family indicates that at least 19 different ETS factors are expressed in human endothelial cells (although not all at high levels) [20], while transcripts for 12 ETS factors are present in endothelial cells of zebrafish [21]. Although it appears that no single ETS protein is truly endothelial specific, 7 Ets genes (Elk3, Erg, Ets1, Ets2, Etv2, Etv6 and Fli1) are highly expressed in embryonic endothelial cells [5,11,12,22]. From an evolutionary perspective, it is interesting that all of the positive regulatory factors are closely related. Phylogenetically, Ets1, Ets2 and Etv2 are members of a distinct subgroup based on primary sequence conservation in the ETS domain [23]. Furthermore, Ets1/Fli1 and Ets2/Erg genes are adjacent in the genome (Fig. 1B). Below, we will review in more detail the contributions of Ets1, Ets2, Fli1, Erg, Tel, Elk3 and Etv2 to development of the endothelium. While recent studies suggest that Etv2 is by far the most important of these genes during embryogenesis, we will discuss this factor last, because it is less well characterized than other family members.
2.1. Ets1 (E26 transformation specific-1)
Ets1, the founding Ets gene, is the best-studied member of the ETS family. Ets1 is expressed at high levels in embryonic endothelial cells, while lower levels of expression are maintained in the resting endothelium of the adult [24–27]. Ets1 expression is induced in response to hypoxia, pro-inflammatory and angiogenic stimuli [28–30]. A large number of vascular genes are reported to be direct targets of ETS1 regulation, including Kdr, Flt1, Tek, Angpt2, Nrp1, Vwf, Pecam1, and Cdh5 [14,16,31–36].
Studies using antisense oligonucleotides in cells in culture and the chicken chorioallantoic membrane assay (CAM) were the first to demonstrate a role for ETS1 in vascular biology [26,37]. Cultured endothelial cells treated with Ets1 antisense oligonucleotides displayed defective tube formation and migration, while transfection of Ets1 antisense oligonucleotides in the CAM assay resulted in reduction in the diameter and number of blood vessels. Expression of a dominant-negative ETS1 construct in vitro impairs proliferation, migration, invasion, and tube formation of endothelial cells, whereas introduction of dominant-negative ETS1 into the eye suppressed retinal angiogenesis in mice [38,39]. In zebrafish, morpholino knockdown of ETS1 caused a loss in trunk circulation at 24 hours post fertilization (hpf) and minor intersegmental vessel sprouting defects however, overall development of the vascular network was relatively normal [27]. Gene ablation studies in mouse demonstrate that ETS1 is dispensable for vascular development. The majority of ETS1 deficient mice are viable, fertile and display no obvious blood vessel defects [40]. Ets1 null mice show increased perinatal mortality, however the cause has not been identified. As will be discussed in more detail below, the lack of severe ETS1 vascular phenotypes is likely due to overlapping functions of other ETS family members, including ETS2.
2.2. Ets2 (E26 transformation specific-2)
Ets2 is expressed in intersomitic blood vessels of E12.5 mice and embryonic endothelial cells of zebrafish [21,25,41]. ETS2 has been shown to transactivate a range of endothelial promoters in vitro, including Angpt2, Flt1, Kdr and Anpep [16,31,35,42]. Endothelial cells treated with Ets2 siRNA oligonucleotides were unable to form capillary networks in Matrigel experiments [42]. Mice lacking ETS2 function die before E8.5 due to defective development of the extra-embryonic tissues [43] (Table 1), but when the extraembryonic defects of the Ets2 knockout animals were bypassed experimentally, the animals were viable and fertile. However, double knockouts of Ets1 and Ets2, die around E13, due to severe defects in embryonic angiogenesis [44], suggesting a redundant role for these two factors during vascular development.
2.3. Fli1 (Friend leukemia integration site-1)
Fli1 is expressed at high levels in the primitive cells of the endothelial lineage suggesting an early role in vascular development. In mice, Fli1 transcripts are observed at E7.5 in cells of the mesoderm thought to give rise to hematopoietic and endothelial cells [45]. Subsequent expression is detected in the blood islands of the yolk sac at E8 and later in endothelial cells located throughout the embryo. In Xenopus and zebrafish, Fli1 is initially expressed in blood and endothelial precursor cells and is later observed throughout the embryonic vasculature [27,46–48]. Consistent with a role in regulating primitive endothelial specification, over-expression of a constitutively active form of Fli1 in zebrafish embryos induced expression of the early vascular markers Kdr, Etv2 and Fli1 itself [48].
Morpholino experiments conducted in Xenopus showed that knockdown of FLI1 function reduced, but did not eliminate, expression of vascular markers such as Kdr and Aplnr [21]. In zebrafish, Fli1 morphants displayed partial loss of trunk circulation and hemorrhaging in the head [21,27]. Similar to fish, targeted deletion of Fli1 in mice resulted in embryonic lethality by E12.5 due to hemorrhaging caused by poor blood vessel integrity [49]. However, specification of the endothelial lineage and early vascular patterning were effectively normal. Overall, ablation of FLI1 function results in relatively mild vascular phenotypes suggesting that other ETS factors, such as the closely related ETS protein, ERG and perhaps ETS1 and ETS2, partially compensate for the loss of FLI1. Its seems likely that FLI1 plays a more important role in blood development than vascular development since FLI1 deficient mice exhibit impaired hematopoiesis [49].
2.4. Erg (Ets-related gene)
The Erg gene is constitutively expressed in all adult and embryonic endothelial cells [23]. ERG is a strong activator of many vascular markers in vitro and in vivo. Expression of Erg cultured cells is sufficient to induce Cdh5, Icam2, Flt1, Vwf, and Hmox1 [28,31,50,51], while in Xenopus, ERG can strongly activate expression of Aplnr and Kdr at ectopic regions in the embryo [19,52].
ERG function is required for several different aspects of vascular development including regulation of endothelial homeostasis, survival and differentiation. Inhibition of ERG function in human umbilical vein endothelial cells (HUVECs) using antisense oligonucleotides, results in the loss of cell-cell contacts leading to endothelial cell death [51]. Furthermore, HUVECs with reduced levels of ERG exhibit a down-regulation of Sparc, Thbs1, Vwf, Rhoa, Icam2 and Cdhn5, which are involved in many different vascular processes [51,53]. Homozygous mice harboring a germline mutation in Erg die by E13.5 due to defects in definitive hematopoiesis [54]. Although the early events of vascular development appeared normal in these mice, blood vessels of the primitive vascular network were noticeably dilated. In zebrafish embryos, knockdown of Erg using morpholinos resulted in hemorrhaging in the head vasculature and subtle defects in the patterning of the intersomitic vessels (ISV) [21]. Expression of Cdh5, which is an ERG target in HUVECs [51], was down-regulated just before hemorrhaging occurred in Erg morphants, suggesting a role in the stabilization of endothelial cell junctions.
Taken together, these results suggest that Erg contributes towards activation of differentiation markers in the endothelial lineage. Like FLI1 however, ERG is not essential for expression of endothelial differentiation markers or for initial assembly of the vascular network.
2.5. Etv6 (Tel, Translocated Ets leukemia)
Etv6 is one of the few ETS proteins that functions as a transcriptional repressor. Etv6 is somewhat unusual amongst ETS family members because it is able to form homodimers in vivo, and its repressor activity is directly linked to its ability to self-associate [55]. Furthermore, TEL can bind to FLI1 protein and inhibit its transcriptional activity [56]. In the mouse, Etv6 is expressed strongly in the yolk sac vasculature [57]. Targeted deletion of Etv6 results in defective yolk sac angiogenesis and embryonic lethality between E10.5 and E11.5 [57] (Table 2). Analysis of the yolk sac blood vessels at E9.5 suggests that the initial events of vascular development occur normally, however maintenance and remodeling of the primary vascular network is compromised. For example, branching angiogenesis of the vitelline veins is absent in two thirds of ETV6 deficient embryos. By E10.5, branching vessels of the yolk sac are no longer detected in mutant mice, while blood vessels within the embryo appear histologically normal. These studies suggest that ETV6 is required for maintenance and elaboration of the complex vascular network in the yolk sac, not for the initiation of yolk sac vasculogenesis. ETV6 function appears to be dispensible in the embryo itself, but to answer this question definitively, it would be necessary to create a conditional mutant that specifically targeted embryonic vessels.
Table 2.
Ets knockout/MO phenotypes
| Ets factor | Mutation | Phenotype | References |
|---|---|---|---|
| Etv2 (Etsrp) | Mouse KO | Embryonic lethal by E9–9.5, loss of endothelial and hematopoietic lineage, complete absence of blood and vascular markers | [62] |
| Xenopus MO | Loss of endothelial specification, hematopoeitic lineage unaffected | [22] | |
| Zebrafish MO | Loss of endothelial specification, hematopoietic lineage unaffected | [63] | |
|
| |||
| Fli1 | Mouse KO | Embryonic lethal by E12.5, hemorrhaging and poor vessel integrity, but endothelial specification appears unaffected | [49] |
| Xenopus MO | Loss of endothelial and blood specification | [48] | |
| Zebrafish MO | Reduced trunk circulation at 24 hpf but regained at 48 hpf, hemorrhaging in the head | [27] [21] |
|
|
| |||
| Erg | Mouse KO | Embryonic lethal by E13.5, defects in definitive hematopoiesis, but development of the embryonic vasculature is unaffected | [54] |
| Zebrafish MO | Hemorrhaging in the head, minor ISV patterning defects, vasculature relatively normal | [21] [67] |
|
|
| |||
| Ets1 | Mouse KO | Viable, fertile, display normal vasculature defects in lymphoid lineage | [40] [78] |
| Zebrafish MO | Decerease in ISV sprouting, but otherwise functional vessels | [27] | |
| CAM assay | Chick antisense oligo Inhibited angiogenesis, blood vessel number and diameter were considerably reduced | [37] | |
|
| |||
| Ets2 | Mouse KO | Extraembryonic lethal by E8.5, defects in extraembryonic tissues, neither amnion nor chorion membranes formed | [43] |
|
| |||
| Ets1/Ets2 | Mouse KO | Embryonic lethal by E11.5–15.5, defective angiogenesis | [44] |
|
| |||
| Etv6 (Tel) | Mouse KO | Embryonic lethal by E10.5–11.5, defective yolk sac angiogenesis | [57] |
|
| |||
| Elk3 (Net) | Mouse hypermorph | Postnatal lethal, respiratory failure, dilated thoracic lymphatic vessels, egr1 upregulated in vasculature structures | [60] |
2.6. Elk3 (Net)
During early mouse development, Elk3 is highly expressed in embryonic endothelial cells starting at around E7.5 and expression persists in the vascular endothelium throughout development [58]. Elk3 is strongly expressed in endothelial cells of early Xenopus embryos [Xenbase database, 59]. Mouse embryos lacking ELK3 function display vascular defects and die shortly after birth due to respiratory failure [60,61]. An up-regulation of the immediate early gene Egr1 is observed in the heart and pulmonary arteries of mutant mice, suggesting that ELK3 represses Egr1 expression in vivo [60]. Furthermore, loss of Elk3 expression results in inhibition of angiogenesis in in vitro and ex vivo experiments [61]. ELK3 can function as a transcriptional repressor or activator depending on the circumstances. Under basal conditions ELK3 is a strong repressor of gene transcription; however, in response to Ras signaling, ELK3 is phosphorylated and converted to a transcriptional activator [23,61].
2.7. Etv2 (Ets variant 2)
Recent studies in mouse, Xenopus and zebrafish demonstrate that ETV2 (Etrsp/Er71) is without question the most important transcriptional regulator of embryonic endothelial development.
In mouse, Etv2 expression is transiently detected, starting at E7.5, in the yolk sac blood islands, a region that consists of both blood and endothelial precursors. Expression in the major blood vessels of the embryos is observed from E8.25 to E9.5 [62]. Endothelial expression of Etv2 is rapidly down-regulated during subsequent vascular development and is undetectable in adult endothelial tissue. In differentiating embryonic stem cells, Etv2 expression is observed prior to expression of endothelial marker genes, including the crucial VEGF receptor, Kdr (also called VEGFR2 or Flk1). Similar to the embryo, Etv2 expression in ES cells is transient and transcripts become undetectable several days after differentiation [62]. In zebrafish at the 2-somite stage, Etv2 expression is localized in endothelial precursor cells in two stripes within the lateral plate mesoderm and also in developing hematopoietic tissues. By 36 hpf, Etv2 expression is down-regulated in axial vessels but is still visible in vessels that are newly developing [21,63]. Consistent with the mouse and zebrafish data, Xenopus Etv2 is expressed shortly after gastrulation in the developing blood islands and subsequently is strongly expressed in endothelial precursor cells throughout the embryo [22]. Again, Etv2 expression in Xenopus is transient and transcripts become undetectable by tadpole stages [22].
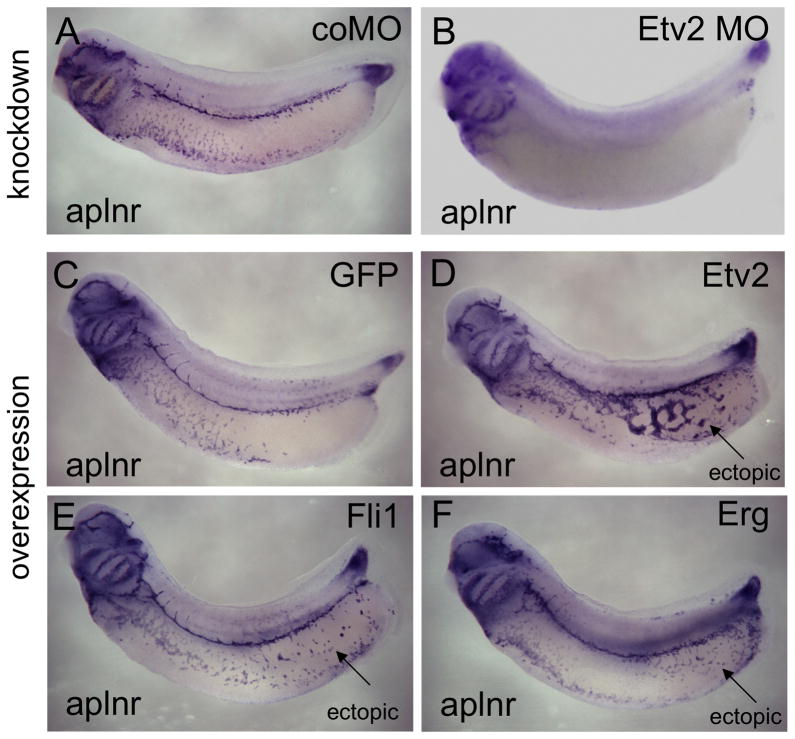
Targeted ablation of Etv2 in mice results in embryonic lethality at E9 to E9.5 due to complete loss of embryonic blood and vascular structures [62] (Table 1). Etv2 mutant embryos show no detectable expression of the VEGF receptor Kdr [62] and consequently, the Etv2 null animals exhibit the most severe vascular phenotype reported for any vascular gene, and much more severe than ablation of any other ETS factor. Analysis of the zebrafish Etv2 mutant, y11, shows that ETV2 is required for expression of a number of endothelial marker genes including Kdr, Fli1b and Flt4 [27] (Table 2). Complementing these studies, morpholino-mediated knockdown of ETV2 function in frogs and fish results in similar phenotypes observed in Etv2 deficient mice and y11 zebrafish, with near complete elimination of endothelial marker expression [22,27,63] (Table 2, Fig. 2). Loss of ETV2 function appears to have different effects on hematopoiesis in different organisms. In mouse, ETV2 is essential for general hematopoiesis, while in zebrafish ETV2 is required for development of the myeloid but not the erythroid lineages [62,64]. In Xenopus is appears that ETV2 is not required for development of either the erythroid or myeloid lineage [22].
Fig. 2.
Phenotypes of ETS factor loss of function and gain of function experiments in the Xenopus embryo. All embryos are assayed by in situ hybridization for expression of the vascular marker, aplnr, at stages 30–32. A. Embryo treated with control MO (coMO) showing wild type expression of aplnr in endothelial precursor cells. B. Embryo treated with translation blocking morpholino specific for Etv2 (Etv2 MO). Note severe inhibition of aplnr expression throughout the embryo. C. Control embryo injected with mRNA encoding GFP, showing wild type pattern of aplnr expression in endothelial cells D. Experimental embryo injected with mRNA encoding ETV2. Note ectopic expression of aplnr in the posterior ventral regions of the embryo (indicated by arrow). E. Experimental embryo injected with mRNA encoding Fli1. Ectopic expression of aplnr has been induced (arrow). F. Experimental embryo injected with mRNA encoding Erg. Ectopic expression of aplnr has been induced (arrow).
Forced expression of ETV2 in mouse embryonic stem cells caused increased expression in Pecam1, Tek and Cdh5 [62]. Consistent with these results, over-expression of Etv2 mRNA in Xenopus and zebrafish robustly activates a number of vascular markers in non-mesodermal tissues [22,63] (Fig. 2). Microarray analysis of zebrafish embryos over-expressing ETV2 showed transcriptional up-regulation of a very large number of endothelial genes, including some previously unidentified genes, suggesting a global induction of the endothelial gene program [65,66]. Overall, these studies across species indicate that ETV2 has a conserved function as a potent activator of the endothelial gene transcription program.
3. Redundancy among ETS family members
At least seven ETS proteins, ELK3, ERG, ETS1, ETS2, ETV2, ETV6 and FLI1 are expressed at high levels in the endothelium during early development. Several studies have shown that ETS proteins bind to the same consensus target sequence and are capable of transactivating the same promoter. This raises the possibility that ETS proteins serve largely redundant functions during early endothelial development. Further evidence for overlapping function of ETS proteins comes from germline deletions and knockdown experiments in mouse and zebrafish [27,44,67] (see Table 2 for a complete list). These studies tend to confirm that ETS factors possess redundant function, since single homozygous mutants exhibit rather minor phenotypes (with the exception of Etv2), while combinatorial knockdowns result in more severe phenotypes including embryonic lethality. In zebrafish, morpholino knockdown of four Ets genes (Ets1, Erg, Fli1 and Etv2) resulted in an almost complete loss of endothelial cells, whereas knockdown of any of the four ETS factors individually, resulted in less severe phenotypes [27]. ETS1 and ETS2 provide another example of overlapping function in the developing vasculature. Ets1 knockout mice have defects in the lymphoid lineage, but otherwise display a normal vasculature and are viable and fertile [40]. Ets2 knockout mice display extraembryonic tissue defects but if this deficiency is rescued, subsequent development of the animals is grossly normal [43]. The lack of a severe phenotype in the embryo proper of either of these single mutants suggested that ETS1 and ETS2 might have redundant functions. Evidence for ETS1/ETS2 functional redundancy came from double-mutant mice, which displayed severe defects in angiogenesis and die by E11.5–15.5 [44]. Functional redundancy of other ETS factors during endothelial development has not been rigorously tested in the mouse model. For example, we do not know whether FLI1 and ERG are functionally equivalent. Perhaps the most revealing experiments would be knock-in studies where one ETS protein coding region was substituted for another. Such an approach would ensure that gene expression levels remained tightly regulated and would therefore directly test whether different ETS proteins possess equivalent gene regulatory activity.
4. How do ETS proteins achieve transcriptional specificity?
This section addresses two related puzzles concerning transcriptional regulation by ETS proteins. First, how do ETS proteins recognize specific endothelial target genes? Second, if different ETS proteins do indeed possess different functional activities (i.e. they are not all perfectly redundant), how do different proteins recognize their specific target promoters? There are at least 3 mechanisms that might be used to achieve specificity and modulate ETS binding. These mechanisms include (1) increased information from sequences flanking the core DNA-binding site, (2) post-translational modifications, and (3) interactions with other proteins. As discussed in more detail below, these regulatory mechanisms, might limit promiscuous binding of ETS proteins to irrelevant fragments of DNA (that might contain the core ETS binding site) and function to enhance the interaction between the ETS factor and a specific endothelial promoter or enhancer.
4.1. Regulation of ETS binding specificity by flanking nucleotides
ETS proteins all recognize a core binding site corresponding to 5’-GGA(A/T)-3’ [6]. Because of the variability in the fourth position and the fact that the site is asymmetric, an ETS binding site will occur more than once every 100 nucleotides within a random DNA sequence. Therefore, the consensus site alone provides remarkably little target specificity. It has been suggested that different ETS proteins might exhibit a preference for different sequences flanking the core ETS binding site. Indeed, biochemical studies suggest that flanking nucleotides play a role in the binding specificity of individual ETS proteins [7,68]. Using a systematic approach to determine ETS family DNA-binding profiles in vitro and in vivo, Wei et al. [7] were able to classify ETS protein binding properties into 4 distinct categories and identify amino acid residues within the DNA-binding domains that are critical for the differences in specificity between the classes. However, while these studies did reveal subtle differences in binding site preference between different classes of ETS proteins, all of the endothelial expressed ETS factors fall into the same category (class I) and are therefore likely to exhibit identical binding specificity. If for example, ERG regulates different genes than FLI1, distinct binding site differences between the proteins are not likely to be sufficient to explain the specificity. Furthermore, the slight preference for additional nucleotides in an extended ETS binding site does not explain how ETS proteins in the vasculature specifically target endothelial genes for transcriptional activation.
4.2. Regulation of ETS activity by post-translational modification
Phosphorylation
The transcriptional properties of ETS factors can be modulated by phosphorylation. The precise effects of phosphorylation depend on the specific ETS protein involved, the signaling pathway responsible and the precise domain that is modified. In the case of ETS1 and ETS2, phosphorylation by Ras/MAPK enhances transcriptional activation [69]. ELK3 can switch from being a transcriptional repressor to an activator following Ras/MAPK-dependent phosphorylation [70] and ELK1 has enhanced DNA binding and transcriptional activity after phosphorylation as a result of MAPK activation [71]. ETS factors can also be phosphorylated by other signaling pathways. Upon calcium activation, phosphorylation of ETS1 by calcium-calmodulin-dependent protein kinase II (CaMKII, a serine/threonine kinase) reinforces the inhibitory conformation of ETS1 [72]. In this inhibitory conformation, the DNA-recognition helices are sterically obscured, preventing ETS1 from associating with DNA. While these studies provide a useful precedent for regulation of ETS activity by phosphorylation, there is currently no evidence that any of these modifications play a role during early endothelial development or that modifications can alter binding site specificity.
Acetylation, ubiquitination and sumoylation
Different ETS factors are subject to modification by aceylation, ubiquitination and sumoylation in different cellular contexts [73]. For simplicity, we will just consider examples of modifications that are characterized for ETS1 protein. Addition of a Sumo moiety to ETS1 reduces transcriptional activity of the protein, but does not effect its stability [74]. Independent of sumoylation, ubiquitination of ETS1 leads to increased degradation, indirectly resulting in reduced transcriptional activity [74]. ETS1 is also reported to undergo acetylation in response to TGF-beta signaling, but the consequences of this modification for ETS1 transcriptional activity are unclear [75]. Once again, the importance of these possible post-translational modifications for ETS factor activity during endothelial development has not been explored.
4.3. Regulation of ETS activity by protein-protein interactions
A hallmark of eukaryotic gene regulation is combinatorial control by multiple different transcription factors. Protein partnerships can provide target gene specificity, enhance DNA binding or transcriptional activity, release or reinforce autoinhibition, or alter subcellular localization. While many ETS binding partners have been identified (see Table 3 for a partial list), very few are recognized as contributing to endothelial specific activation. ETS proteins can partner with diverse classes of transcription factors including AP1, EP300, SRF, MYB, NFKB1, RUNX1, GATA1 and GATA3, KLF2, FOXC2, and PAX5 to regulate diverse developmental and disease processes. Since a number of these proteins are ubiquitously expressed, they have the potential to function as ETS partners in all tissue types, including endothelium. Almost no studies have investigated to what extent these protein-protein interactions might be shared between ETS family proteins, or whether they are specific for individual ETS factors.
Table 3.
Ets interacting proteins
| Ets factor | Partner Protein | Function Affected | References |
|---|---|---|---|
| Ets1 | Runx1 (CEB-alpha-2) | Relieves autoinhibition, enhanced DNA affinity | [79] |
| Pax5 | Conformational change recognizes suboptimal Ets site | [80] | |
| CBP/p300 | Co-activator | [81] | |
| MafB | Inhibits Ets1 activation by binding DNA binding domain | [82] | |
| EAP1/Daax | Inhibits Ets1 activation by binding to the Pointed-domain | [83] | |
| AP1 (cFos/cJun) | Transcriptional activation | [84], [85] | |
| NFkappaB | Transcriptional activation | [86] | |
| TFE3 (bHLH zippers) | Relieves autoinhibition, enhanced DNA affinity | [87] | |
| USF (bHLH zippers) | Relieves autoinhibition, enhanced DNA affinity | [87] | |
| Ets1 | Homodimerization relieves autoinhibition, transcriptional activation | [77] | |
| Ets2 | Unknown | [88] | |
| Ets2 | AP1 (cFos/cJun) | Transcriptional activation | [84], [85] |
| P300 | Co-activator | [89] | |
| c-Myb | Transcriptional activation | [90], [91] | |
| Erg | Antagonist to transcriptional activation by Ets2 | [88] | |
|
| |||
| Etv2 | FoxC2 | Transcriptional activation | [18] |
|
| |||
| TSGA | Transcriptional repression | [92] | |
| Erg | cJun | Transcriptional activation | [93] |
| Ets2 | Antagonist to transcriptional activation by Ets2 | [86] | |
| Fli1 | Unknown | [94] | |
| Spi1 | Unknown | [94] | |
|
| |||
| Klf2 | Transcriptional activation | [19] | |
|
| |||
| Fli1 | SRF | Cooperative DNA binding | [95] |
| CBP | Inhibits CBP-dependent transcriptional activation | [96] | |
| Etv6 | Inhibits Fli1-dependent transcriptional activation | [56] | |
| Gata1 | Cooperative DNA binding, transcriptional activation | [97] | |
| Runx1 | Transcriptional activation | [98] | |
| PIASxalpha/ARIP3 | Corepressor, alters Fli1 localization | [99] | |
| Etv6 | Etv6 | Transcriptional repression | [76] |
| Fli1 | Antagonist to transcriptional activation by Fli1 | [56] | |
To date, there are only two examples of cooperativity between an ETS factor and an interacting protein during early vascular development. First, De Val et al [18] demonstrated that ETS and FOX (Forkhead) family proteins work cooperatively to regulate endothelial gene expression. Studies using mouse, zebrafish, Xenopus, and cell lines demonstrated the presence of a novel FOX:ETS motif in many endothelial enhancers including Kdr, Tek, Mef2c, Tal1, Cdn5 and Notch4. This motif consists of a non-canonical FOX binding site immediately adjacent to an ETS binding site. Experiments in Xenopus and cell lines indicated that Etv2 and FoxC2 can synergize in activation of a number of endothelial marker genes. Second, studies in Xenopus have demonstrated that the Kruppel-like factor (KLF2) cooperates with Erg to activate vascular genes such as Kdr, Aplnr and Cdn5 [19]. While KLF family members were previously recognized as important regulators of endothelial gene expression, this study was the first to demonstrate cooperativity between KLF and ETS proteins. While both the ETS/FOXC2 and ETS/KLF2 cooperative interactions appear to be important, it seems likely that most endothelial genes are not regulated by these specific interactions. Therefore, further work is required to determine whether other ETS partner proteins are involved in regulation of gene expression in the endothelium.
Finally, studies have shown that ETS proteins can homodimerize, or can heterodimerize with other ETS family members, functioning to either relieve or enhance repression [76,77]. In one example of ETS heterodimerization, ETV6 binds to FLI1 and inhibits transactivation of promoters that would normally be activated by FLI1 [56]. Unfortunately, the precise mechanism by which this inhibition is achieved is not well understood. An example of ETS homodimerization is provided by ETS1. ETS1 proteins dimerize and bind to a pair of palindromic ETS binding sites separated by exactly 4 nucleotides. Such elements are essential for regulation of the Mmp3 and Trp53 (p53) genes. Additional study of ETS protein dimerization would be worthwhile, because the requirement for precisely spaced, paired ETS binding sites has the potential to provide additional sequence specificity for target gene identification.
5. Conclusion
ETS transcription factors are essential for development and maintenance of the endothelial lineage and for regulation of endothelial cell behavior. Recent studies have revealed a central role for ETV2 during initial specification and differentiation of the endothelium. It seems likely that ETV2 activates the endothelial transcriptional program, which includes other ETS transcription factors, and that these factors assume regulation of endothelial gene expression when ETV2 is downregulated. While the importance of ETS proteins for endothelial development is clear, the mechanism(s) by which target gene specificity is achieved remains obscure.
Acknowledgments
SMM was supported in part by The Hartwell Foundation Fellowship Award and an NIH Institutional Research Service Award in Cardiovascular Research (5-T32-HL007360-32). CTM is supported by a Molecular Cardiovascular Research Fellowship generously provided by the Bellows Foundation and NIH Training Grant (# T32 GM08659). PAK is the Allan C. Hudson and Helen Lovaas Endowed Professor of the Sarver Heart Center at the University of Arizona College of Medicine and is supported by the Sarver Heart Center and by the NHLBI of the NIH, ( # HL093694).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leprince D, Gegonne A, Coll J, de Taisne C, Schneeberger A, Lagrou C, et al. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983;306:395–7. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- 2.Hollenhorst PC, Shah AA, Hopkins C, Graves BJ. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev. 2007;21:1882–94. doi: 10.1101/gad.1561707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bult CJ, Eppig JT, Kadin JA, Richardson JE, Blake JA Mouse Genome Database Group. The Mouse Genome Database (MGD): mouse biology and model systems. Nucleic Acids Res. 2008;36:D724–8. doi: 10.1093/nar/gkm961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–37. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 5.Lelievre E, Lionneton F, Soncin F, Vandenbunder B. The Ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol. 2001;33:391–407. doi: 10.1016/s1357-2725(01)00025-5. [DOI] [PubMed] [Google Scholar]
- 6.Sharrocks AD, Brown AL, Ling Y, Yates PR. The ETS-domain transcription factor family. Int J Biochem Cell Biol. 1997;29:1371–87. doi: 10.1016/s1357-2725(97)00086-1. [DOI] [PubMed] [Google Scholar]
- 7.Wei GH, Badis G, Berger MF, Kivioja T, Palin K, Enge M, et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. Embo J. 2010;29:2147–60. doi: 10.1038/emboj.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 9.Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem. 2004;91:896–903. doi: 10.1002/jcb.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, et al. The Ewing family of tumors--a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–9. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 11.Dejana E, Taddei A, Randi AM. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–95. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gory S, Dalmon J, Prandini MH, Kortulewski T, de Launoit Y, Huber P. Requirement of a GT box (Sp1 site) and two Ets binding sites for vascular endothelial cadherin gene transcription. J Biol Chem. 1998;273:6750–5. doi: 10.1074/jbc.273.12.6750. [DOI] [PubMed] [Google Scholar]
- 14.Iljin K, Dube A, Kontusaari S, Korhonen J, Lahtinen I, Oettgen P, et al. Role of ets factors in the activity and endothelial cell specificity of the mouse Tie gene promoter. FASEB J. 1999;13:377–86. doi: 10.1096/fasebj.13.2.377. [DOI] [PubMed] [Google Scholar]
- 15.Korhonen J, Lahtinen I, Halmekytö M, Alhonen L, Jänne J, Dumont D, et al. Endothelial-specific gene expression directed by the tie gene promoter in vivo. Blood. 1995;86:1828–35. [PubMed] [Google Scholar]
- 16.Kappel A, Schlaeger TM, Flamme I, Orkin SH, Risau W, Breier G. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood. 2000;96:3078–85. [PubMed] [Google Scholar]
- 17.Pimanda JE, Chan WY, Donaldson IJ, Bowen M, Green AR, Göttgens B. Endoglin expression in the endothelium is regulated by Fli-1, Erg, and Elf-1 acting on the promoter and a -8-kb enhancer. Blood. 2006;107:4737–45. doi: 10.1182/blood-2005-12-4929. [DOI] [PubMed] [Google Scholar]
- 18.De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135:1053–64. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meadows SM, Salanga MC, Krieg PA. Kruppel-like factor 2 cooperates with the ETS family protein ERG to activate Flk1 expression during vascular development. Development. 2009;136:115–25. doi: 10.1242/dev.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the ETS family of transcription factors. Nucleic Acids Res. 2004;32:5693–702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Patient R. Genome-wide analysis of the zebrafish ETS family identifies three genes required for hemangioblast differentiation or angiogenesis. Circ Res. 2008;103:1147–54. doi: 10.1161/CIRCRESAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- 22.Salanga MC, Meadows SM, Myers CT, Krieg PA. ETS family protein ETV2 is required for initiation of the endothelial lineage but not the hematopoietic lineage in the Xenopus embryo. Dev Dyn. 2010;239:1178–1187. doi: 10.1002/dvdy.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randi AM, Sperone A, Dryden NH, Birdsey GM. Regulation of angiogenesis by ETS transcription factors. Biochem Soc Trans. 2009;37:1248–53. doi: 10.1042/BST0371248. [DOI] [PubMed] [Google Scholar]
- 24.Stiegler P, Wolff CM, Meyer D, Sénan F, Durliat M, et al. The c-ets-1 proto-oncogenes in Xenopus laevis: expression during oogenesis and embryogenesis. Mech Dev. 1993;41:163–74. doi: 10.1016/0925-4773(93)90046-z. [DOI] [PubMed] [Google Scholar]
- 25.Maroulakou IG, Papas TS, Green JE. Differential expression of ets-1 and ets-2 proto-oncogenes during murine embryogenesis. Oncogene. 1994;9:1551–65. [PubMed] [Google Scholar]
- 26.Iwasaka C, Tanaka K, Abe M, Sato Y. Ets-1 regulates angiogenesis by inducing the expression of urokinase-type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J Cell Physiol. 1996;169:522–31. doi: 10.1002/(SICI)1097-4652(199612)169:3<522::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, et al. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–83. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin F, Ludbrook VJ, Kola I, Campbell CJ, Randi AM. Characterisation of the tumour necrosis factor (TNF)-(alpha) response elements in the human ICAM-2 promoter. J Cell Sci. 1999;112:4695–703. doi: 10.1242/jcs.112.24.4695. [DOI] [PubMed] [Google Scholar]
- 29.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oikawa M, Abe M, Kurosawa H, Hida W, Shirato K, Sato Y. Hypoxia induces transcription factor ETS-1 via the activity of hypoxia-inducible factor-1. Biochem Biophys Res Commun. 2001;289:39–43. doi: 10.1006/bbrc.2001.5927. [DOI] [PubMed] [Google Scholar]
- 31.Wakiya K, Begue A, Stehelin D, Shibuya M. A cAMP response element and an Ets motif are involved in the transcriptional regulation of flt-1 tyrosine kinase (vascular endothelial growth factor receptor 1) gene. J Biol Chem. 1996;271:30823–8. doi: 10.1074/jbc.271.48.30823. [DOI] [PubMed] [Google Scholar]
- 32.Schwachtgen JL, Janel N, Barek L, Duterque-Coquillaud M, Ghysdael J, Meyer D, et al. Ets transcription factors bind and transactivate the core promoter of the von Willebrand factor gene. Onco gene. 1997;15:3091–102. doi: 10.1038/sj.onc.1201502. [DOI] [PubMed] [Google Scholar]
- 33.Lelièvre E, Mattot V, Huber P, Vandenbunder B, Soncin F. ETS1 lowers capillary endothelial cell density at confluence and induces the expression of VE-cadherin. Oncogene. 2000;19:2438–46. doi: 10.1038/sj.onc.1203563. [DOI] [PubMed] [Google Scholar]
- 34.Teruyama K, Abe M, Nakano T, Takahashi S, Yamada S, Sato Y. Neurophilin-1 is a downstream target of transcription factor Ets-1 in human umbilical vein endothelial cells. FEBS Lett. 2001;504:1–4. doi: 10.1016/s0014-5793(01)02724-7. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa Y, Abe M, Yamazaki T, Niizeki O, Shiiba K, Sasaki I, et al. Transcriptional regulation of human angiopoietin-2 by transcription factor Ets-1. Biochem Biophys Res Commun. 2004;316:52–8. doi: 10.1016/j.bbrc.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi F, Okada M, Kato K, Jakt LM, Iwata H. Array-based functional screening for genes that regulate vascular endothelial differentiation of Flk1- positive progenitors derived from embryonic stem cells. Biochim Biophys Acta. 2007;1770:1085–97. doi: 10.1016/j.bbagen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Wernert N, Stanjek A, Hügel A, Giannis A. Inhibition of angiogenesis on the chicken chorioallantoic membrane by Ets 1 antisense oligodeoxyribonucleotides. Verh Dtsch Ges Pathol. 1999;17:603–5. [PubMed] [Google Scholar]
- 38.Nakano T, Abe M, Tanaka K, Shineha R, Satomi S, Sato Y. Angiogenesis inhibition by transdominant mutant Ets-1. J Cell Physiol. 2000;184:255–62. doi: 10.1002/1097-4652(200008)184:2<255::AID-JCP14>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe D, Takagi H, Suzuma K, Suzuma I, Oh H, Ohashi H, et al. Transcription factor Ets-1 mediates ischemia- and vascular endothelial growth factor-dependent retinal neovascularization. Am J Pathol. 2004;164:1827–35. doi: 10.1016/S0002-9440(10)63741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barton K, Muthusamy N, Fischer C, Ting CN, Walunas TL, Lanier LL, Leiden JM. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity. 1998;9:555–63. doi: 10.1016/s1074-7613(00)80638-x. [DOI] [PubMed] [Google Scholar]
- 41.Ristevski S, Tam PP, Hertzog PJ, Kola I. Ets2 is expressed during morphogenesis of the somite and limb in the mouse embryo. Mech Dev. 2002;116:165–8. doi: 10.1016/s0925-4773(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 42.Petrovic N, Bhagwat SV, Ratzan WJ, Ostrowski MC, Shapiro LH. CD13/APN transcription is induced by RAS/MAPK-mediated phosphorylation of Ets-2 in activated endothelial cells. J Biol Chem. 2003;278:49358–68. doi: 10.1074/jbc.M308071200. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto H, Flannery ML, Kupriyanov S, Pearce J, McKercher SR, Henkel GW, et al. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–26. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei G, Srinivasan R, Cantemir-Stone CZ, Sharma SM, Santhanam R, Weinstein M, et al. Ets1 and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood. 2009;114:1123–30. doi: 10.1182/blood-2009-03-211391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mélet F, Motro B, Rossi DJ, Zhang L, Bernstein A. Generation of a novel Fli- 1 protein by gene targeting leads to a defect in thymus development and a delay in Friend virus-induced erythroleukemia. Mol Cell Biol. 1996;16:2708–18. doi: 10.1128/mcb.16.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer D, Wolff CM, Stiegler P, Sénan F, Befort N, Befort JJ, et al. Xl-fli, the Xenopus homologue of the fli-1 gene, is expressed during embryogenesis in a restricted pattern evocative of neural crest cell distribution. Mech Dev. 1993;44:109–21. doi: 10.1016/0925-4773(93)90061-2. [DOI] [PubMed] [Google Scholar]
- 47.Brown LA, Rodaway AR, Schilling TF, Jowett T, Ingham PW, Patient RK, et al. Insights into early vasculogenesis revealed by expression of the ETS- domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech Dev. 2000;90:237–52. doi: 10.1016/s0925-4773(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 48.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18:1234–40. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 49.Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, et al. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–52. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deramaudt BM, Remy P, Abraham NG. Upregulation of human heme oxygenase gene expression by Ets-family proteins. J Cell Biochem. 1999;72:311–21. [PubMed] [Google Scholar]
- 51.Birdsey GM, Dryden NH, Amsellem V, Gebhardt F, Sahnan K, Haskard DO, et al. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baltzinger M, Mager-Heckel AM, Remy P. Xl erg: expression pattern and overexpression during development plead for a role in endothelial cell differentiation. Dev Dyn. 1999;216:420–33. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<420::AID-DVDY10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 53.McLaughlin F, Ludbrook VJ, Cox J, von Carlowitz I, Brown S, Randi AM. Combined genomic and antisense analysis reveals that the transcription factor Erg is implicated in endothelial cell differentiation. Blood. 2001;98:3332–9. doi: 10.1182/blood.v98.12.3332. [DOI] [PubMed] [Google Scholar]
- 54.Loughran SJ, Kruse EA, Hacking DF, de Graaf CA, Hyland CD, Willson TA, et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol. 2008;9:810–9. doi: 10.1038/ni.1617. [DOI] [PubMed] [Google Scholar]
- 55.Lopez RG, Carron C, Oury C, Gardellin P, Bernard O, Ghysdael J. TEL is a sequence-specific transcriptional repressor. J Biol Chem. 1999;274:30132–8. doi: 10.1074/jbc.274.42.30132. [DOI] [PubMed] [Google Scholar]
- 56.Kwiatkowski BA, Bastian LS, Bauer TR, Jr, Tsai S, Zielinska-Kwiatkowska AG, Hickstein DD. The ets family member Tel binds to the Fli-1 oncoprotein and inhibits its transcriptional activity. J Biol Chem. 1998;273:17525–30. doi: 10.1074/jbc.273.28.17525. [DOI] [PubMed] [Google Scholar]
- 57.Wang LC, Kuo F, Fujiwara Y, Gilliland DG, Golub TR, Orkin SH. Yolk sac angiogenic defect and intra-embryonic apoptosis in mice lacking the Ets- related factor TEL. Embo J. 1997;16:4374–83. doi: 10.1093/emboj/16.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayadi A, Suelves M, Dollé P, Wasylyk B. Net, an Ets ternary complex transcription factor, is expressed in sites of vasculogenesis, angiogenesis, and chondrogenesis during mouse development. Mech Dev. 2001;102:205–8. doi: 10.1016/s0925-4773(01)00289-1. [DOI] [PubMed] [Google Scholar]
- 59.Bowes JB, Snyder KA, Segerdell E, Jarabek CJ, Azam K, Zorn AM, et al. Xenbase: gene expression and improved integration. Nucleic Acids Res. 2010;38:D607–12. doi: 10.1093/nar/gkp953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayadi A, Zheng H, Sobieszczuk P, Buchwalter G, Moerman P, Alitalo K, Wasylyk B. Net-targeted mutant mice develop a vascular phenotype and up- regulate egr-1. EMBO J. 2001;20:5139–52. doi: 10.1093/emboj/20.18.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng H, Wasylyk C, Ayadi A, Abecassis J, Schalken JA, Rogatsch H, et al. The transcription factor Net regulates the angiogenic switch. Genes Dev. 2003;17 :2283–97. doi: 10.1101/gad.272503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S. Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111 :4500–10. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomez GA, Veldman MB, Zhao Y, Burgess S, Lin S. Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish. PLoS One. 2009;4:e4994. doi: 10.1371/journal.pone.0004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature- specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238 :1836–50. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- 67.Ellett F, Kile BT, Lieschke GJ. The role of the ETS factor erg in zebrafish vasculogenesis. Mech Dev. 2009;126:220–9. doi: 10.1016/j.mod.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graves BJ, Petersen JM. Specificity within the ets family of transcription factors. Adv Cancer Res. 1998;75:1–55. doi: 10.1016/s0065-230x(08)60738-1. [DOI] [PubMed] [Google Scholar]
- 69.Yang BS, Hauser CA, Henkel G, Colman MS, Van Beveren C, Stacey KJ, et al. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–47. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maira SM, Wurtz JM, Wasylyk B. Net (ERP/SAP2) one of the Ras-inducible TCFs, has a novel inhibitory domain with resemblance to the helix-loop-helix motif. Embo J. 1996;15:5849–65. [PMC free article] [PubMed] [Google Scholar]
- 71.Yang SH, Whitmarsh AJ, Davis RJ, Sharrocks AD. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. Embo J. 1998;17:1740–9. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cowley DO, Graves BJ. Phosphorylation represses Ets-1 DNA binding by reinforcing autoinhibition. Genes Dev. 2000;14:366–76. [PMC free article] [PubMed] [Google Scholar]
- 73.Charlot C, Dubois-Pot H, Serchov T, Tourrette Y, Wasylyk B. A review of post-translational modifications and subcellular localization of Ets transcription factors: possible connection with cancer and involvement in the hypoxic response. Methods Mol Biol. 2010;647:3–30. doi: 10.1007/978-1-60761-738-9_1. [DOI] [PubMed] [Google Scholar]
- 74.Ji Z, Degerny C, Vintonenko N, Deheuninck J, Foveau B, Leroy C, et al. Regulation of the Ets-1 transcription factor by sumoylation and ubiquitinylation. Oncogene. 2007;26:395–406. doi: 10.1038/sj.onc.1209789. [DOI] [PubMed] [Google Scholar]
- 75.Czuwara-Ladykowska J, Sementchenko VI, Watson DK, Trojanowska M. Ets1 is an effector of the transforming growth factor beta (TGF-beta ) signaling pathway and an antagonist of the profibrotic effects of TGF-beta. J Biol Chem. 2002;277:20399–408. doi: 10.1074/jbc.M200206200. [DOI] [PubMed] [Google Scholar]
- 76.Kim CA, Phillips ML, Kim W, Gingery M, Tran HH, Robinson MA, et al. Polymerization of the SAM domain of TEL in leukemogenesis and transcriptional repression. Embo J. 2001;20:4173–82. doi: 10.1093/emboj/20.15.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamber EP, Vanhille L, Textor LC, Kachalova GS, Sieweke MH, Wilmanns M. Regulation of the transcription factor Ets-1 by DNA-mediated homo-dimerization. Embo J. 2008;27:2006–17. doi: 10.1038/emboj.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walunas TL, Wang B, Wang CR, Leiden JM. Cutting edge: the Ets1 transcription factor is required for the development of NK T cells in mice. J Immunol. 2000;164:2857–60. doi: 10.4049/jimmunol.164.6.2857. [DOI] [PubMed] [Google Scholar]
- 79.Goetz TL, Gu TL, Speck NA, Graves BJ. Auto-inhibition of Ets-1 is counteracted by DNA binding cooperativity with core-binding factor alpha2. Mol Cell Biol. 2000;20:81–90. doi: 10.1128/mcb.20.1.81-90.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pufall MA, Graves BJ. Ets-1 flips for new partner Pax-5. Structure. 2002;10:11–4. doi: 10.1016/s0969-2126(01)00701-8. [DOI] [PubMed] [Google Scholar]
- 81.Yang C, Shapiro LH, Rivera M, Kumar A, Brindle PK. A role for CREB binding protein and p300 transcriptional coactivators in Ets-1 transactivation functions. Mol Cell Biol. 1998;18:2218–29. doi: 10.1128/mcb.18.4.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sieweke MH, Tekotte H, Frampton J, Graf T. MafB is an interaction partner and repressor of Ets-1 that inhibits erythroid differentiation. Cell. 1996;85:49–60. doi: 10.1016/s0092-8674(00)81081-8. [DOI] [PubMed] [Google Scholar]
- 83.Li R, Pei H, Watson DK. Regulation of Ets function by protein - protein interactions. Oncogene. 2000;19:6514–23. doi: 10.1038/sj.onc.1204035. [DOI] [PubMed] [Google Scholar]
- 84.Wasylyk B, Wasylyk C, Flores P, Begue A, Leprince D, Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990;346:191–3. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- 85.Bassuk AG, Leiden JM. A direct physical association between ETS and AP- 1 transcription factors in normal human T cells. Immunity. 1995;3:223–37. doi: 10.1016/1074-7613(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 86.Bassuk AG, Anandappa RT, Leiden JM. Physical interactions between Ets and NF-kappaB/NFAT proteins play an important role in their cooperative activation of the human immunodeficiency virus enhancer in T cells. J Virol. 1997;71:3563–73. doi: 10.1128/jvi.71.5.3563-3573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian G, Erman B, Ishii H, Gangopadhyay SS, Sen R. Transcriptional activation by ETS and leucine zipper-containing basic helix-loop-helix proteins. Mol Cell Biol. 1999;19:2946–57. doi: 10.1128/mcb.19.4.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Basuyaux JP, Ferreira E, Stehelin D, Buttice G. The Ets transcription factors interact with each other and with the c-Fos/c-Jun complex via distinct protein domains in a DNA-dependent and -independent manner. J Biol Chem. 1997;272:26188–95. doi: 10.1074/jbc.272.42.26188. [DOI] [PubMed] [Google Scholar]
- 89.Sun HJ, Xu X, Wang XL, Wei L, Li F, Lu J, et al. Transcription factors Ets2 and Sp1 act synergistically with histone acetyltransferase p300 in activating human interleukin-12 p40 promoter. Acta Biochim Biophys Sin (Shanghai) 2006;38:194–200. doi: 10.1111/j.1745-7270.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 90.Dudek H, Tantravahi RV, Rao VN, Reddy ES, Reddy EP. Myb and Ets proteins cooperate in transcriptional activation of the mim-1 promoter. Proc Natl Acad Sci U S A. 2009;89:1291–5. doi: 10.1073/pnas.89.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shapiro LH. Myb and Ets proteins cooperate to transactivate an early myeloid gene. J Biol Chem. 1995;270:8763–71. doi: 10.1074/jbc.270.15.8763. [DOI] [PubMed] [Google Scholar]
- 92.Knebel J, De Haro L, Janknecht R. Repression of transcription by TSGA/Jmjd1a, a novel interaction partner of the ETS protein ER71. J Cell Biochem. 2006;99:319–29. doi: 10.1002/jcb.20945. [DOI] [PubMed] [Google Scholar]
- 93.Verger A, Buisine E, Carrere S, Wintjens R, Flourens A, Coll J, et al. Identification of amino acid residues in the ETS transcription factor Erg that mediate Erg-Jun/Fos-DNA ternary complex formation. J Biol Chem. 2001;276:17181–9. doi: 10.1074/jbc.M010208200. [DOI] [PubMed] [Google Scholar]
- 94.Carrere S, Verger A, Flourens A, Stehelin D, Duterque-Coquillaud M. Erg proteins, transcription factors of the Ets family, form homo, heterodimers and ternary complexes via two distinct domains. Oncogene. 1998;16:3261–8. doi: 10.1038/sj.onc.1201868. [DOI] [PubMed] [Google Scholar]
- 95.Dalgleish P, Sharrocks AD. The mechanism of complex formation between Fli-1 and SRF transcription factors. Nucleic Acids Res. 2000;28:560–9. doi: 10.1093/nar/28.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramakrishnan R, Fujimura Y, Zou JP, Liu F, Lee L, Rao V, et al. Role of protein-protein interactions in the antiapoptotic function of EWS-Fli-1. Oncogene. 2004;23:7087–94. doi: 10.1038/sj.onc.1207927. [DOI] [PubMed] [Google Scholar]
- 97.Eisbacher M, Holmes ML, Newton A, Hogg PJ, Khachigian LM, Crossley M, et al. Protein-protein interaction between Fli-1 and GATA-1 mediates synergistic expression of megakaryocyte-specific genes through cooperative DNA binding. Mol Cell Biol. 2003;23:3427–41. doi: 10.1128/MCB.23.10.3427-3441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang H, Yu M, Akie TE, Moran TB, Woo AJ, Tu N, et al. Differentiation- dependent interactions between RUNX-1 and FLI-1 during megakaryocyte development. Mol Cell Biol. 2009;29:4103–15. doi: 10.1128/MCB.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van den Akker E, Ano S, Shih HM, Wang LC, Pironin M, Palvimo JJ, et al. FLI-1 functionally interacts with PIASxalpha, a member of the PIAS E3 SUMO ligase family. J Biol Chem. 2005;280:38035–46. doi: 10.1074/jbc.M502938200. [DOI] [PubMed] [Google Scholar]