Abstract
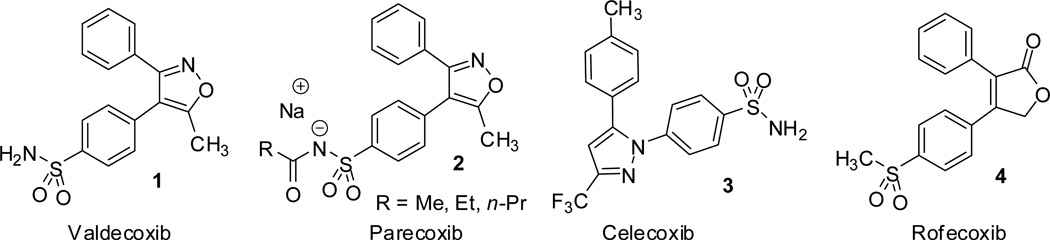
Non-steroidal anti-inflammatory drugs are widely used therapeutic agents in the treatment of inflammation, pain and fever. Cyclooxygenase catalyzes the initial step of biotransformation of arachidonic acid to prostanoids, and exist as three distinct isozymes; COX-I, COX-II and COX-III. Selective COX-II inhibitors are a class of potential anti-inflammatory, analgesic, and antipyretic drugs with reduced gastrointestinal (GI) side effects compared to nonselective inhibitors. 3,4-diarylisoxazole scaffold is recurrently found in a wide variety of NSAIDs, protein kinase inhibitors, hypertensive agents, and estrogen receptor (ER) modulators. In the present review, we document on the recent synthetic strategies of 3,4-diarylisoxazolyl scaffolds of valdecoxib and its relevant structural analogues.
Keywords: Isoxazoles, Valdecoxib, Nitrile oxides, Heterocycles, Regioselectivity and Cycloaddition
1. Introduction
The classical non-steroidal anti-inflammatory drugs (NSAIDs) are therapeutic agents widely used in the treatment of inflammation, pain and fever.1,2,3 These compounds are primarily used in the inhibition of cyclooxygenase (COX), normally referred to as prostaglandin synthase.3,4 Prostaglandins are lipid derived fatty acids virtually present in all human tissues and produced by most of the cells in the body.3 Cyclooxygenase (COX) catalyzes the first step in the biotransformation of arachidonic acid to prostanoids,5–7 and usually exist in three distinct isoforms: COX-I, COX-II, COX-III,8–11 as well as two smaller COX-I derived proteins (partial COX-1 or PCOX-I proteins).11,12 While COX-I is expressed in all healthy tissues reconciling physiological responses, COX-II is induced by inflammatory stimuli,13–15 and is responsible for the conversion of arachindonic acid to prostaglandin H2, a potential key mediator for the inflammation.16 Further COX-III and PCOX-Ia protein are derived from the COX-I which retain intron one and has a frameshift mutation.12 Selective inhibition of COX-II provided a potential class of anti-inflammatory, analgesic, and antipyretic drugs with reduced gastrointestinal (GI) side effects compared to nonselective inhibitors.5,17–20 Recent studies also indicated that inhibiting COX-II is not only a key approach for the treatment of several types of cancers,21 and also can be used to postpone the clinical manifestation of Alzheimer’s disease.22 3,4-diarylisoxazole scaffold23 is one of the frequently found pharmacophore in a wide variety of NSAIDs,24–26 protein kinase inhibitors,27 and hypertensive agents.28 One of the most familiar examples of 3,4-diarylisoxazoles is Valdecoxib (4-(5-methyl-3-phenyl-4-isoxazolyl) benzenesulfonamide), commercialized under the brand name Bextra. The phenylsulfonamide group located at the para- position is known to interact effectively with the COX-II side pocket through slow tight-binding kinetics.29–31 The syntheses of isoxazoles usually involve the 1,3-dipolar cycloaddition of nitrile oxides with alkynes32,33 or alkyne surrogates,34 or through Suzuki cross coupling reaction.21 In the present review, we document on the recent synthetic methodologies to access these diarylisoxazolyl derivatives of valdecoxib 1 in the context of recent applications to organic synthesis and methodology.
2.1 Coupling & Electrophilic Cyclization reactions
3,4-diarylisoxazoles are popular examples known for high COX-II selectivity and potency, and are represented by 4-(5-methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide moiety. Although several literature reports exist to synthesize substituted isoxazoles,34–36 and selectivity in synthesizing 3,4-diarylisoxazoles are restricted and subjected to the availability of the starting materials.37–39
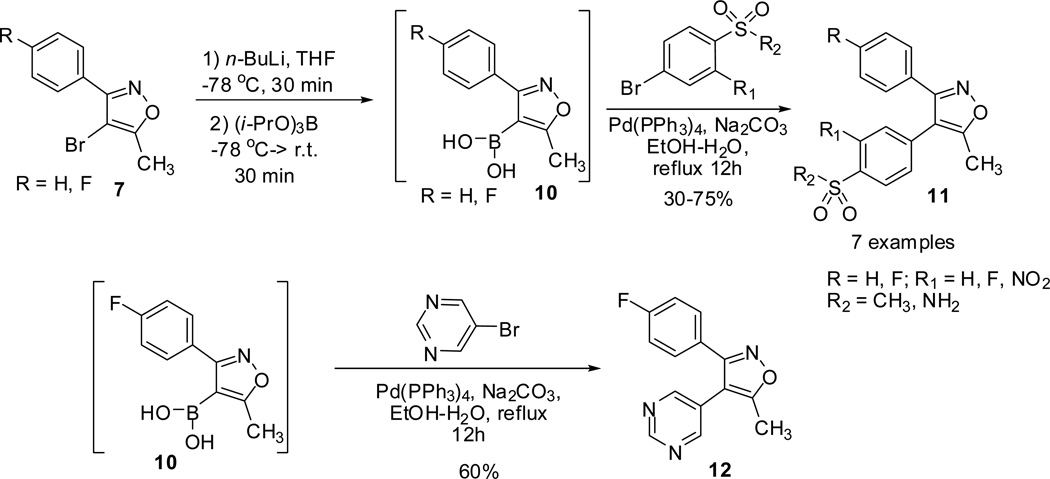
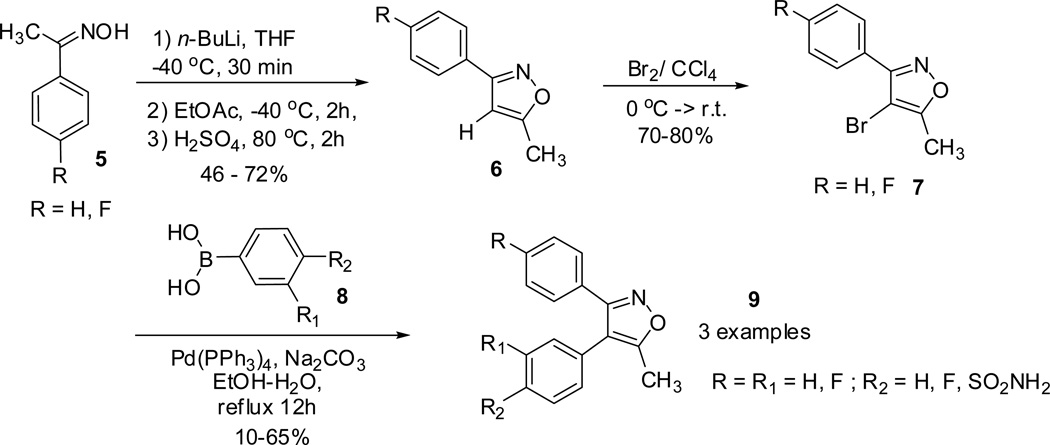
Toyokuni and co-workers23 reported the synthesis of 3,4-diarylisoxazoles (9, 11 and 12) through Suzuki cross coupling arylation of the C-4 position of the 3-arylisoxazoles.40,41 Reaction of substituted phenyl-ethanone oxime 5 in presence of n-BuLi generated the enolate, which on subsequent condensation with EtOAc followed by dehydration, furnished the 3-aryl-5-methylisoxazole 6.42–44 Electrophilic bromination followed by Suzuki coupling reaction of 4-bromoisoxazole 7 with arylboronic acids 8 led to the formation of the 3,4-diarylisoxazoles 9.
In addition, the treatment of 4-bromoisoxazole 7 with triisopropyl borate provided the corresponding isoxazole boronic acids 10. Reaction of isoxazole boronic acids 10 with several arylbromides (bearing sulfonamide or 4-methylsulfonyl groups) and 5-bromopyrimidine proceeded successfully and furnished valdecoxib and analogues (11 and 12). However, poor yields were observed for arylbromides substituted with vicinal nitro and sulfonamide groups. The nitro and sulfonamide residues functioned as bidentate chelating ligand for Pd and interfered the catalytic cycle (scheme 2).21
Scheme 2.
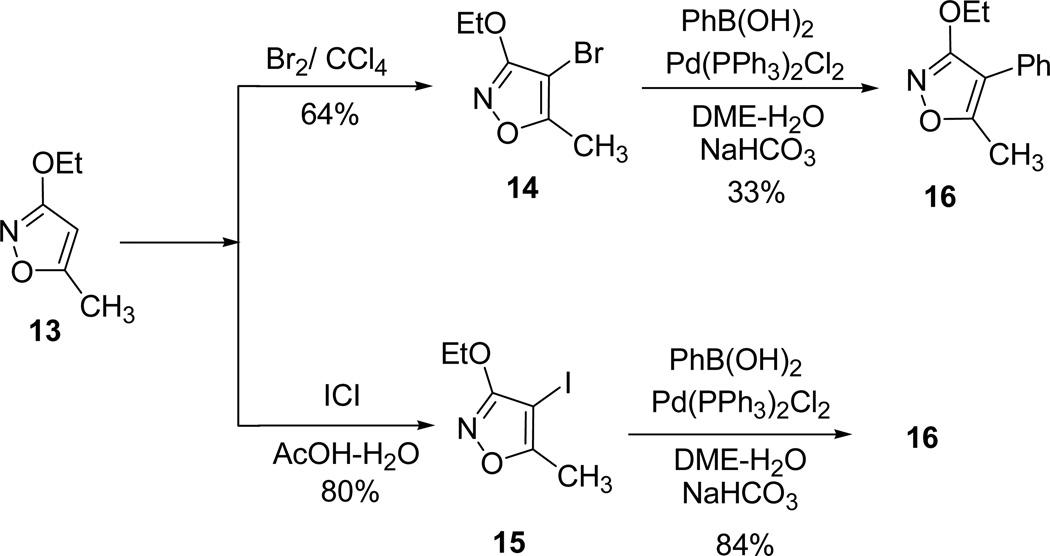
Krogsgaard-Larsen and his group documented the synthesis of 4-aryl and 4-heteroaryl substituted 3-alkoxyisoxazoles as immediate precursors for the synthesis of pharmacologically interesting 3-isoxazolyl containing aminoacids.40 Reaction of 3-ethoxy-5-methylisoxazole45 13 in presence of Br2 in CCl4 (or ICl/AcOH) generated the 4-halosubsituted isoxazoles 14 (15) (Scheme 3).
Scheme 3.
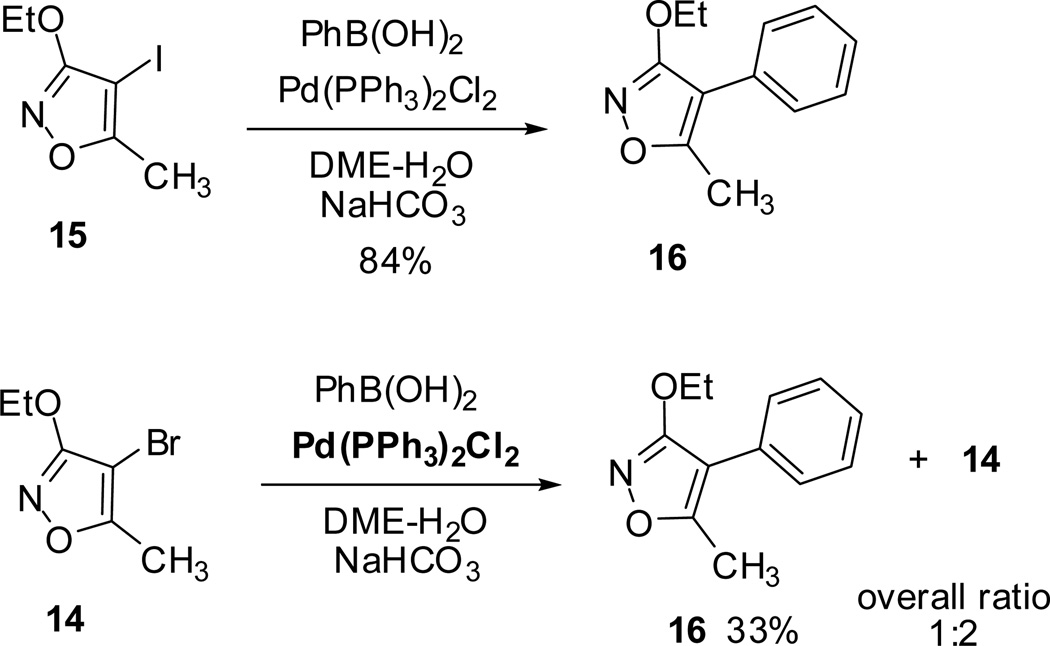
Suzuki-Miyaura coupling reaction of 4-substituted isoxazoles (14 and 15) with phenylboronic acid catalyzed by Pd(PPh3)2Cl2 afforded the corresponding 3-ethoxy-5-methyl-4-phenylisoxazole 16. In addition the reaction with 4-bromoisoxazole 14 was partially complete and the starting materials were isolated in the 1:2 ratio (Scheme 4).
Scheme 4.
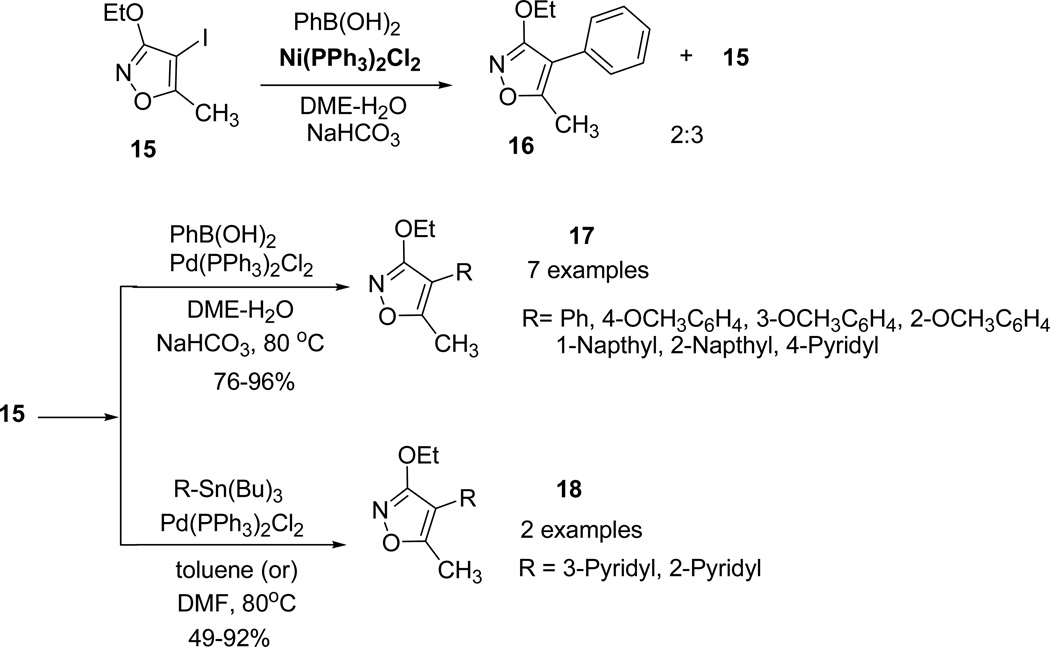
However, when Pd(PPh3)2Cl2 was replaced with Ni(PPh3)2Cl2, it failed to undergo completion and resulted in the isolation of the 3-ethoxy-5-methyl-4-phenylisoxazole 16 along with 15. Additional Suzuki-Miyaura and Stille cross-coupling reactions of 3-ethoxy-5-methyl-4-iodoisoxazole 15 with phenylboronic acids and tributyltin reagents furnished the desired coupling products (17 and 18) in moderate to excellent yields (Scheme 5).40
Scheme 5.
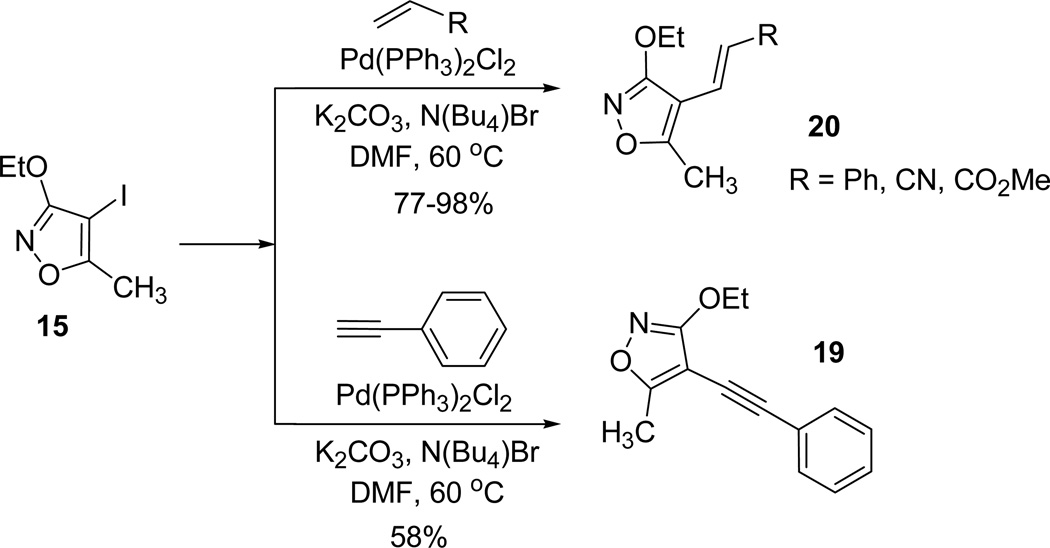
Heck coupling reactions of 3-ethoxy-5-methyl-4-iodoisoxazole 15 were further attempted to study the arylation and vinylation of alkyne and olefine derivatives (Scheme 6).
Scheme 6.
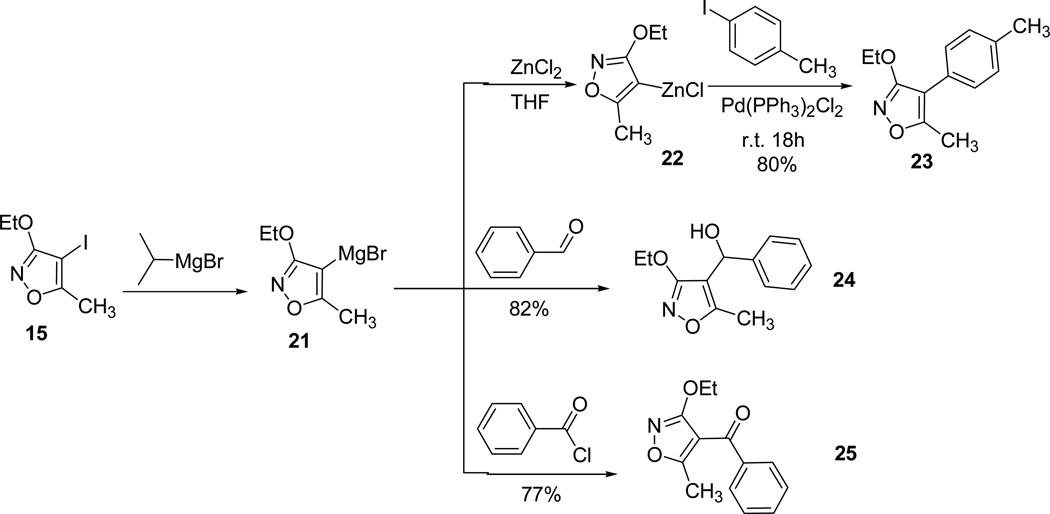
Finally, attempts to incorporate different functionalities with alkyllithium reagents primarily resulted in the deprotonation of methyl group of 3,5-disubsituted isoxazole 13.46 To avoid the undesired deprotonation, 4-iodoisoxazole 15 was converted into the Grignard intermediate 21 using i-PrMgBr,47 later followed by transmetallation 22 and Pd-coupling reaction furnished the desired coupling product 23. Similar reactions of the intermediate heteromagnesium bromide were smoothly converted to the final products (24 and 25) following treatment with benzaldehyde and benzoylchloride, respectively (Scheme 7).40
Scheme 7.
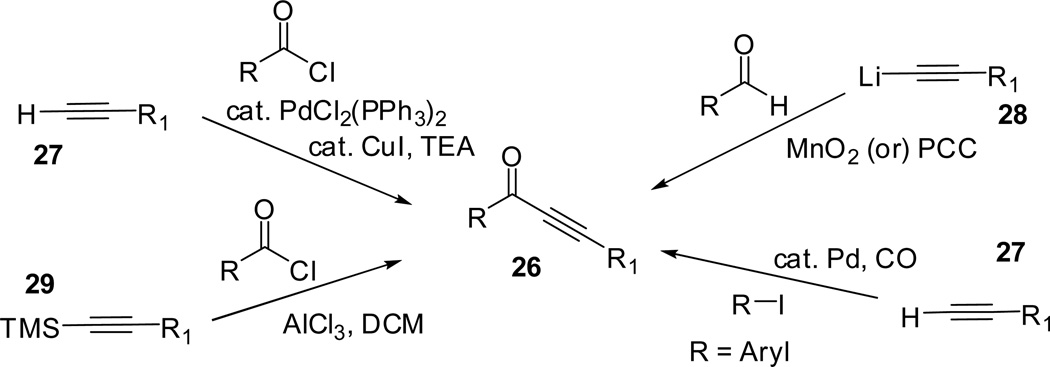
Larock and co-workers reported an efficient methodology toward the construction of several isoxazoles through electrophilic cyclization of 2-alkyn-1-one O-methyl oximes.48,49 The reaction protocol involved the ICl promoted cyclization of methyl oximes, which was successfully employed for the synthesis of 4-iodoisoxazoles. The ynones 26 required for the synthesis of O-methyl oximes 31 were prepared by coupling of the terminal acetylene 27 with an acid chloride or an aryl iodide, or by the reaction of an acetylide 28 with aldehyde followed by oxidation of the alcohol, or by the treatment of silylated acetylene 29 with an acid chloride in the presence of AlCl3 (Scheme 8).
Scheme 8:49.
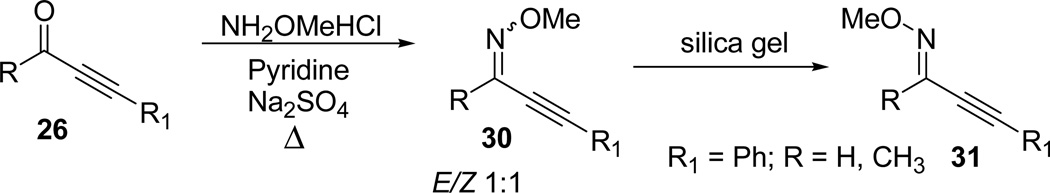
The reaction of ynone 26 with methoxylamine hydrochloride, pyridine, and Na2SO4 (or MgSO4/MeOH) provided the O-methyl oxime 30.50 When 30 is unsubstituted or substituted with a methyl group (R= H, Me), 2-alkyn-1-one O-methyl oxime tend to isomerize to 31 when passed through silica gel and often resulted in a mixture of E/Z isomers in 1:1 ratio. However, with increase of the size of the R group, the Z isomer was predominantly the major isolated product (Scheme 9).49
Scheme 9.
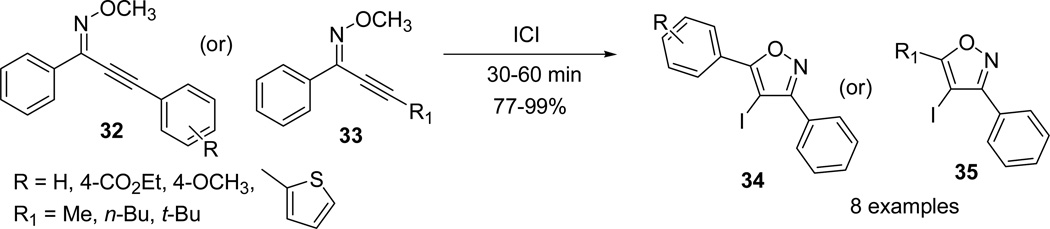
The ICl promoted electrophilic cyclizations of Z-O-methyl oximes (32/33) were performed with a wide variety of aryl and alkyl substitutions or substituted heteroaromatic systems. The desired 4-iodoisoxazoles (34/35) were isolated in very good to excellent yields depending on the nature of the substituent. Substitution of alkynyl moiety with aliphatic and aryl substituents had a little effect on the yield of the reaction. Further, the electron-deficient and electron-rich substituents at the para-position did not significantly alter the yields. On the other hand, insertion of thiophene heterocycle at the alkynyl unit slightly lowered the yield (Scheme 10).49
Scheme 10.
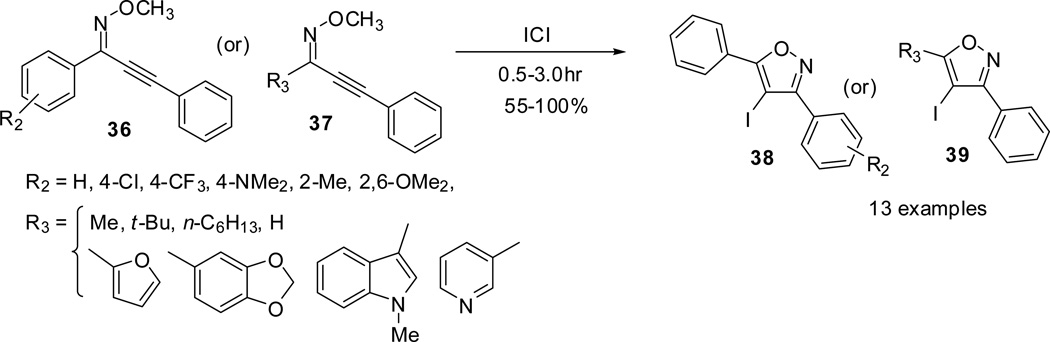
Similarly, the effect of the substituents attached to the imine carbon of the 2-alkyn-1-one O-methyl oximes (36/37) was also investigated. The para-substituted electron-deficient groups provided the isoxazoles in excellent yields (38/39). The electron-rich NMe2 group in para position required extended reaction times and an excess of ICl to achieve excellent yield. Other alkyl and heteroaryl substituents of the 1-alkynone were quite successful and the products were isolated in excellent yields irrespective of the size (Scheme 11).49
Scheme 11.
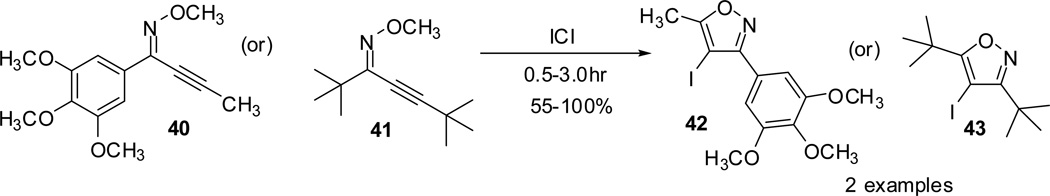
Additional attempts to examine the effect of steric hindrance exerted by the introduction of bulky substituents (40/41) proved to be negligible on product formation and the desired isoxazoles (42/43) were isolated in good to excellent yields (Scheme 12).
Scheme 12.
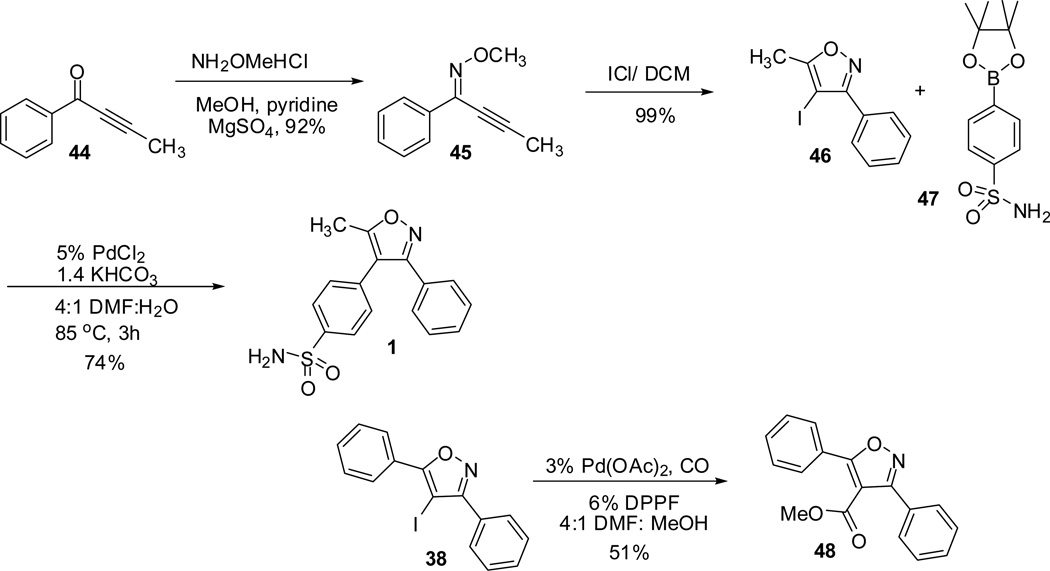
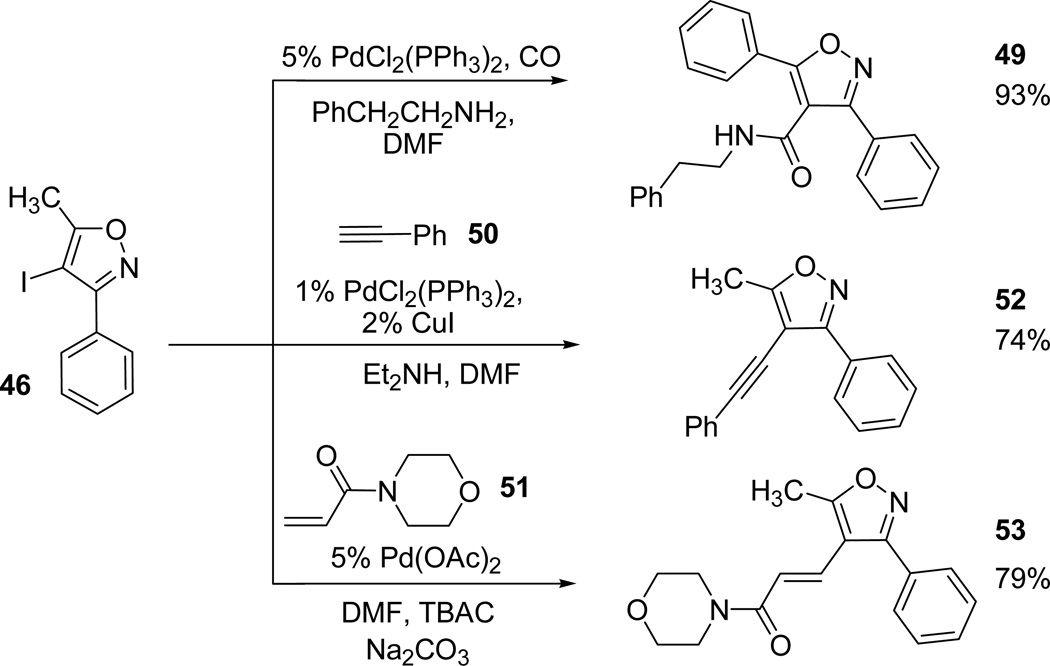
The readily synthesized 4-iodoisoxazoles (35 and 39) are useful precursors to synthesize valdecoxib 1 through Suzuki cross-coupling with the corresponding boronic acid ester. Reaction of 4-iodoisoxazole 46 with boronic ester 47 under mild Suzuki conditions (5 mol % PdCl2 catalyst, 1.4 equiv of KHCO3; DMF:H2O (4:1), 85°C) afforded the valdecoxib 1 in 74% yield. Similar reaction of 4-iodoisoxazole 38 in catalytic amounts of Pd(OAc)2, ferrocene, carbon monoxide, in DMF:MeOH (4:1) gave the isoxazole methyl ester 48 (Scheme 13).48,49
Scheme 13.
Further, the 4-iodo-5-methyl-3-phenylisoxazole 46 was converted to the corresponding phenethylamide 49 by using catalytic amounts of PdCl2(PPh3)2, carbon monoxide, and 2-phenethyl amine.50 Additional Heck51 and Sonogashira52 coupling reactions of 4-iodo-5-methyl-3-phenylisoxazole 46 with phenyl acetylene 50 and N-acryloylmorpholine 51 provided the desired 4-acetlyated isoxazole 52 and α,β-unsaturated amide 53 in high yields (Scheme 14).
Scheme 14.
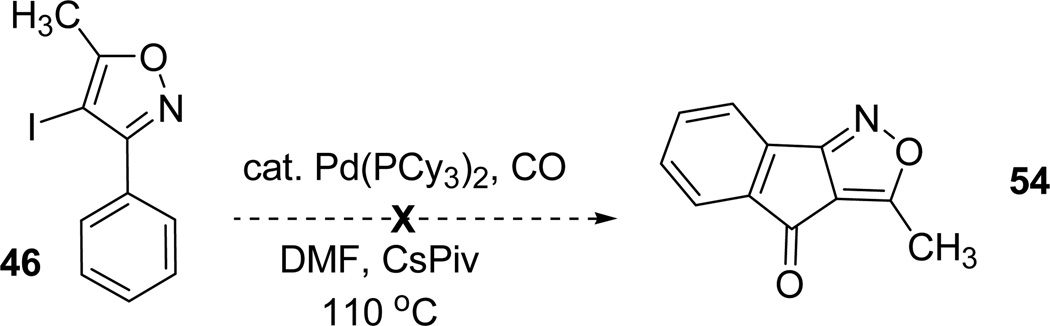
However, the carbonylative cyclization catalyzed in presence of palladium furnished the reduced isoxazole product than expected cyclized product 54 (Scheme 15).49
Scheme 15.
2.3 Condensation reactions
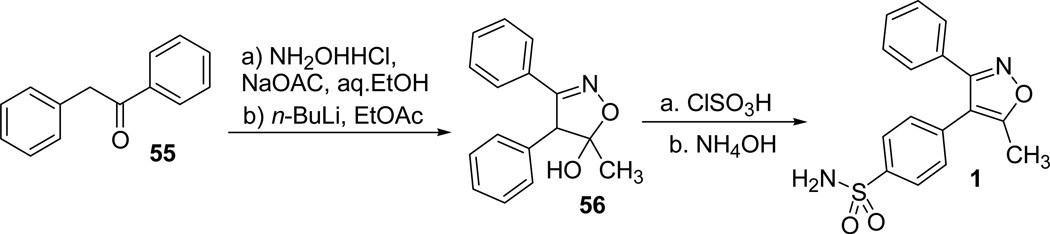
Talley et al38 reported the initial synthesis of valdecoxib 1 and its hydroxymethyl analogue 59, and the concise route to access 1 is outlined in scheme 14. Treatment of deoxybenzoin 55 with hydroxylamine hydrochloride in presence of sodium acetate furnished the desired oxime. Deprotonation of the oxime with n-BuLi followed by condensation with ethyl acetate afforded the intermediate isoxazoline 56. Reaction of the isoxazoline 56 with chlorosulfonic acid followed by the reaction with ammonium hydroxide afforded the valdecoxib 1 (Scheme 16).
Scheme 16.
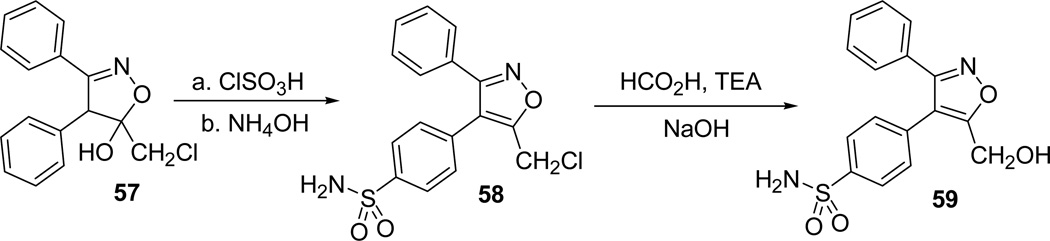
Similarly, the hydroxymethyl analogue of valdecoxib 59 was synthesized following the same protocol. Condensation of dianion of the deoxybenzoin with methyl chloroacetate provided the isoxazoline derivative 57. Chlorosulfonation and treatment of the isoxazoline 57 with sulfonyl chloride with aqueous ammonia afforded the sulfonamide derivative 58. Final treatment of sulfonamide derivative with formic acid in presence of triethylamine followed by hydrolysis with aqueous NaOH53 furnished the hydroxymethyl analogue of valdecoxib 59 (Scheme 17).38
Scheme 17.
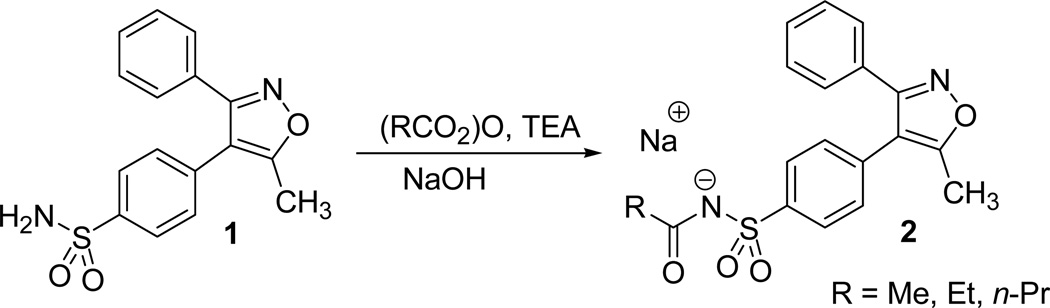
In another report, Talley and his coworkers reported the synthesis of parecoxib sodium 2, a water soluble prodrug of valdecoxib.17 Parecoxib sodium was identified as a highly potent and selective inhibitor of prostaglandins from COX-2. Acylation of the valdecoxib sulfonamide 1 with acid anhydride in presence of triethylamine afforded the desired sodium salt of the isoxazole derivative 2 (Scheme 18).
Scheme 18.
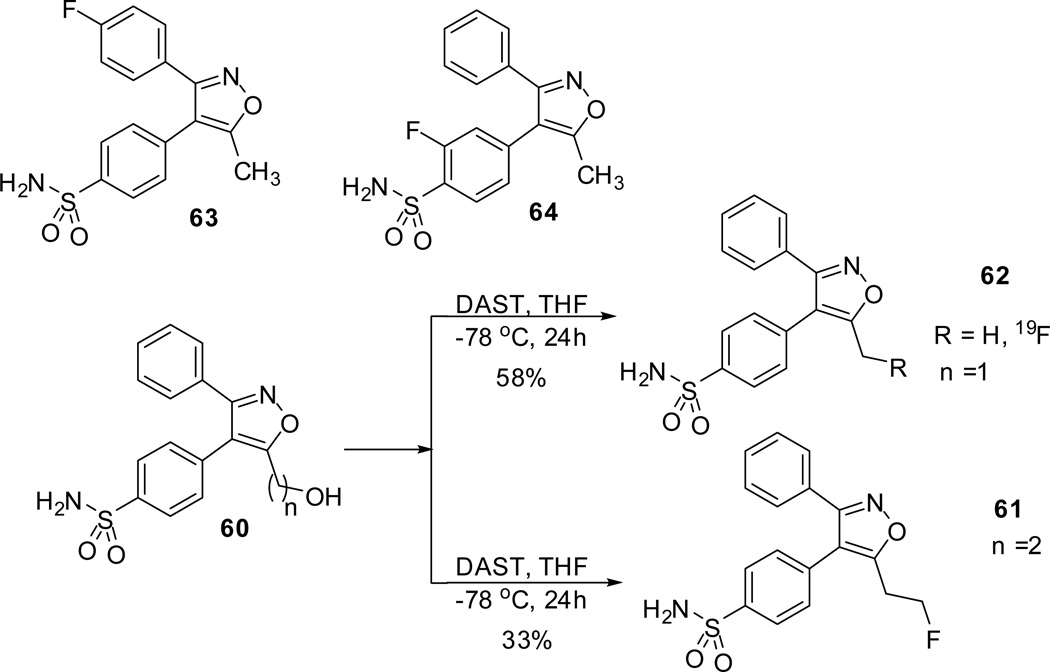
Syntheses of radiolabelled COX-II inhibitors with short lived positron emission are attractive targets for synthesis and find several applications in imaging techniques. Positron emission tomography abbreviated as PET is a powerful non-invasive, molecular imaging technique that found extensive biomedical applications.54 Toyokuni et al reported the synthesis of fluoroalkyl and fluroaryl analogues of valdecoxib as a potential PET imaging probe for COX-II.8 The non radioactive fluoroalkyl analogues of valdecoxib 61 and 62 were synthesized by direct fluorination of the corresponding hydroxy analogue 60 with DAST (diethylaminosulfur trifluoride) and other relevant fluoroaryl analogues (63 and 64) were synthesized by earlier reports (Scheme 19).23,37,38
Scheme 19.
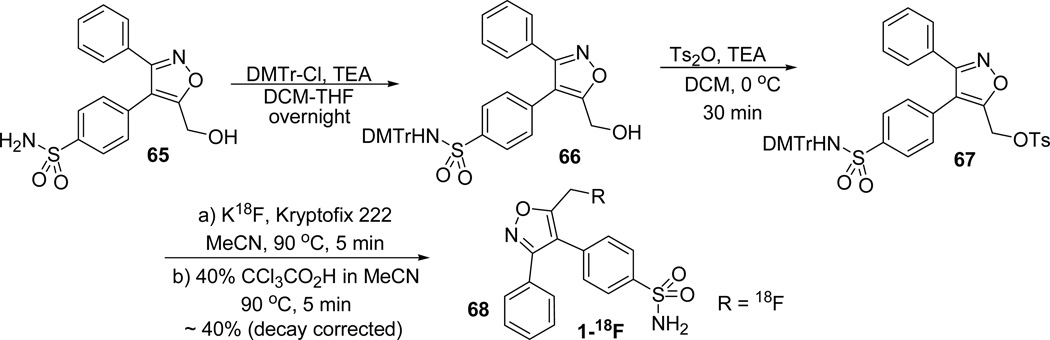
The radiosynthesis of the [18F]fluoromethyl analogue 1-18F (68) of valdecoxib was accomplished as shown in Scheme 18. The sulfonamide group of the hydroxymethyl isoxazole 65 was selectively protected using 4,4'-dimethoxytrityl (DMTr) group 66. The hydroxyl group was tosylated with tosylic anhydride to give the tosylated isoxazole 67. The tosyl group was substituted with fluorine and following deprotection under acidic conditions furnished the desired 1-18F (68; Scheme 20).8
Scheme 20.
2.3 1,3-Dipolar Cycloadditions
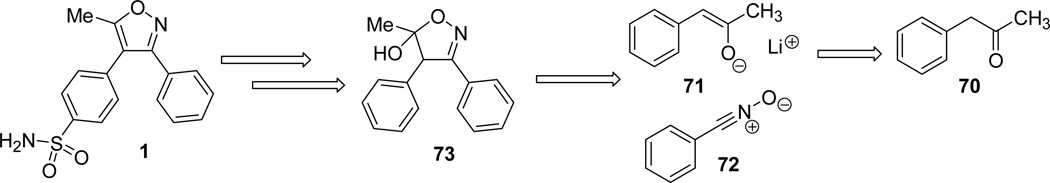
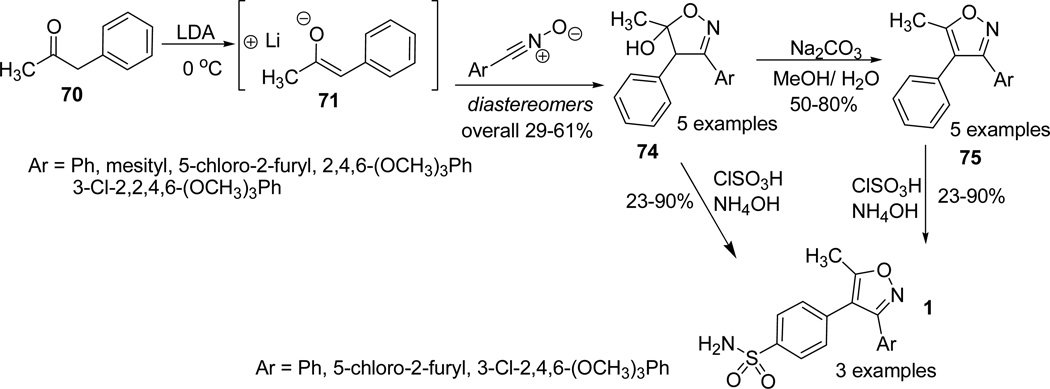
Scilimati and his group31 reported the synthesis of a series of 3,4-diarylisoxazoles analogues of valdecoxib (Fig. 2). The synthetic protocol involved the generation of a thermodynamically stable enolate 71 from phenylacetone 70 in presence of LDA at 0°C.55 The readily formed 71, reacted with a wide variety of substituted arylnitrile oxides and afforded the intermediate 3-aryl-5-hydroxy-5-methyl-4-phenyl-2-isoxazoline diastereomer 74. The hydroxyisoxazoline underwent aromatization to the analogous isoxazole 75 through dehydration process.31,56 However, the reaction of mesityl and 5-chloro-2-furyl substituted nitrile oxides with enolate 71 provided unstable isoxazolines, which on partial dehydration (or silica gel separation) furnished the isoxazoles 75. The readily synthesized 5-methyl-3,4-diphenylisoxazole 75 or the intermediate hydroxyl-isoxazoline 74 under ClSO3H/NH4OH conditions provided the valdecoxib analogues 1 (Scheme 21).57
Figure 2.
Scheme 21.
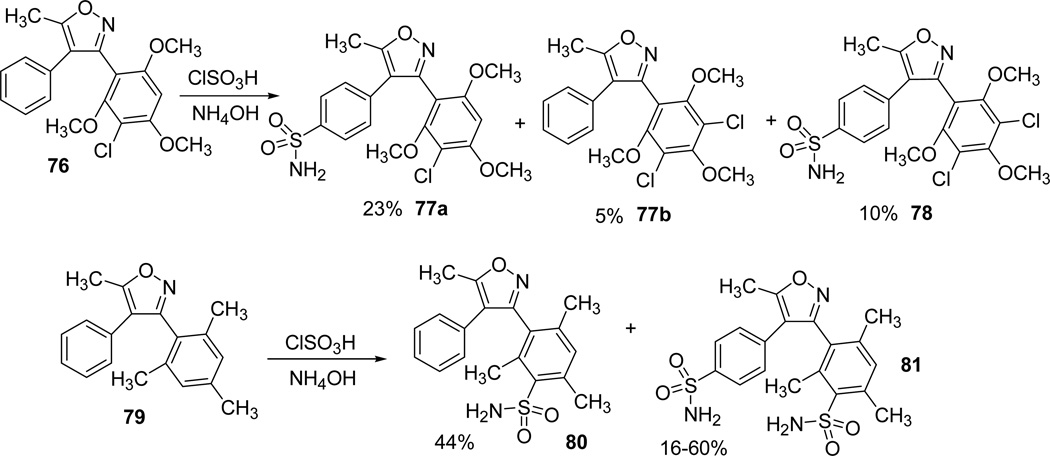
Similarly, other potential analogues of valdecoxib 1 were synthesized by adopting the same protocol. While analogues (Ar = Ph, 5-chloro-2furyl) were synthesized in high to excellent yields, analogue 77a (Ar = 3-Cl-2,4,6-(OCH3)3Ph) was isolated in low yields. Further, in case of mesityl substituted analogue 79, ClSO3H/NH4OH reaction took place at the mesityl ring 80 and 81, due to the activation of electron rich methyl groups on the phenyl ring. However, in case of 81 the reaction wasn’t exclusive and also occurred at the C-4 phenyl ring of the isoxazole (Scheme 22).31.
Scheme 22.
Erdélyi et al3 reported the synthesis and pharmacological evaluation of an active metabolite N-hydroxyvaldecoxib. Chlorosulfonation of 5-methyl-3,4-diphenylisoxazole38 gave a mixture of ortho- and para-chlorosulfonylisoxazoles (83 and 84), which following recrystallization and treatment with hydroxylamine hydrochloride gave the unstable para- substituted N-Hydroxy-4-(5-methyl-3-phenyl-isoxazol-4-yl)-benzenesulfonamide isomer 85. The intermediate 85 was transformed into a more stable monohydrate form 86 and recrystallized from L-ascorbic acid in aqueous ethanol (Scheme 23).3
Scheme 23.
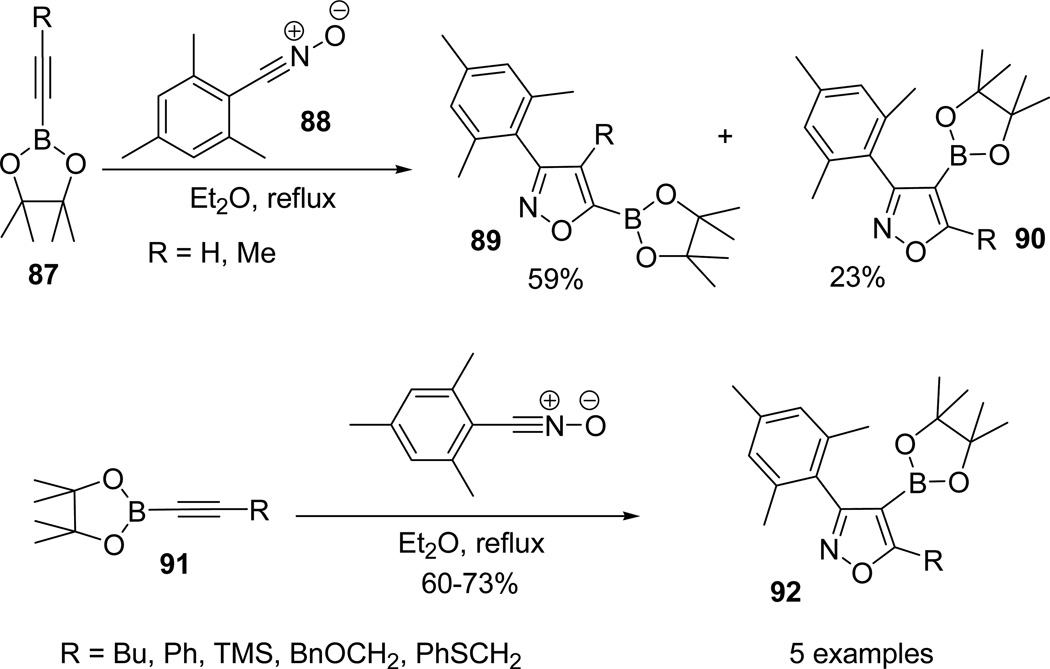
Organoboron reagents58 are the most sought synthetic intermediates59 primarily used in the construction of C-C bond via Pd-catalyzed cross coupling reactions under relatively mild conditions.60 Harrity and his coworkers documented the regiochemistry of boron containing alkynes by investigating the 1,3-dipolar cycloaddition reactions of nitrile oxides with various alkynylboronates.61–63 1,3-Dipolar cycloaddition reaction of alkynylboronate 87 with mesitylcarbonitrile oxide 88 proceeded smoothly and furnished a mixture of isoxazoleboronates 89 and 90. In addition, 1,3-dipolar reaction of mesitylcarbonitrile oxide 88 with methyl substituted alkynylboronate (87; R= Me) resulted in complete reversal of regioselectivity and isolated 90 as single regioisomer. Similar reactions of mesitylcarbonitrile oxide 88 with substituted alkynylboronates 91 provided the isoxazole-4-boronic esters 92 regioselectively (Scheme 24).63
Scheme 24.
The application of alkyl 93 and substituted arylnitrile oxides 94 also provided the 4-substituted boronic esters 95/96 with excellent regioselectivity.32,64 This regioselective 1,3-dipolar reaction toward the synthesis of 3,4,5-trisubstituted isoxazoles can selectively be used to incorporate the boronates at C-4 with excellent levels of regioselectivity by permitting a reasonable variety of substituents to be introduced at C-5 position of the isoxazole (Scheme 25).63
Scheme 25.
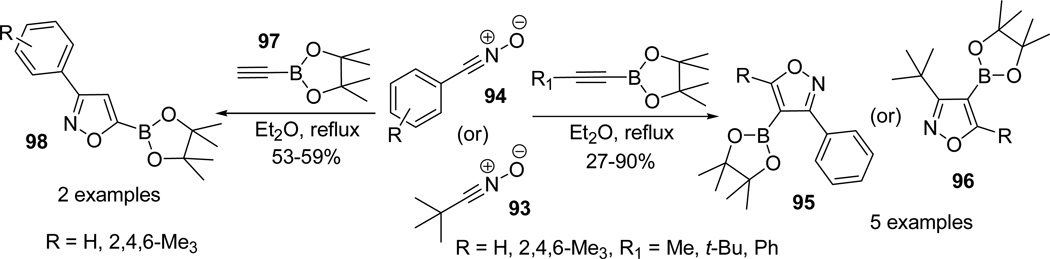
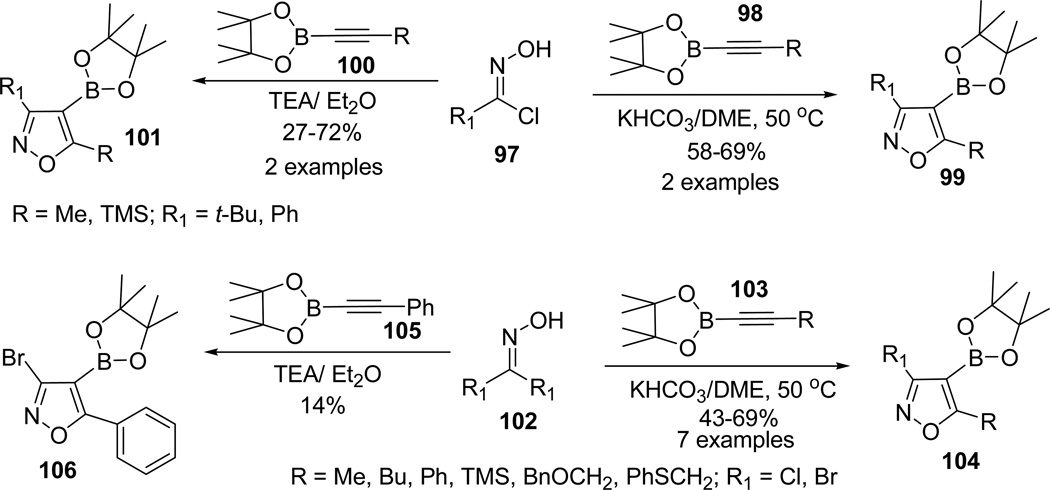
In efforts to promote the dipolarophile cycloaddition over competiting dimerization of nitrile oxides,65 the reaction conditions of the 1,3-dipolar reaction were optimized (KHCO3/DME suspension) by decreasing the relative solubility to aid in slow generation of nitrile oxides.66 1,3-dipolar reaction of aryl and alkyl nitrile oxides 97 under either set of conditions with substituted ethynylboronate 98/100 proceeded smoothly and furnished isoxazole-4-boronates as a single regioisomers 99/101. However, attempted similar cycloadditions using triethylamine with aryl and alkyl nitrile oxides 97 resulted in a low yield of isoxazoles 101 (Scheme 26).63
Scheme 26.
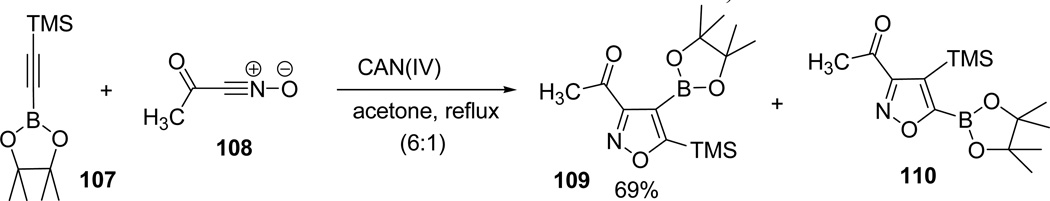
The reaction scope was further broadened by extending the cycloadditions reactions with halonitrile oxides 102 generated in situ from the corresponding dihaloformaldoximes. The formations of the 3-haloisoxazoles 104/106 were found to be more efficient in KHCO3/DME suspension than compared to triethylamine conditions. After the efficient optimization of the cycloadditions in presence of mild bases, the reaction scope was further tested in acidic conditions. Reaction of TMS substituted alkyne 107 with acetylnitrile oxide 10867 provided a 6:1 mixture of 3-acetylisoxazole regioisomers 109 and 110 (Scheme 27).63
Scheme 27.
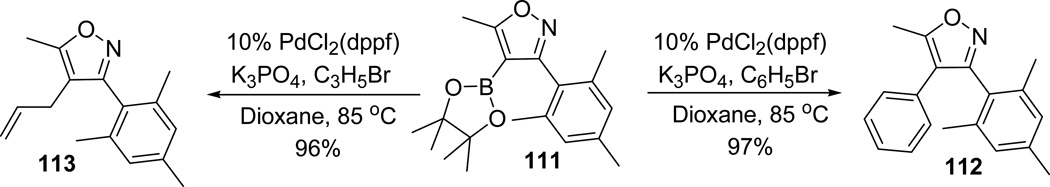
The readily prepared isoxazoleboronates 111 are useful precursors for the Suzuki coupling reactions. Isoxazoleboronic ester 111 underwent Pd-catalysed coupling with bromobenzene and allyl bromides efficiently to yield the coupling products 112 and 113 in excellent yields. This efficient two step route is of great significance for the regioselective construction of an array of highly substituted isoxazoles (Scheme 28).61,63
Scheme 28.
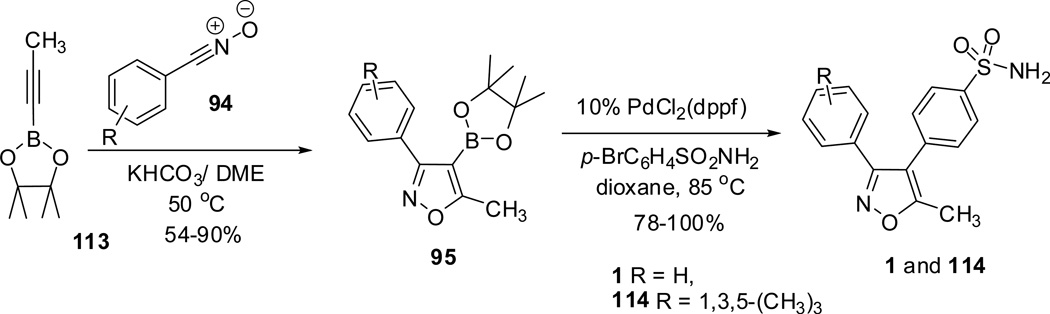
The scope of this reaction methodology63 was explored to a significant extent toward the synthesis of nonsteroidal antinflammatory drug; valdecoxib 1 and relevant analogues 114. Valdecoxib 1 and its mesityl-substituted analogue 114 were synthesized by the cycloaddition of alkyne 113 with benzo- and mesitonitrile oxides 94 to afford intermediate isoxazole-4-boronates 95.62 These isoxazole-4-boronates 95 when subjected to Suzuki coupling reaction with pbromobenzene sulfonamide furnished the targeted drug valdecoxib 1 and its mesityl-substituted analogue 114 in excellent yields (Scheme 29). The reaction protocol is of greater significance in the synthesis of valdecoxib analogues (especially 114) compared to earlier report,31 where the reaction suffered from undesired chlorosulfonation, steric effects, and low product formation.
Scheme 29.
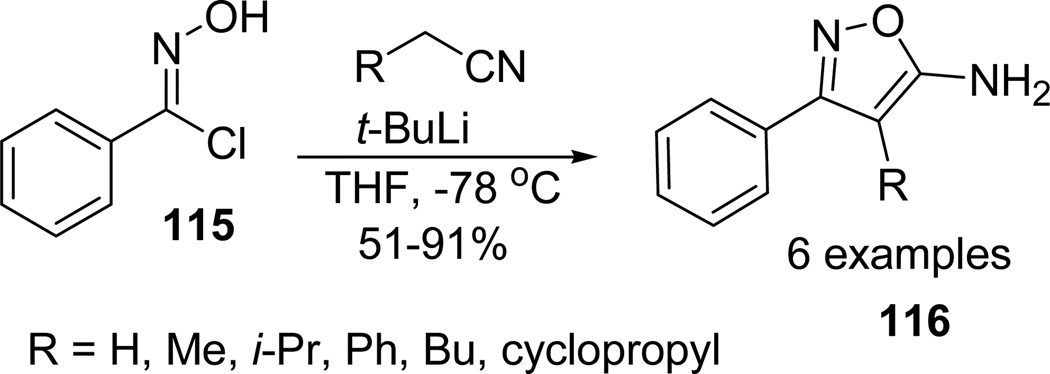
Bourbeau et al68 reported the synthesis of 4-alkyl-5-aminisoxazoles through nucleophilic addition of lithiated alkyl nitriles to hydroximoyl chlorides. The hydroximoyl chlorides are highly electrophilic and can easily react with lithiated nitriles at low temperature reaction conditions. α-Chlorobenzaldoxime 115 reacted with a variety of lithiated nitriles to provide the desired 5-aminoisoxazoles 116 (Scheme 30).69,70
Scheme 30.
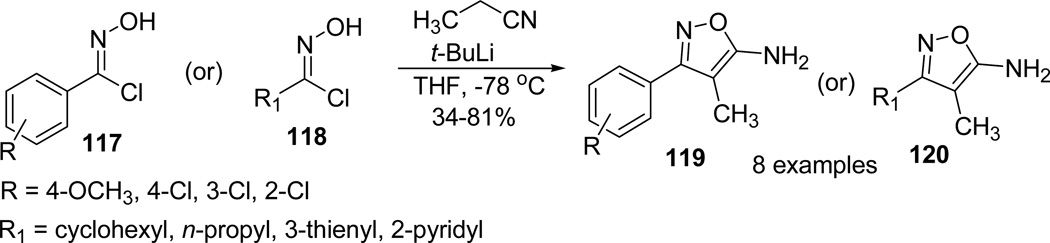
Similarly lithiated propionitrile was treated with an array of substituted hydroximoyl chlorides 117/118 to examine the effect of substitution on the rate of reaction.68 All the reactions proceeded smoothly and furnished the desired aminoisoxazoles 119/120 in moderate to high yields (Scheme 31). The plausible reaction mechanism involved the addition of lithiated nitrile to in situ generated nitrile oxides, followed by cyclization to afford the 5-aminoisoxazoles (or) involve a concerted pathway analogous to oxime chlorides and alkynes.70
Scheme 31.
Conclusions
In summary, the review provided a compact overview of different synthetic methodologies to synthesize diarylisoxazole derivatives of valdecoxib and synthetic analogues. The chemistry of isoxazoles8,23,32,34,38 is of great interest for synthetic organic and medicinal chemists over several decades. Of the several existing protocols, (a) 1,3-dipolar cycloaddition reactions of sydnones offered excellent regio-control to synthesize valdecoxib and analogues.61–63 This methodology has the advantage of avoiding the issues associated with undesired chlorosulfonation, steric effects, and low product yield;31 (b) in addition to the cycloadditions, electrophilic cyclizations48,49 and cross-coupling reactions23,40 are equally important and serve as alternative approaches for the synthesis of diarylisoxazoles.
Figure 1.
Examples of pharmaceutically relevant diaryl heterocycle scaffolds
Scheme 1.
Acknowledgements
We thank the State of Florida, Executive Office of the Governor’s Office of Tourism, Trade, and Economic Development; NIH (1R03DA025850-01A1, Nefzi); NIH (5P41GM081261-03, Houghten) and NIH (3P41GM079590-03S1, Houghten) for their generous financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Reitz DB, Isakson PC. Curr. Pharm. Des. 1995;1:211. [Google Scholar]
- 2.Chavatte P, You S, Marot C, Baurin N, Lesieur D. J. Med. Chem. 2001;44:3223. doi: 10.1021/jm0101343. [DOI] [PubMed] [Google Scholar]
- 3.Erdélyi P, Fodor T, Varga AK, Czugler M, Gere A, Fischer J. Bioorg. Med. Chem. 2008;16:5322. doi: 10.1016/j.bmc.2008.02.088. [DOI] [PubMed] [Google Scholar]
- 4.Vane JR. Nature (New Biol) 1971;231:232. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 5.Herschman HR. Biochim. Biophys. Acta. 1996;1299:125. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 6.Turini ME, DuBois RN. Annu. Rev. Med. 2002;53:35. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 7.Simmons DL, Botting RM, Hla T. Pharmacol. Rev. 2004;56:387. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 8.Toyokuni T, Dileep Kumar JS, Walsh JC, Shapiro A, Talley JJ, Phelps ME, Herschman HR, Barrio JR, Satyamurthy N. Bioorg. Med. Chem. Lett. 2005;15:4699. doi: 10.1016/j.bmcl.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 9.Botting R. Thrombosis Res. 2003;110:269. doi: 10.1016/s0049-3848(03)00411-0. [DOI] [PubMed] [Google Scholar]
- 10.Warner TD, Mitchell JA. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13371. doi: 10.1073/pnas.222543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons DL. Thrombosis Res. 2003;110:265. doi: 10.1016/s0049-3848(03)00380-3. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13926. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie W, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2692. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. J. Biol. Chem. 1991;266:12866. [PubMed] [Google Scholar]
- 15.O’Banion MK, Sadowski HB, Winn V, Young DAA. J. Biol. Chem. 1991;266:23261. [PubMed] [Google Scholar]
- 16.Vane JR, Botting RM. Adv. Prostaglandin, Thromboxane, Leukot. Res. 1995;23:41. [PubMed] [Google Scholar]
- 17.Talley JJ, Bertenshaw SR, Brown DL, Carter JS, Graneto MJ, Kellogg MS, Koboldt CM, Yuan J, Zhang YY, Seibert K. J. Med. Chem. 2000;43:1661. doi: 10.1021/jm000069h. [DOI] [PubMed] [Google Scholar]
- 18.Talley JJ. Selective Inhibitors of COX-2. In: King FD, Oxford A, editors. Progress in Medicinal Chemistry. Amsterdam: Elsevier; 1999. pp. 201–234. [DOI] [PubMed] [Google Scholar]
- 19.Kalgutkar AS. Exp. Opin. Ther. Patents. 1999;8:831. [Google Scholar]
- 20.Vane JR, Bakhle YS, Botting RM. Annu. Rev. Pharmacol. Toxicol. 1998;38:97. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 21.Subbaramaiah K, Zakim D, Weksler BB, Dannenberg AJ. Proc. Soc. Exp. Biol. Med. 1997;216:201. doi: 10.3181/00379727-216-44170. [DOI] [PubMed] [Google Scholar]
- 22.Pasinetti GM. J. Neurosci. Res. 1998;54:1. doi: 10.1002/(SICI)1097-4547(19981001)54:1<1::AID-JNR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Dileep Kumar JS, Ho MM, Leung JM, Toyokuni T. Adv. Synth. Catal. 2002;344:1146. [Google Scholar]
- 24.Talley JJ. Prog. Med. Chem. 1999;13:201. doi: 10.1016/s0079-6468(08)70048-1. [DOI] [PubMed] [Google Scholar]
- 25.Micetich RG, Shaw CC, Rastogi RB. EP 26928. 1981 [Google Scholar]; Chem. Abstr. 1981;95:115518. [Google Scholar]
- 26.Hagiwara Y, Tanaka M, Kajitani A, Yasumoto S. JP 03220180. 1991 [Google Scholar]; Chem. Abstr. 1991;116:128944. [Google Scholar]
- 27.Green J, Bemis G, Grillot A-L, Ledeboer M, Salituro F, Harrington E, Gao H, Baker C, Cao J, Hale M. WO 0112621. 2001 [Google Scholar]; Chem. Abstr. 2001;134:178569. [Google Scholar]
- 28.Dannhardt G, Dominiak P, Laufer S. Arzneim.-Forsch. 1993;43:441. [PubMed] [Google Scholar]
- 29.Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Nature. 1996;384:644. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 30.Walker MC, Kurumbail RG, Kiefer JR, Moreland KT, Koboldt CM, Isakson PC, Seibert K, Gierse JK. Biochem. J. 2001;357:709. doi: 10.1042/0264-6021:3570709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Nunno L, Vitale P, Scilimati A, Tacconelli S, Patrignani P. J. Med. Chem. 2004;47:4881. doi: 10.1021/jm040782x. [DOI] [PubMed] [Google Scholar]
- 32.Sandanayaka VP, Youjun Y. Org. Lett. 2000;2:3087. doi: 10.1021/ol006256r. [DOI] [PubMed] [Google Scholar]
- 33.Croce PD, La Rosa C, Zecchi G. J. Chem. Soc. Perkin Trans 1. 1985:2621. [Google Scholar]
- 34.(a) Dadiboyena S, Xu J, Hamme AT., II Tetrahedron Lett. 2007;49:1295. doi: 10.1016/j.tetlet.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu J, Hamme AT., II Synlett. 2008:919. [Google Scholar]
- 35.Grunanger P, Vita-Finzi P. In: The Chemistry of Heterocyclic Compounds. Taylor EC, Weissberger A, editors. Vol. 49. New York: John Wiley & Sons; 1991. pp. 125–264. [Google Scholar]
- 36.Katritzky AR, Wang M, Zhang S, Voronkov MV, Steel PJ. J. Org. Chem. 2001;66:6787. doi: 10.1021/jo0101407. [DOI] [PubMed] [Google Scholar]
- 37.Talley JJ. 5,859,257. US Patent. 1999; Chem. Abstr. 1999;130:110269. [Google Scholar]
- 38.Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K. J. Med. Chem. 2000;43:775. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- 39.Habeeb AG, Rao PNP, Knaus EE. Drug Dev. Res. 2000;51:273. [Google Scholar]
- 40.Kromann H, Sløk FA, Johansen TN, Krogsgaard-Larsen P. Tetrahedron. 2001;57:2195. [Google Scholar]
- 41.Chemler SR, Trauner D, Danishefsky SJ. Angew. Chem. Int. Ed. 2001;40:4544. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama M, Tsuji K, Kushida M. J. Chem. Soc. Perkin Trans. 1. 1986:67. [Google Scholar]
- 43.Moriya O, Urata Y, Endo T. J. Chem. Soc. Chem. Commun. 1991:884. [Google Scholar]
- 44.Bunnelle WH, Singam PR, Narayanan BA, Bradshaw CW, Liou JS. Synthesis. 1997:439. [Google Scholar]
- 45.Madsen U, Nielson EØ, Curtis DR, Beattie DT, Krogsgaard-Larsen P. Acta. Chem. Scand. 1990;44:96. doi: 10.3891/acta.chem.scand.44-0096. [DOI] [PubMed] [Google Scholar]
- 46.Bowden G, Crank G, Ross WJ. J. Chem. Soc. (C) 1968:172. [Google Scholar]
- 47.(a) El Borai M, Hassanein M. Org. Prep. Proc. Int. 1982;14:409. [Google Scholar]; (b) Turner RM, Lindell SD. J. Org. Chem. 1991;56:5739. [Google Scholar]; (c) Kondo Y, Yoshidam A, Sato S, Sakamoto T. Heterocycles. 1996;42:105. [Google Scholar]; (d) Carver DS, Lindell SD, Saville-Stones EA. Tetrahedron. 1997;53:14481. [Google Scholar]; (e) Boymond L, Rottlander M, Cahiez G, Knochel P. Angew. Chem. Int. Ed. Engl. 1998;37:1701. doi: 10.1002/(SICI)1521-3773(19980703)37:12<1701::AID-ANIE1701>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; (f) Jetter MC, Reitz AB. Synthesis. 1998:829. [Google Scholar]; (g) Felding J, Kristensen J, Bjerregaard T, Sander L, Vedsø P, Begtrup M. J. Org. Chem. 1999;64:4196. [Google Scholar]
- 48.Waldo JP, Larock RC. Org. Lett. 2005;23:5203. doi: 10.1021/ol052027z. [DOI] [PubMed] [Google Scholar]
- 49.Waldo JP, Larock RC. J. Org. Chem. 2007;72:9643. doi: 10.1021/jo701942e. and ref. 19–22 cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beak P, Basha A, Kokko B, Loo D. J. Am. Chem. Soc. 1986;108:6016. doi: 10.1021/ja00279a058. [DOI] [PubMed] [Google Scholar]
- 51.(a) Friesen RW, Ducharme Y, Ball RG, Blouin M, Boulet L, Cote B, Frenette R, Girard M, Guay D, Huang Z, Jones TR, Laliberte F, Lynch JJ, Mancini J, Martins E, Masson P, Muise E, Pon DJ, Siegl PKS, Styhler A, Tsou NN, Turner MJ, Young RN, Girard Y. J. Med. Chem. 2003;46:2413. doi: 10.1021/jm0204542. [DOI] [PubMed] [Google Scholar]; (b) Schoenberg A, Heck RF. J. Org. Chem. 1974;39:3327. [Google Scholar]
- 52.(a) Sonogashira K. Chapter 5. In: Diederich F, Stang PJ, editors. Metal-Catalyzed Cross-Coupling Reactions. Weinheim, Germany: Wiley-VCH; 1998. pp. 203–229. [Google Scholar]; (b) Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975:4467. [Google Scholar]; (c) Alonso F, Beletskaya IP, Yus M. Tetrahedron. 2005;61:11771. [Google Scholar]; (d) Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem. Int. Ed. 2005;44:4442. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]; (e) Negishi E, de Meijere A, editors. Handbook of Organopalladium Chemistry for Organic Synthesis. Vol. 1. New York: Wiley-Interscience; 2002. p. 1223. [Google Scholar]
- 53.Alexander J, Renyer ML, Veerapanane HA. Synth. Commun. 1995;25:3875. [Google Scholar]
- 54.(a) Phelps ME. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9226. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fowler JS, Wolf AP. Acc. Chem. Res. 1997;30:181. [Google Scholar]
- 55.Di Nunno L, Scilimati A, Vitale P. Tetrahedron. 2002;58:2659. [Google Scholar]
- 56.Di Nunno L, Scilimati A. Tetrahedron. 1987;43:2181. [Google Scholar]
- 57.Talley JJ, Brown D, Nagarajan S, Carter JS, Weier RM, Stealey MA, Collins PW, Rogers RS, Seibert K. 5,633,272. U.S. Patent. 1997; CAN 127:65756. 1997 [Google Scholar]
- 58.Murata M, Oyama T, Watanabe S, Masuda Y. J. Org. Chem. 2000;65:164. doi: 10.1021/jo991337q. [DOI] [PubMed] [Google Scholar]
- 59.Vaultier M, Carboni B. In: Comprehensive Organometallic Chemistry II. Abel EW, Stone FGA, Wilkinson G, editors. Vol. 11. Oxford: Elsevier; 1995. p. 191. [Google Scholar]
- 60.(a) Masahiro M. Angew. Chem. Int. Ed. 2004;43:2201. [Google Scholar]; (b) Bellina F, Carpita A, Rossi R. Synthesis. 2004:2419. [Google Scholar]; (c) Kotha S, Lahiri K, Kashinath D. Tetrahedron. 2002;58:9633. [Google Scholar]; (d) Hassan J, Sevignon M, Gozzi C, Schultz E, Lemaire M. Chem. Rev. 2002;102:1359. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]; (e) Suzuki A. Pure. Appl. Chem. 1994;66:213. [Google Scholar]
- 61.Davies MW, Wybrow RAJ, Johnson CN, Harrity JPA. Chem. Commun. 2001:1558. doi: 10.1039/b103319k. [DOI] [PubMed] [Google Scholar]
- 62.Moore JE, Davies MW, Goodenough KM, Wybrow RAJ, York M, Johnson CN, Harrity JPA. Tetrahedron. 2005;61:6707. [Google Scholar]
- 63.Moore JE, Goodenough KM, Spinks D, Harrity JPA. Synlett. 2002:2071. [Google Scholar]
- 64.(a) Sauers RR, Hadel LM, Scimone AA, Stevenson TA. J. Org. Chem. 1990;55:4011. [Google Scholar]; (b) Hojo M, Tomita K, Hosomi A. Tetrahedron Lett. 1993;34:485. [Google Scholar]; (c) Fouli FA, Habashy MM, El-Kafrawy AF, Youseef AJA, El-Adly MM. J. Prakt. Chem. 1987;329:1116. [Google Scholar]; (d) Bianchi G, Cogoli A, Grunanger P. J. Organomet. Chem. 1966;6:598. [Google Scholar]
- 65.Grundmann C. Synthesis. 1970:344. [Google Scholar]
- 66.Chiarino D, Napoletano M, Sala A. Tetrahedron Lett. 1986;27:3181. [Google Scholar]
- 67.Itoh K-i, Horiuchi CA. Tetrahedron. 2004;60:1671. [Google Scholar]
- 68.Bourbeau MP, Rider JT. Org. Lett. 2006;8:3679. doi: 10.1021/ol061260+. [DOI] [PubMed] [Google Scholar]
- 69.Becalli EM, Manfredi A, Marchesini A. J. Org. Chem. 1985;13:2372. [Google Scholar]
- 70.Baraldi PG, Barco A, Bennetti S, Pollini GP, Simoni D. Synthesis. 1987:857. [Google Scholar]