Retinal mitochondria fusionγÇôfission and protein import machinery are severely affected in diabetes, and reversal of hyperglycemia fails to provide any benefit to these abnormalities, suggesting their role in the development and in the continued progression of diabetic retinopathy.
Abstract
Purpose.
Mitochondrial function is controlled by membrane structure. In diabetes, retinal mitochondria are dysfunctional, and reversal of hyperglycemia fails to inhibit such changes. The goal of this study was to use anatomic and molecular biologic techniques to investigate the effect of diabetes on mitochondrial membrane structure.
Methods.
Wistar rats were maintained in poor glycemic control (PC; GHb 11.2%) or good glycemic control (GC; GHb 5.5%) for 12 months or in PC for 6 months, followed by GC for an additional 6 months. The structure of the retinal mitochondria in the microvascular region was evaluated by electron microscopy (TEM) and gene expressions of mitochondrial structure–related proteins by rat mitochondrial PCR array. Representative genes were validated by real-time PCR, and their protein expression by Western blot. The results were confirmed in the retina obtained from human donors with diabetic retinopathy.
Results.
TEM showed enlarged mitochondria with partial cristolysis in the retinal microvasculature from PC rats, compared with those from normal rats. Among 84 genes, 6 retinal genes were upregulated and 12 were downregulated. PCR confirmed alternations in the gene expressions of fusion (Mfn2), carrier (Timm44 and Slc25a21), Akt1, and fission proteins (Dnm1l). Protein levels of Mfn2 and Dnm1l were consistent with their mRNA levels, but their mitochondrial abundance was decreased. Reversal of hyperglycemia failed to normalize these changes. Retinas from donors with diabetic retinopathy also presented similar patterns of changes in the gene and protein expressions.
Conclusions.
Mitochondrial structural and transport proteins play an important role in the development of diabetic retinopathy and also in the metabolic memory phenomenon associated with its continued progression.
Diabetic retinopathy remains the leading cause of blindness in young adults. Research to understand how the disease develops has explored several hyperglycemia-initiated pathways, but the molecular mechanism remains elusive. In diabetes, retinal mitochondria become dysfunctional, their DNA (mtDNA) is damaged, and capillary cell apoptosis is increased, and these abnormalities are observed before histopathology can be detected, suggesting a major role of mitochondria in the development of diabetic retinopathy.1–3
Mitochondria are highly dynamic organelles; depending on the energy demand, they can branch, retract, and increase the number of cristae; change their shape and fuse; or increase in size.4,5 Their function is regulated by cycle of fusion and fission, and alterations in membrane structure and morphology are regarded as significant components of mitochondrial dysfunction.6 Fission of mitochondria is generally related with apoptosis and fusion with inhibition of apoptosis.7,8 These fission (e.g., dynamin 1-like protein, Dnm1l/Drp1) and fusion (e.g., mitofusin 2, Mfn2; Mfn1) proteins are GTPases and are energy dependent.6 How diabetes affects mitochondrial fusion and fission proteins in the retina is not clear.
Mitochondria are equipped with approximately 1500 proteins, but only 13 proteins are encoded by mtDNA. Thus, most proteins, encoded by nuclear DNA, have a mitochondrial leading sequence in their preproteins.9 These proteins are imported into the mitochondria via the translocase of the outer and inner membrane (TOM and TIM, respectively) complexes. The TOM complex has a central component, Tom40, with three preprotein receptors: Tom20, Tom22, and Tom70.9 Tom34 is on the outer membrane and is mostly a cytosolic chaperon-binding mature preprotein.10,11 In the matrix side of the inner membrane, Tim44 serves as a docking protein for heat shock protein 70 (Hsp70).12 Our recent work has shown that, in diabetes, the enzymes involved in repairing damaged retinal mtDNA, do not reach the mitochondria, despite the increase in their mRNA levels,3 suggesting impaired mitochondrial membrane structure and transport machinery.
Good glycemic control, if instituted in the early stage of diabetes, prevents the development of retinopathy, but if reinstituted after a period of poor glycemic control, fails to halt its progression, suggesting a “metabolic memory” phenomenon. Our studies have demonstrated that the reversal of hyperglycemia fails to prevent mtDNA damage and the repair machinery remains subnormal.3 The purpose of this study was to investigate whether the retinal mitochondrial dysfunction observed in diabetes correlates with the ultrastructure of the mitochondria and the genes associated with mitochondrial membrane structure, including the fusion–fission and transport proteins. Further, we investigated whether these changes are reversed when the hyperglycemic insult is terminated. The effect of diabetes on the ultrastructure of the mitochondria was evaluated in the microvascular region of the retina by transmission electron microscope (TEM). Retinas from rats maintained in various glycemic controls were analyzed with a rat mitochondrial PCR array for mitochondrial biogenesis and structure–function. The PCR array results were validated by quantifying the mRNA and protein levels of the key genes by real-time PCR and Western blot analysis, respectively. The key proteins involved in maintaining mitochondrial structure and transport machinery were also confirmed in the retina from donors with diabetic retinopathy.
Methods
Animals
Wistar rats (male, 200g) were randomly assigned to remain normal or diabetic. Diabetes was induced by streptozotocin (55 mg/kg body weight), and 3 days after induction, the rats were divided into three groups: those maintained in poor glycemic control for 12 months (PC group, glycated hemoglobin, GHb >11.0%); those maintained in poor glycemic control for 6 months, followed by GC for 6 additional months (Rev group); and those maintained in good glycemic control for the entire 12 months (GC group; GHb<5.5%). Glycemic control was maintained as per our published methods.3,13,14 Rats in poor glycemic control received 1-2U insulin (Humulin; Eli Lilly, Indianapolis, IN) four to five times a week, and those in GC received insulin twice daily (a total of 7–8 units). Blood glucose was measured every week, and GHb was quantified every 2 months with a kit from Helena Laboratories (Beaumont, TX). The protocols conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. At the end of the experiment, the rats were euthanized by carbon dioxide asphyxiation, the retinas were removed immediately and either used to prepare mitochondria or stored in liquid nitrogen for future use.
Human Retina
Retinas were isolated from human eyes, enucleated between 6 and 9 hours postmortem (obtained from the Midwest Eye Banks, Ann Arbor, MI, and managed in accordance with the Declaration of Helsinki). Diabetic donors, 54 to 75 years of age with a history of diabetes for 10 or more years, had documented proliferative retinopathy. Age-matched nondiabetic donors served as controls (Table 1).
Table 1.
Human Donor Characteristics
| Age (y) | Duration of Diabetes (y) | Cause of Death | |
|---|---|---|---|
| Nondiabetic donors | |||
| 1 | 44 | — | Intracranial hemorrhage |
| 2 | 70 | — | Cerebrovascular accident |
| 3 | 72 | — | Myocardial infarction |
| 4 | 55 | — | Subarachnoid hemorrhage |
| 5 | 77 | — | Myocardial infarction |
| Donors with diabetic retinopathy | |||
| 1 | 75 | 25 | Pulmonary edema |
| 2 | 54 | 10 | Congestive heart failure |
| 3 | 69 | 10 | Respiratory failure |
| 4 | 61 | 10 | Acute myocardial infarction |
| 5 | 70 | 10 | Coronary artery disease |
| 6 | 47 | 27 | Acute myocardial infarction |
| 7 | 59 | 16 | Renal failure |
Subcellular Fractionation of the Retina
The retinas were sonicated in buffer containing 30 mM Tris-HCl (pH 7.5), 2 mM ethylene glycol tetraacetic acid, 1 mM EDTA, and 1% Triton X-100 and were centrifuged at 800g for 5 minutes to remove the cell debris. The resultant supernatant served as the total homogenate. Mitochondria were prepared from freshly isolated retinas (Mitochondrial Isolation Kit; Thermo Scientific; Rockford, IL), according to the manufacturer's instructions. Briefly, the retina was homogenized in reagent A (containing1% bovine serum albumin), and an equal amount of reagent C was added to the homogenate before centrifuging it at 350g for 10 minutes at 4°C. The supernatant was centrifuged at 13,000g for 15 minutes at 4°C, and the pelleted mitochondrial fraction was washed with reagent C and resuspended in mitochondrial buffer (25 mM Tris-HCl [pH 7.4], 250 mM sucrose, and 2 mM EDTA). Protein concentration was measured by bicinchoninic acid reagent (Sigma-Aldrich, St. Louis, MO).
Electron Microscopy
The mitochondrial ultrastructure was evaluated by TEM. The enucleated eyes were dissected along the equators and immediately incubated with 2.5% glutaraldehyde solution in 100 mM phosphate buffer (pH 7.4) for 24 hours. The retina was postfixed with 1% buffered osmium tetroxide and dehydrated with graded ethanol solutions. The samples were embedded in 812 resin (Electron Microscope Science, Hatfield, PA), and ultrathin transverse sections (70–80 nm) of selected areas near the retinal microvasculature were prepared. The sections were stained with uranyl acetate and lead citrate and viewed by TEM (model EM 900; Carl Zeiss Meditec, Oberkochen, Germany). At least eight random images were recorded at 20,000× magnification from each independent preparation. Mitochondria in the endothelial cells were analyzed by Image J software to calculate their total area (in square micrometers) and density (number/square micrometer of cytosol area)15 (ImageJ software, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
RNA Purification
RNA was isolated (Trizol; Invitrogen, Carlsbad, CA), treated with DNase I, and purified according to the instructions provided by the manufacturer (RNeasy Mini Kit; Qiagen, Valencia, CA).
Rat Mitochondrial PCR Array
First-strand cDNA was synthesized (RT2 First Strand Kit C-03; SABiosciences, Frederick, MD) by using 300 ng rat retinal RNA each. Gene expression was measured with a rat mitochondrial PCR array plate and qPCR master mix (PRAN-087 and RT2 Master Mix; SA Biosciences) as SYBR-quantitative real-time PCR (7500 PCR System; Applied Biosystem, Inc. [ABI], Foster City, CA). PCR conditions included denaturation at 95°C for 10 minutes, 40 cycles of denaturation at 95°C for 15 seconds, annealing and extension at 60°C for 60 seconds, followed by 95°C for 15 seconds, 60°C for 60 seconds, 95°C for 15 seconds and 60°C for 15 seconds. All the samples passed the reverse transcription control and genomic DNA contamination control. PCR values were normalized to the Ct value from β-actin by using the ΔΔCt method in the same sample. Relative fold changes were calculated by setting the mean fraction of normal rats to 1.
Quantitative Real-Time PCR
The first-strand cDNA was synthesized by high-capacity cDNA reverse transcription (ABI). Expression of the genes Mfn2 (NM_130894.3), Timm44 (NM_017267.1), Slc25a21 (NM_133614.2), Akt1 (NM_033230.1), and Dnm1l (NM_053655.3) in rat retina and Mfn2 (NM_001127660.1), Timm44 (AF026030.1), Timm9 (NM_012460.2), Slc25a21 (NM_030631.3), Slc25a12 (NM_003705.3), Akt1 (NM_032375.3), and Dnm1l (NM_012062.3) in human retina was measured by using commercial primers (Taqman; ABI). Internal controls included β-actin for rat and 18S rRNA for human samples.
Western Blot Analysis
Protein (30–60 μg) was separated on a 4% to 20% gradient polyacrylamide gel, blotted onto nitrocellulose membranes, blocked, and incubated with the primary antibodies against the target proteins (Mfn2, Slc25a21, Slc25a12, and Dnm1l, obtained from Abcam, Cambridge, MA; or Tim44 and Tom34, obtained from Santa Cruz Biotechnology, Santa Cruz, CA). Loading standards were β-actin for homogenate and Cox IV for mitochondria. The band intensity was quantified with gel-digitizing software (Un-Scan-It; Silk Scientific, Orem, UT), and the expression of the target protein was calculated relative to the expression of the loading control.
Statistical Analysis
Results are presented as the mean ± SD and analyzed by the nonparametric Kruskal-Wallis test followed by the Mann-Whitney test for multiple group comparison. Similar conclusions were achieved by ANOVA with the Fisher or Tukey test. Significance was set at P < 0.05.
Results
Severity of Hyperglycemia
Rats in poor glycemic control had significantly higher GHb values and lower body weights than did their age-matched normal rats (GHb 11.2% ± 1.8% vs. 5.3% ± 2.1%, body weight 310 ± 77g vs. 464 ± 61g, respectively; P < 0.05). The rats maintained in GC had GHb values and body weights (5.5% and 420 ± 34 g) similar to those of normal rats. In the Rev group, during the 6 months of the PC period, GHb and body weight were similar to those in the PC group, and during the following 6 months of the GC period, the values became similar to those of the normal or GC group.
Rat Retina
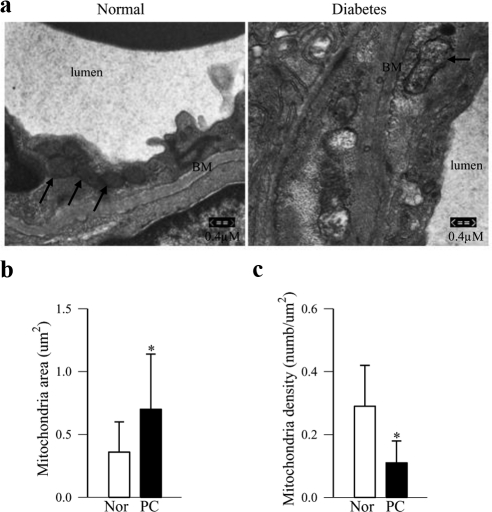
Electron microscopy revealed heterogeneous mitochondria in the retinal endothelium region with diverse size and morphology. Overall, >30% mitochondria in the retinal endothelial cell region in the PC rats were larger with an electron-lucent matrix, and their cristae showed partial cristolysis compared with the intact and tightly packed lamellar cristae in the mitochondria from normal rat retina (Figs. 1a, 1b). However, the density of the mitochondria per square millimeter of cytosol was decreased by twofold in the retinal endothelium of the PC rats, compared with that in the normal animals (Fig. 1c).
Figure 1.
Morphology of the mitochondria in retinal microvasculature by TEM. (a) Sections prepared from the area near the retinal microvasculature were stained with uranyl acetate and lead citrate and viewed by TEM. Eight or more images/sections were recorded at 20,000× magnification. The photomicrograph is representative of three to four rat retinas per group; arrows: endothelial cell mitochondria. BM, basement membrane. Average mitochondrial (b) area, and (c) mitochondrial density in retinal endothelial cells were analyzed using Image J software (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/index.html). The values are represented as the mean ± SD of 20 or more mitochondria per group. *P < 0.05 compared to values obtained from normal rats.
Among 84 genes related with mitochondrial biogenesis and function in the mitochondrial PCR array, diabetes downregulated 12 genes in the retina. Genes from the fusion–fission family Mfn2 and Fis1; the import proteins Tomm34, Tomm40, Timm17b, and Timm44; the chaperon protein Hsp60; the small molecule transport and import/fission proteins Slc25a21 and LC691853; the membrane polarization and potential proteins Ucp2 and Gclc; and the apoptotic protein Akt1 were significantly downregulated in the retinas from PC rats compared with the values obtained from age-matched normal rats (Table 2). In the same diabetic animals, six retinal genes (fission protein Dnm1l, transport proteins Timm9 and Timm10, small molecule transport protein Slc25a12, and apoptotic proteins Bbc3 and Sh3hlb1) were upregulated by 1.6- to 2.7-fold (Table 3).
Table 2.
Effect of Glycemic Control on Downregulation of Retinal Genes
| Gene Symbol | Description | Fold Decrease |
|||
|---|---|---|---|---|---|
| PC | Rev | GC | |||
| Fusion-fission | Mfn2 | Mitofusin 2 | 1.67 ± 0.20* | 1.75 ± 0.49*† | 1.05 ± 0.22 |
| Fis1 | Fission 1 | 1.52 ± 0.12* | 1.68 ± 0.21*† | 0.96 ± 0.24 | |
| Outer membrane translocation | Tomm34 | TOM 34 | 1.38 ± 0.19* | 1.96 ± 0.48*† | 1.10 ± 0.20 |
| Tomm40b | TOM 40b | 1.35 ± 0.08* | 1.82 ± 0.46*† | 0.97 ± 0.13 | |
| Inner membrane translocation | Timm17b | TIM 17b | 1.20 ± 0.04* | 1.37 ± 0.17*† | 0.63 ± 0.15 |
| Timm44 | TIM 44 | 1.63 ± 0.12* | 1.55 ± 0.27*† | 0.79 ± 0.15 | |
| Small molecule transport | Slc25a21 | Mitochondrial oxodicarboxylate carrier | 1.54 ± 0.41* | 1.81 ± 0.60*† | 0.99 ± 0.37 |
| Mitochondrial transport/membrane polarization and potential | Ucp2 | Uncoupling protein 2 (mitochondrial, proton carrier) | 1.98 ± 0.27* | 1.57 ± 0.27*† | 1.12 ± 0.41 |
| Mitochondrial transport/mitochondrion protein import | Hspd1 (Hsp60) | Heat shock protein 60 | 1.69 ± 0.49* | 2.21 ± 0.68*† | 1.10 ± 0.22 |
| Apoptotic genes | Akt1 | V-akt murine thymoma viral oncogene homolog 1 | 1.56 ± 0.11* | 1.43 ± 0.09*† | 0.86 ± 0.07 |
| Membrane polarization and potential | Gclc | Glutamate-cysteine ligase catalytic subunit | 1.49 ± 0.15* | 1.67 ± 0.21*† | 1.08 ± 0.18 |
| Mitochondrial protein import/fission and fusion | LOC691853 (Cox10) | COX10 homolog, cytochrome c oxidase assembly protein | 1.64 ± 0.43* | 2.16 ± 0.87*† | 0.97 ± 0.28 |
The values are represented mean ± SD from five to six rats in each group, and the values obtained from normal rats are set as 1. PC, poor glycemic control for 12 months; Rev, poor glycemic control for 6 months followed by good glycemic control for 6 additional months; GC, good glycemic control for 12 months.
P < 0.05 compared to values from the normal group.
P > 0.05 compared to values from the PC group.
Table 3.
Effect of Glycemic Control on Upregulation of Retinal Genes
| Gene Symbol | Description | Fold Increase |
|||
|---|---|---|---|---|---|
| PC | Rev | GC | |||
| Fission and fusion | Dnm1l | Dynamin 1-like | 2.02 ± 0.20* | 2.08 ± 0.53*† | 0.84 ± 0.3 |
| Inner membrane translocation | Timm10 | TIM10 | 1.95 ± 0.45* | 1.30 ± 0.14*† | 1.18 ± 0.38 |
| Timm9 | TIM9 | 1.78 ± 0.49* | 1.23 ± 0.02*† | 1.17 ± 0.38 | |
| Small molecule transport | Slc25a12 | Mitochondrial aspartate/glutamate member 12 | 1.76 ± 0.36* | 1.69 ± 0.37*† | 1.14 ± 0.06 |
| Apoptotic genes | Bbc3 | Bcl-2 binding component 3 (puma) | 2.67 ± 0.56* | 2.77 ± 1.25*† | 1.68 ± 0.38 |
| Sh3glb1 | SH3-domain GRB2-like endophilin B1 (bif, interacts with Bax) | 1.63 ± 0.38* | 1.48 ± 0.27*† | 1.06 ± 0.47 | |
P < 0.05 and compared to values from normal groups.
P > 0.05 compared to values from PC groups.
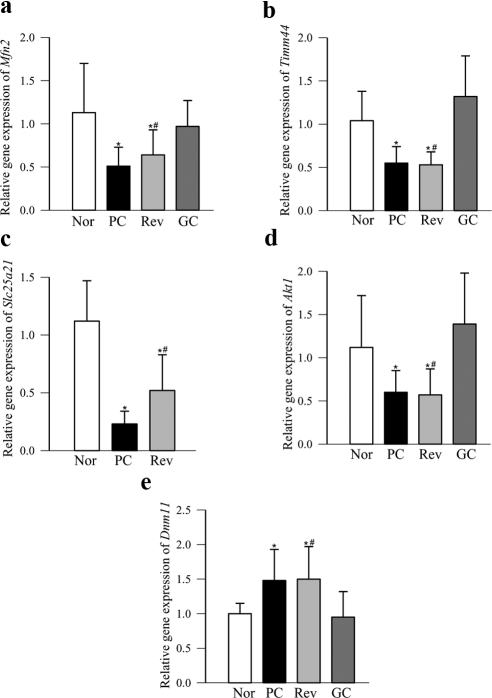
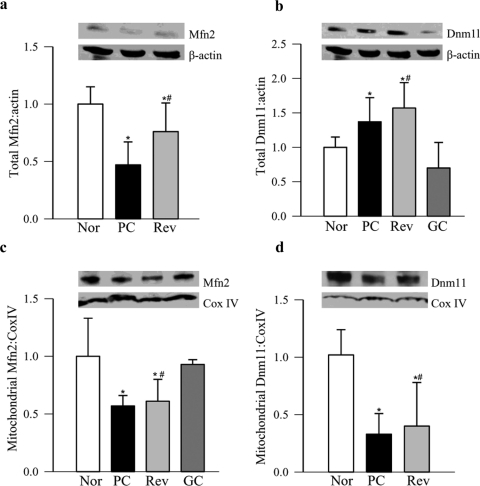
Real-time PCR confirmed a diabetes-induced decrease in retinal Mfn2, Timm44, Slc25a21 and Akt1, and an increase in Dnm1l transcripts (Fig. 2). Figures 3a and 3b clearly show that protein expression of Mfn2 decreased and that of Dnm1l increased by ∼30% in the retinas obtained from diabetic rats. To determine whether these proteins are transported to the mitochondria, we quantified their expressions in the retinal mitochondria. As expected, mitochondrial expression of Mfn2 also decreased by ∼40%, but surprisingly, in the same animals, Dnm1l decreased significantly (Figs. 3c, 3d).
Figure 2.
Validation of gene expression by real-time PCR. Expression of representative genes (a, Mfn2; b, Timm44; c, Slc25a21; d, Akt1; e, Dnm1l) from the mitochondrial PCR array was performed by quantifying their expression with commercial primers. The expression of each gene was normalized with that of β-actin by the ΔΔCt method, and the fold change was calculated compared with the values obtained from normal rats (set at 1). The relative gene expression in five to six rats in each group is represented as the mean ± SD. *P < 0.05 and †P > 0.05 compared with values from normal rat retina or PC rat retina, respectively. Nor, normal; PC, poor glycemic control for 12 months; Rev, poor glycemic control for 6 months followed by good glycemic control for 6 additional months; GC, good glycemic control for 12 months.
Figure 3.
Protein expression in the retina and their mitochondria. Total protein expressions of (a) Mfn2 and (b) Dnm1l were determined in the retinal homogenate by Western blot analysis, with β-actin as the loading control. Expression of (c) Mfn2 and (d) Dnm1l was quantified in the mitochondrial fraction using CoxIV as the loading control. The Western blots are representative of four or more rats in each group. The accompanying histograms represent the expression of Mfn2 or Dnm1l adjusted to the loading controls (β-actin for total and CoxIV for mitochondria).
Reinstitution of good glycemic control after 6 months of poor glycemic control (Rev group) failed to provide any benefit to the 12 genes that were downregulated in diabetes and six genes that were upregulated (Tables 2, 3). PCR and measures of protein expressions in retinal homogenate confirmed that result, and the values obtained from the rats in the Rev group were not different from those in the rats in the PC group (Figs. 2, 3). However, when good glycemic control was initiated soon after induction of diabetes, diabetes-induced alterations (downregulation or upregulation) in the gene transcripts were prevented. The values obtained from the rats in the GC group were significantly different from those in the rats in the PC or Rev groups (Tables 2, 3; Figs. 2, 3), thus suggesting that the effects of GC on the alterations in retinal fusion–fission and import/transport/carrier proteins are not influenced by the high insulin dose administered to maintain good glycemic control and thus support the importance of early and sustained good glycemic control to prevent further progression of diabetic retinopathy.
Diabetic Donors
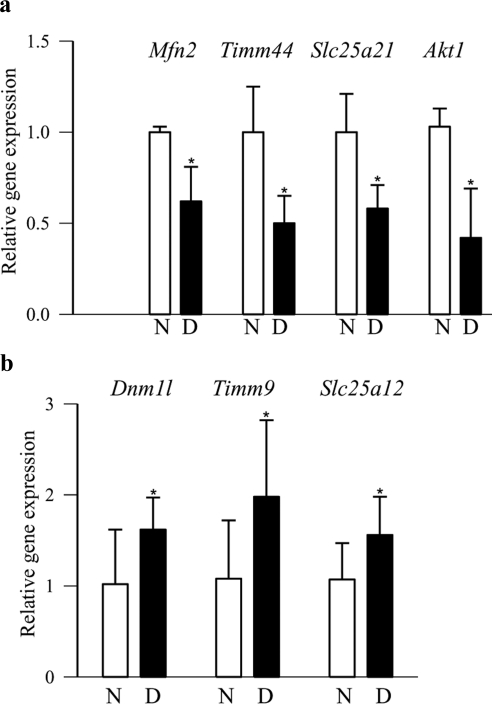
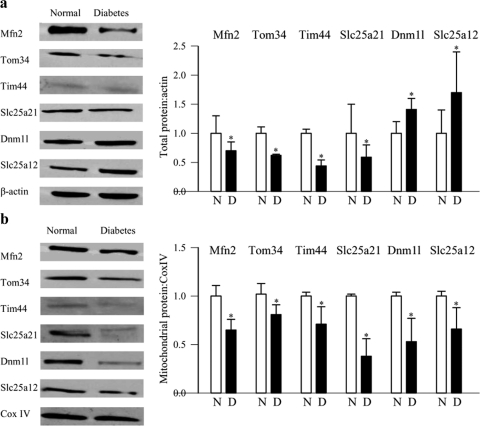
As with diabetic rodents, gene transcripts of the fusion protein Mfn2, the transport proteins Timm44 and Slc25a21, and the apoptotic protein Akt1 were significantly downregulated in the retinas obtained from donors with diabetic retinopathy compared with their age-matched nondiabetic donors (Fig. 4a). Similarly, gene transcripts of the fission protein Dnm1l and the transport proteins Timm9 and Slc25a12 were upregulated by 1.5- to 2-fold (Fig. 4b). Western blot results confirmed the downregulation of Mfn2, Tom34, Tim44, and Slc25a21 and also the upregulation of Dnm1l and Slc25a12 (Fig. 5a). Despite the increased expression of Dnm1l and Slc25a12, their accumulation in the retinal mitochondria was significantly decreased in the donors with diabetic retinopathy (Fig. 5b), and this result was inconsistent with those obtained from rats maintained in PC for 12 months, a duration in which early signs of retinopathy can be seen.
Figure 4.
Expressions of representative genes in human donors with diabetic retinopathy. Expressions of a group of representative (a) downregulated (Mfn2, Timm44, Slc25a21, and Akt1) and (b) upregulated (Dnm1l, Timm9, and Slc25a12) genes were quantified in the retina from donors with diabetic retinopathy and age-matched nondiabetic donors by real-time PCR with 18S used as the internal control. The values are represented as the mean ± SD from five nondiabetic donors and seven diabetic donors. *P < 0.05, compared with nondiabetic donors. N, nondiabetic donors; D, donors with diabetic retinopathy.
Figure 5.
Retinal protein expression in the donors with diabetic retinopathy. Expression of Mfn2, Tom34, Tim44, Slc25a21, Dnm1l, and Slc25a12 was determined in the retinal (a) homogenate and (b) mitochondria by Western blot technique using β-actin and CoxIV as loading controls for homogenate and mitochondria samples, respectively. Western blot analyses are representative of five to seven donors in each group, and the accompanying histograms represent expression of the proteins adjusted to the loading controls (β-actin for total and CoxIV for mitochondria). *P < 0.05 compared with the values obtained from nondiabetic donors.
Discussion
Mitochondria are very dynamic organelles, and their function depends on the normal fusion–fission process. This is the first report to show that the mitochondrial ultrastructure in the retinal microvasculature is damaged in diabetes; the mitochondria are heterogeneous, and approximately 30% of them are enlarged with partial cristolysis. Furthermore, diabetes has a significant effect on the regulators and mediators of mitochondrial fusion–fission and molecular transport complexes in the retina. The important fission protein Fis1, the fusion protein Mfn2, the small molecule transport protein Slc25a21, the mitochondrial import/chaperon Hsp60, and the apoptotic Akt1 were downregulated. In contrast, genes for fission protein Dnm1l, transport proteins Timm9 and Timm10, the small molecule transport protein Slc25a12, and the apoptotic proteins Bbc3 and Sh3hlb1 were upregulated. Despite the upregulation of gene expressions, their mitochondrial abundance remained subnormal, and these abnormalities continued even after termination of hyperglycemic insult. Similar patterns were observed in the retina from donors with diabetic retinopathy, thus suggesting the role of mitochondria fusion–fission and transport machinery in the development of diabetic retinopathy.
Mitochondria are double-membrane organelles with the inner membrane characterized by numerous convolution-cristae and proapoptotic stimuli remodel cristae. Mitochondrial morphology is crucial for cell physiology, and changes in mitochondrial shape have been linked to cell death. Some of the classic hallmarks of mitochondrial permeabilization include swollen and electron-opaque mitochondria with loss of cristae.16,17 In our study, mitochondria in the retinal microvasculature region were enlarged, and their cristae structure was altered, suggesting a role in the apoptotic phenomenon. In support of that notion, diabetes has been shown to increase mitochondria permeability and induce apoptosis of retinal capillary cells, a phenomenon that is followed by the histopathology characteristic of diabetic retinopathy.1,18–20
The mitochondrial fusion–fission process is important in constantly changing energy demands of the cell. Their shape is regulated by balance between fusion and fission, and function is controlled by multiple proteins that mediate remodeling of the outer and inner mitochondrial membranes. Fusion allows the spreading of metabolites, protein, and DNA throughout the network and protects cells from the toxic effects of damaged mitochondrial DNA by allowing functional complementation between two adjacent mitochondria.21 For maintaining mitochondrial morphology and metabolism, Mfn2 is considered crucial, and deficiency is lethal.22 In contrast, inactivation of fusion induces the loss of mtDNA, and mitochondria fission is related to autophagy and Bax-mediated apoptosis.7,8 Fission proteins Fis1 and Dnm1l are the core components of the mitochondrial fission machinery. Excessive mitochondrial fission, induced by oxidative and nitrosative stress, serves as an important and early event in neurodegenerative diseases.23 In our study, diabetes decreased the gene expression of Mfn2 in the retina. Our data, however, show a dichotomy: Diabetes also decreased the expression of Fis1 but increased the expression of Dnm1l. Although Fis1 is located throughout the outer membrane, Dnm1l is in the cytosol and is recruited at the point of fission.24 Others have shown that Fis1 does not directly activate Bax, but it can induce calcium-dependent mitochondrial dysfunction, and this bifunctional protein can independently regulate mitochondrial fragmentation.25 The assembly of Dnm1l is necessary for fission activity, and apoptotic stimulation recruits it to the mitochondrial outer membrane, where it is co-localized with Bax and Mfn2 at the fission site.26 The recruitment of Dnm1l to mitochondria could be facilitated in a mitochondrial fission factor (Mff)–dependent manner and mitochondrial-dynamic proteins of 49 and 51 kDa (MiD49/51), anchored in the mitochondrial outer membrane, help directly recruit Dnm1l to the mitochondrial surface. However, recruitment could also be facilitated in an Mff/Mfn2-independent manner by interacting with MiD51, which could act both as an inhibitor of fission and a promoter of fusion.27–30 The role of these proteins in regulating Dnm1l in the pathogenesis of diabetic retinopathy remains unclear. Our results clearly suggest that the fusion–fission machinery of the retinal mitochondria is severely dysfunctional in diabetes, and this factor may contribute to the alterations in the ultrastructure of the mitochondria and also to the decreased number of mitochondria. In support of this conclusion, others have shown increased Dnm1l in neuron and endothelial cells with fragmented mitochondria in diabetic rodents,31,32 and our recent study has documented that the mitochondria copy number is significantly decreased in the retinas of rats that are diabetic for 12 months.33
The translocase complexes TOM and TIM help in the import of most of the proteins into the mitochondria, and they cooperate to achieve efficient transport of proteins to the matrix or into the inner membrane. As stated above, these complexes have multiple membrane protein subunits. The TOM complex is distributed throughout the outer membrane: Tom 40 is a pore-forming protein, and Tom70, Tom20, and Tom22 serve as the receptor protein. Tom34 is the ancillary component of the TOM and is found both in cytosol and in the mitochondrial membrane. However, TIM complexes differ in their substrate specificity, Tim23 mediates mainly the import of preproteins with a positively charged matrix-targeting signal, Tim22 facilitates the insertion of hydrophobic proteins to the inner membrane, and Tim44 tethers mitochondrial matrix Hsp70 to the translocon.9 We showed that the transcripts of Tomm34, Tomm40b, Timm44, and Timm17b (part of Tim23 complex) were downregulated. The transport protein Tim44 is shown to facilitate the import of superoxide dismutase and glutathione peroxidase, the enzymes that are subnormal in the retina in diabetes,34,35into the mitochondria.12 In contrast, transcripts of Timm10 and Timm9, the two components of the mitochondrial intermembrane space assembly, were upregulated. Their upregulation fails to compensate for the decreased transport functions experienced by the retina in diabetes. This is in agreement with our recent work demonstrating that, despite increased transcripts of mtDNA repair enzymes, these nuclear-encoded proteins do not reach the mitochondria efficiently.3
The data presented here show that the small molecule transport Slc25a21 is decreased in the retina in diabetes. This protein is involved in the uptake of 2-oxoadipate into the mitochondrial matrix, with 2-oxoglutarate released from the mitochondrial matrix into the cytosol. It is used not only to transport metabolites, but also as a potential carrier for glutathione (GSH).36,37 Consistent with decreased Slc25a21, GSH levels in retinal mitochondria are subnormal in diabetes.2,3 On the other hand, another small molecule transport protein, Slc25a12, essential for exchange of glutamate and aspartate between cytosol and mitochondria,38 is upregulated, and its mitochondrial abundance is decreased, suggesting that insertion of slc25a12 into the inner mitochondria and the transport of TCA cycle substrates to the mitochondria are both subnormal in diabetes. As shown in Table 2, diabetes also downregulates the membrane polarizing proteins Ucp2 and Gclc, this is consistent with the impaired mitochondrial membrane potential seen in the pathogenesis of diabetic retinopathy and is supported by the report showing a glucose-induced decrease in the Gclc gene in mouse endothelial cells.39 In the pathogenesis of diabetic retinopathy mitochondria-mediated apoptosis of retinal capillary cells is considered to precede the development of diabetic retinopathy.18,40 Here, the results show that the apoptotic factor Sh3glb1, which interacts with Bax in cytosol and facilitates its translocation to the mitochondria,41 was upregulated in the retina in diabetes. In addition, Bbc3, which induces apoptosis and helps recruit Dnm1l to the mitochondria,42 also increased, and Akt1, an antiapoptotic factor, decreased. In support of these results, diabetes increased the translocation of Bax to the mitochondria in the retina and its capillary cells, initiating the apoptotic machinery.18,43
In our study, the alteration in the fusion and fission, transport machinery, and apoptosis persisted for at least 6 months after reversal of hyperglycemia, suggesting that the reversal does not provide any benefit to the mitochondria transport and biogenesis. This finding is consistent with that in our previous studies showing continued increased retinal mtDNA damage and accelerated apoptosis after termination of the hyperglycemic insult.3,40 However, when good glycemic control is initiated immediately after induction of diabetes (GC group), the gene profile remains similar to those in normal rats. This further strengthens the importance of early good glycemic control in patients and suggests that the persistent gene changes observed after the reversal of hyperglycemia (Rev group) were not influenced by the high amount of insulin administered to maintain the desired glycemic control.
In summary, we demonstrate that mitochondria ultrastructure, fusion-fission and protein import machinery are severely affected in the retina in diabetes. This contributes to dysfunctional mitochondria, and accelerates apoptosis, ultimately leading to the development of diabetic retinopathy. Similar impairments in the retina from donors with diabetic retinopathy strongly implicate them in the development of diabetic retinopathy. Reversal of hyperglycemia in rats failed to correct these alterations, implying that such alterations in the mitochondrial fusion–fission and transport proteins are persistent and have an important role in the metabolic memory phenomenon associated with the continued progression of diabetic retinopathy. Future molecular strategy targeting the fusion–fission system and the protein import machinery may be promising for halting the development of diabetic retinopathy.
Acknowledgments
The authors thank James Hatfield (VA Medical Center, Detroit, MI) for help with the electron microscopy and Doug Putt and Yakov Shamailov for technical assistance.
Footnotes
Supported in part by grants from the National Institutes of Health, Juvenile Diabetes Research Foundation, Thomas Foundation, and Research to Prevent Blindness.
Disclosure: Q. Zhong, None; R.A. Kowluru, None
References
- 1. Kern TS, Tang J, Mizutani M, et al. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978 [PubMed] [Google Scholar]
- 2. Madsen-Bouterse S, Zhong Q, Mohammad G, Ho YS, Kowluru RA. Oxidative damage of mitochondrial DNA in diabetes, and its protection by manganese superoxide dismutase. Free Radic Res. 2010;44:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madsen-Bouterse SA, Mohammad G, Kanwar M, Kowluru RA. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antioxid Redox Signal. 2010;13:797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins TJ, Bootmann MD. Mitochondria are morphologically heterogeneous within cells. J Exp Biol. 2003;206:1993–2000 [DOI] [PubMed] [Google Scholar]
- 5. Benard G, Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal. 2008;10:1313–1342 [DOI] [PubMed] [Google Scholar]
- 6. McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–R560 [DOI] [PubMed] [Google Scholar]
- 7. Cassidy-Stone A, Chipuk JE, Ingerman E, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanaka A, Youle RJ. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol Cell. 2008;29:409–410 [DOI] [PubMed] [Google Scholar]
- 9. Schmidt O, Pfanner N, Meisinger C. Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol. 2010;11:655–667 [DOI] [PubMed] [Google Scholar]
- 10. Mukhopadhyay A, Avramova LV, Weiner H. Tom34 unlike Tom20 does not interact with the leader sequences of mitochondrial precursor proteins. Arch Biochem Biophys. 2002;400:97–104 [DOI] [PubMed] [Google Scholar]
- 11. Yang CS, Weiner H. Yeast two-hybrid screening identifies binding partners of human Tom34 that have ATPase activity and form a complex with Tom34 in the cytosol. Arch Biochem Biophys. 2002;400:105–110 [DOI] [PubMed] [Google Scholar]
- 12. Matsuoka T, Wada J, Hashimoto I, et al. Gene delivery of Tim44 reduces mitochondrial superoxide production and ameliorates neointimal proliferation of injured carotid artery in diabetic rats. Diabetes. 2005;54:2882–2890 [DOI] [PubMed] [Google Scholar]
- 13. Kowluru RA. Effect of re-institution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52:818–823 [DOI] [PubMed] [Google Scholar]
- 14. Zhong Q, Kowluru RA. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertoni-Freddari C, Fattoretti P, Giorgetti B, et al. Preservation of mitochondrial volume homeostasis at the early stages of age-related synaptic deterioration. Ann N Y Acad Sci. 2007;1096:138–146 [DOI] [PubMed] [Google Scholar]
- 16. Knels L, Worm M, Wendel M, et al. Effects of advanced glycation end products-inductor glyoxal and hydrogen peroxide as oxidative stress factors on rat retinal organ cultures and neuroprotection by UK-14304. J Neurochem. 2008;106:1876–1887 [DOI] [PubMed] [Google Scholar]
- 17. Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11:678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–5334 [DOI] [PubMed] [Google Scholar]
- 19. Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47:1594–1599 [DOI] [PubMed] [Google Scholar]
- 20. Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811 [DOI] [PubMed] [Google Scholar]
- 21. Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;11:870–879 [DOI] [PubMed] [Google Scholar]
- 22. Zheng M, Xiao RP. Role of mitofusin 2 in cardiovascular oxidative injury. J Mol Med. 2010;88:987–991 [DOI] [PubMed] [Google Scholar]
- 23. Knott AB, Bossy-Wetzel E. Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann N Y Acad Sci. 2008;1147:283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ding H, Jiang N, Liu H, et al. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800:250–256 [DOI] [PubMed] [Google Scholar]
- 25. Scorrano L, James D, Huber D, et al. The mitochondrial fission protein hFis1 requires the endoplasmic reticulum gateway to induce apoptosis. Mol Biol Cell. 2006;17:4593–4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karbowski M, Lee YJ, Gaume B, et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Otera H, Wang C, Cleland MM, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao J, Liu T, Jin S, et al. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J. 2011;30:2762–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schuldt A. Membrane dynamics: MIEF1 mingles with mitochondria. Nat Rev Mol Cell Biol. 2011;22:464–465 [DOI] [PubMed] [Google Scholar]
- 31. Edwards JL, Quattrini A, Lentz SI, et al. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;160:160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia. 2010;53:1783–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Santos JM, Tewari S, Goldberg AFX, Kowluru RA. Mitochondria biogenesis and the development of diabetic retinopathy. Free Radic Biol Med. 2011;51:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kowluru RA, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or experimental galactosemia, IV: antioxidant defense system. Free Radic Biol Med. 1997;22:587–592 [DOI] [PubMed] [Google Scholar]
- 35. Obrosova IG, Fathallah L, Greene DA. Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: effect of DL-alpha-lipoic acid. Eur J Pharmacol. 2000;398:139–146 [DOI] [PubMed] [Google Scholar]
- 36. Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fiermonte G, Dolce V, Palmieri L, et al. Identification of the human mitochondrial oxodicarboxylate carrier: bacterial expression, reconstitution, functional characterization, tissue distribution, and chromosomal location. J Biol Chem. 2001;276:8225–8230 [DOI] [PubMed] [Google Scholar]
- 38. Satrústegui J, Contreras L, Ramos M, et al. Role of aralar, the mitochondrial transporter of aspartate-glutamate, in brain N-acetylaspartate formation and Ca(2+) signaling in neuronal mitochondria. J Neurosci Res. 2007;85:3359–3366 [DOI] [PubMed] [Google Scholar]
- 39. Urata Y, Yamamoto H, Goto S, et al. Long exposure to high glucose concentration impairs the responsive expression of gamma-glutamylcysteine synthetase by interleukin-1beta and tumor necrosis factor-alpha in mouse endothelial cells. J Biol Chem. 1996;271:15146–15152 [DOI] [PubMed] [Google Scholar]
- 40. Kowluru RA, Chan PS. Metabolic memory in diabetes-from in vitro oddity to in vivo problem: role of apoptosis. Brain Res Bull. 2010;87:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cuddeback SM, Yamaguchi H, Komatsu K, et al. Molecular cloning and characterization of Bif-1: a novel Src homology 3 domain-containing protein that associates with Bax. J Biol Chem. 2001;276:20559–20565 [DOI] [PubMed] [Google Scholar]
- 42. Wang JX, Li Q, Li PF. Apoptosis repressor with caspase recruitment domain contributes to chemotherapy resistance by abolishing mitochondrial fission mediated by dynamin-related protein-1. Cancer Res. 2009;69:492–500 [DOI] [PubMed] [Google Scholar]
- 43. Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7:1581–1587 [DOI] [PubMed] [Google Scholar]