Abstract
Purpose
Heat shock protein-90 (HSP-90), a molecular chaperone required by numerous oncogenic kinases (e.g. HER-2, EGFR, Raf-1, v-Src, AKT) for conformational stability, has attracted wide interest as a novel target for cancer therapy. HSP-90 inhibition induces degradation of HSP-90 client proteins, leading to a combinatorial inhibition of multiple oncogenic signaling pathways with consecutive growth arrest and apoptosis. MET, a tyrosine kinase which is constitutively active in tumor cells with MET oncogene amplification, has recently been identified as another HSP-90 client.
Experimental Design
Aim of our study was to assess the efficacy of SNX-2112, a synthetic HSP-90 inhibitor, in 3 different MET-amplified tumor cell lines (GTL-16, MKN-45 and EBC-1) as well as PR-GTL-16 cells, a GTL-16 subline selected for resistance to the highly selective MET kinase inhibitor PHA-665752.
Results
In all cell lines, SNX-2112 led to degradation of MET, HER-2, EGFR and AKT as well as abrogation of Ras/Raf/MEK/MAPK and PI3K/AKT signaling, followed by complete cell cycle arrest. SNX-5542, an orally bioavailable prodrug of SNX-2112, displayed significant antitumor efficacy in vivo in nude mice bearing MET-amplified tumor xenografts. Importantly, HSP-90 inhibition maintained its antitumor efficacy in PR-GTL-16 cells both in vitro and in vivo, suggesting that HSP-90 inhibition could be a particularly valuable strategy in MET-amplified tumors which have acquired resistance to MET kinase inhibition.
Conclusions
Our study provides evidence for the efficacy of HSP-90 inhibition in MET-amplified cancer cells, particularly when MET kinase inhibitor resistance has emerged.
Keywords: MET, HSP-90 inhibition, tyrosine kinase inhibition, resistance
INTRODUCTION
The MET proto-oncogene encodes a heterodimeric receptor tyrosine kinase that consists of an extracellular α-chain and a transmembrane β-chain (1, 2). Hepatocyte growth factor binds to MET with high affinity and induces receptor dimerization with consecutive triggering of MET tyrosine kinase activity (3, 4). MET downstream signaling includes the phosphoinositide-3-kinase (PI3K)/AKT, Ras/Raf/MEK/mitogen-activated protein kinase (MAPK) and phospholipase C-γ pathways (3).
In tumors where MET is overexpressed due to genomic amplification, MET becomes constitutively active, rendering the malignant cells highly dependent on MET signaling for proliferation and survival (“MET oncogene addiction”) (5, 6). Several selective small molecule MET inhibitors have been developed and have shown promising antitumor efficacy against MET-amplified tumor cells both in vitro as well as in mouse models (7–9). However, as with other highly selective kinase inhibitors, acquired resistance may develop, which prompted us to investigate possible therapeutic alternatives.
A large number of oncogenic kinases (e.g. HER-2, EGFR, v-Src, Raf1, cyclin dependent kinase-4 and AKT) require heat shock protein-90 (HSP-90) for conformational stability (10–17). Given the critical roles played by these HSP-90 clients in tumor cell signaling, proliferation and survival, inhibition of HSP-90 has emerged as a potent antitumor treatment strategy (18). The underlying mechanism involves proteasomal degradation of HSP-90 client proteins leading to disruption of the tumor cell signaling network with consecutive cell cycle arrest and apoptosis.
Previous studies have shown that multiple HSP-90 clients are activated in MET oncogene-addicted cancer cells either through MET-dependent downstream signaling or receptor cross-talk (e.g. Raf1, AKT, EGFR) (5–7, 19). Furthermore, MET itself has recently been implicated as an HSP-90 client (20–24). We therefore hypothesized that HSP-90 inhibition could be a particularly promising treatment strategy in MET-amplified cancer cells. Moreover, due to its combined effect on multiple signal transduction pathways, we hypothesized that HSP-90 inhibition could also overcome acquired resistance to small molecule MET inhibition in these malignancies. In the present study, we have tested the effects of SNX-2112, a novel synthetic HSP-90 inhibitor (25–27), in 3 different tumor cell lines with MET amplification (EBC-1 [non small-cell lung cancer], GTL-16 [gastric cancer], MKN-45 [gastric cancer]) as well as PR-GTL-16 cells which we selected for acquired resistance to PHA-665752, a highly selective MET kinase inhibitor. In all cells, degradation of MET was observed together with degradation of the HSP-90 clients HER-2, EGFR and AKT. MET degradation was paralleled by loss of MET phosphorylation, abrogation of downstream PI3K/AKT and Ras/Raf/MEK/MAPK signaling as well as by cell cycle arrest. HSP-90 inhibition using SNX-5542, an orally bioavailable prodrug of SNX-2112, also displayed significant antitumor activity in vivo in nude mice bearing MET-amplified xenografts with minimal toxicity. Importantly, HSP-90 inhibition maintained its in vitro and in vivo antitumor efficacy in PR-GTL-16 tumor cells with acquired resistance to PHA-665752, providing a strong rationale for the use of HSP-90 inhibition in MET-amplified tumors that have become resistant to selective MET kinase inhibition.
MATERIALS AND METHODS
Cell lines
Human GTL-16 gastric cancer cells were a gift from Dr. Silvia Giordano (Institute for Cancer Research and Treatment, Torino School of Medicine, Italy). MKN-45 gastric cancer cells were obtained from the RIKEN BRC Cell Bank (RIKEN BioResource Center, Ibaraki, Japan). EBC-1 non small-cell lung cancer cells were from the Health Science Research Resources Bank (Japan Health Sciences Foundation, Tokyo, Japan). NCI-H820 cells were obtained from the American Type Culture Collection. GTL-16 and PR-GTL-16 cells were grown in Dulbecco's Modified Eagle's Medium (DMEM), MKN-45 and NCI-H820 cells were grown in RPMI-1640, and EBC-1 cells were grown in Eagle's Minimal Essential Medium + 2mM L-glutamine + 1mM sodium pyruvate + 0.1 mM non essential amino acids. All media were supplemented with 10% FCS and maintained at 37°C in a humidified atmosphere containing 5% CO2.
Chemicals
PHA-665752, PD173074 and PD330631 were provided by Pfizer Global Research and Development (La Jolla, CA). Gefitinib (ZD-1839; Iressa) was obtained from AstraZeneca Pharmaceuticals (Wilmington, DE). Recombinant human fibroblast growth factor-3 (FGF-3) was purchased from R&D Systems (Minneapolis, MN). SNX-2112 (for chemical structure see Supplementary Figure 1) and SNX-5542 were obtained from Serenex, Inc. (Durham, NC). SNX-2112 was dissolved in DMSO for in vitro studies, whereas SNX-5542 was formulated in 5% dextrose in water for in vivo studies.
Western Blot
After removal of growth medium, tissue culture flasks were placed on ice and washed twice with ice-cold Tris-buffered saline (TBS). Cells were scraped off the culture flasks, centrifuged and placed in ice-cold Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA) containing protease and phosphatase inhibitors (Halt™ Protease and Phosphatase Inhibitor Cocktails [Pierce, Rockford, IL]). After shaking for 15 min. at 4°C, the lysates were centrifuged at 20000g for 15 min. and stored at minus 70°C until further use. For Western Blotting, equal amounts of protein (50 μg) were boiled in Laemmli buffer for 5 min., resolved by 10% SDS-polyacrylamide gel electrophoresis (Invitrogen, Carlsbad, CA) and electrophoretically transferred onto a polyvinylidene difluoride membrane (BioRad, Hercules, CA). After blocking nonspecific binding sites with 5% nonfat dry milk in TBS + 0.05% Tween 20 (TBS-T), the membrane was incubated with the respective primary antibodies. After 3 washes with TBS-T, the membrane was incubated for 1 h at room temperature with a horseradish peroxidase-linked secondary antibody, followed by several washes with TBS-T. The immunocomplexes were visualized using the ECL Plus detection system (GE Healthcare, Uppsala, Sweden).
Antibodies
Antibodies against MET (C-12), EGFR (1005) and cyclin D1 (M-20) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phospho-MET (Tyr1230/1234/1235), p44/42 MAPK, phospho-p44/42 MAPK (Thr202/Tyr204), AKT, phospho-AKT (Ser473) were from Cell Signaling Technology. Anti-HER-2 antibody (clone e2-4001 + 3B5) was from Lab Vision (Fremont, CA).
Cell proliferation assays
Cellular proliferation was measured using a commercially available 5-bromo-2-deoxyuridine (BrdU) cell proliferation assay (Roche, Nutley, NJ). Briefly, the cells were seeded in triplicate in flat-bottom 96-well plates at 5000 cells/well and allowed to adhere for 48h. Thereafter, the cells were treated for 24h, as indicated. After incubation with BrdU labeling reagent for 2h, the cells were fixed and BrdU incorporation into newly synthesized DNA was assessed by incubation with an anti-BrdU peroxidase-conjugated antibody for 90 min., followed by addition of substrate solution and colorimetric detection at 450 and 690 nm, respectively. IC50 values were calculated by four-parameter curve fitting using SigmaPlot 11.0 software.
Cell cycle analysis
Cells were seeded in 100 mm dishes at a density of 5 × 105 cells per dish. Forty-eight hours later, the cells were treated with drug or vehicle (dimethyl sulfoxide) for 24h. Both adherent and floating cells were harvested and stained with ethidium bromide. Quantitation of the cell cycle distribution was performed by flow cytometric analysis.
Apoptosis
Induction of apoptosis was assessed using the Annexin V FITC Apoptosis Detection Kit from BD Pharmingen (BD Biosciences, San Jose, CA). Briefly, the cells were treated with SNX-2112 for 24 h, stained with Annexin V-FITC + PI at room temperature according to the manufacturer's instructions and finally subjected to flow cytometric analysis.
Creation of PHA-665752-resistant GTL-16 cells in vitro
To generate GTL-16 cells resistant to the MET tyrosine kinase inhibitor PHA-665752, cells were exposed to increasing concentrations of PHA-665752 in vitro. After 6 months, a resistant subclone (PR-GTL-16) was obtained which grew in the presence of up to 2 μM PHA-665752 with similar growth kinetics to untreated GTL-16 cells. After several passages in the absence of PHA-665752, PR-GTL-16 cells retained their resistant phenotype as determined by repeated cell proliferation assays. Parental GTL-16 cells were maintained concomitantly without PHA-665752 and no significant change in the sensitivity to PHA-665752 was noted.
RNA isolation, labeling and microarray analysis
Total RNA was isolated from tissue using the RNeasy Kit (Qiagen, Valencia, CA). The quality of the RNA was ensured before labeling by analyzing 20–50 ng of each sample using the RNA 6000 NanoAssay and the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). Samples with a 28S/18S ribosomal peak ratio of 1.8–2.0 and a RIN number >7.0 were considered suitable for labeling. For samples meeting this standard, 2 μg of total RNA was used for cDNA synthesis using an oligo-dT-T7 primer and the SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). Synthesis, linear amplification, and labeling of cRNA were accomplished by in-vitro transcription using the MessageAmp aRNA Kit (Ambion, Austin, TX) and biotinylated nucleotides (Enzo Diagnostics, Farmingdale, NY). Ten micrograms of labeled and fragmented cRNA were then hybridized to the Human HG-U133A2.0 GeneChip (Affymetrix, Santa Clara, CA) at 45°C for 16 h. After washing, the chips were processed according to the manufacturer's instructions and scanned with a high-numerical aperture and flying objective lens (FOL) in the GS3000 scanner (Affymetrix). The image was quantified using GCOS version 1.4 (GeneChip Operating Software, Affymetrix).
RT-PCR
TaqMan® Gene Expression Assays for MET, FGF-3 and 18s ribosomal RNA were purchased from Applied Biosystems (Foster City, CA). Gene expression was measured using the ABI Prism 7900HT Sequence Detection System from Applied Biosystems. RT-PCR of cDNA specimens was conducted as described previously (28).
siRNA
For siRNA experiments, cells were seeded in triplicate in 96-well plates at 5000 cells/well in antibiotic-free complete medium and were allowed to adhere for 24 h at 37°C. Thereafter, the cells were transfected with siGenome ON-TARGET plus FGF-3 or Non-Silencing Pool #3 siRNA (Dharmacon, Chicago, IL) according to the manufacturer's instructions. Untransfected cells were used for control. After 48 h, cell proliferation was determined using a BrdU cell proliferation assay.
Animal studies
Four- to 6-week-old nu/nu athymic BALB/c mice were obtained from the National Cancer Institute-Frederick Cancer Center and maintained in pressurized ventilated caging at the Sloan-Kettering Institute. All studies were done in compliance with Institutional Animal Care and Use Committee guidelines. Tumors were established by flank injection of 1×107 cells suspended 1:1 (volume) with reconstituted basement membrane (Matrigel, Collaborative Research, Bedford, MA). For efficacy studies, mice (5 per group) with established tumors were selected. Fourteen days after inoculation, mice were treated with SNX-5542 using the indicated doses. Tumor dimensions were measured with vernier calipers and tumor volumes were calculated using the formula π/6 × larger diameter × (smaller diameter)2.
Statistical analysis of xenograft experiments
Because of right-skewed distribution of tumor volume measurements, a logarithmic transformation of the tumor volume was performed. For data depiction, mean and corresponding 68% confidence intervals (CI) were calculated and transformed back to the original scale (which would correspond to mean ± SE for normally distributed data). Group differences in tumor volume for the different cell lines and effects over time were modeled with a linear mixed model with repeated measures. A logistic transformation was applied to the dependent variable tumor volume to achieve homoscedasticity of residuals. Group differences, effect of time and a group time-interaction were modeled and tested. All p-values are two-sided and p≤0.05 was considered significant. Calculations were performed using the statistical software package SAS (r) (SAS Institute Inc., Cary, NC, USA, Version 9.2).
RESULTS
HSP-90 inhibition induces degradation of MET and other HSP-90 client proteins in tumor cells with MET amplification
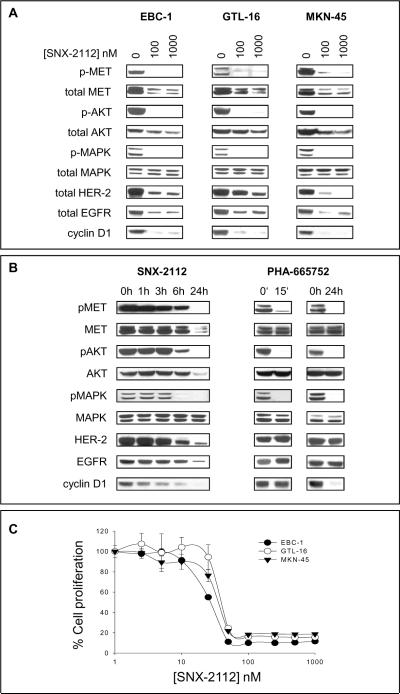
We first assessed the effects of the synthetic HSP-90 inhibitor SNX-2112 on MET expression in 3 different MET-amplified tumor cell lines. As shown in Figure 1A, SNX-2112 induced a dose-dependent degradation of MET in all cell lines, together with degradation of the HSP-90 client proteins HER-2, EGFR and AKT. Degradation of MET was paralleled by complete dephosphorylation of MET, inhibition of downstream MAPK and AKT activation as well as loss of cyclin D1 expression, indicating cell cycle arrest.
Figure 1. HSP-90 inhibition induces degradation of MET and inhibition of proliferation in tumor cells with MET amplification.
(A) Tumor cells were treated with SNX-2112 for 24 h as indicated, followed by Western Blot analysis. (B) Comparative kinetics of HSP-90 and selective MET kinase inhibition in GTL-16 gastric cancer cells. Cells were either treated with 1000 nM SNX-2112 or 400 nM PHA-665752 as indicated, followed by Western Blot analysis. (C) Cells were treated for 24 h as indicated, followed by cell proliferation analysis. Data are depicted as mean ± SE.
SNX-2112-induced dephosphorylation of MET, AKT and MAPK occurs approximately 6 h after initiation of treatment, while selective MET inhibition using the small molecule inhibitor PHA-665752 causes immediate dephosphorylation of MET and downstream signaling pathways
As shown in Figure 1B, dephosphorylation of MET, AKT and MAPK does not occur before 6 h of SNX-2112 treatment, whereas PHA-665752, a highly selective small molecule inhibitor of MET, causes complete dephosphorylation of these proteins as early as 15 minutes after onset of treatment. However, in contrast to the HSP-90 inhibitor, PHA-665752 does not affect the total levels of MET, AKT, HER-2 and EGFR within the cells. After 24 h of treatment, complete loss of cyclin D1 expression is observed both in SNX-2112- and PHA-665752-treated cells (Figure 1B).
Effects of SNX-2112 on cell proliferation of tumor cells with MET amplification
Next, we assessed the effects of SNX-2112 on cell proliferation of MET-amplified cell lines. In all cells, a potent growth-inhibitory effect was observed. IC50 values for the individual cell lines were 25.2 (EBC-1), 30.3 (MKN-45) and 35.6 nmol/l (GTL-16) (Figure 1C).
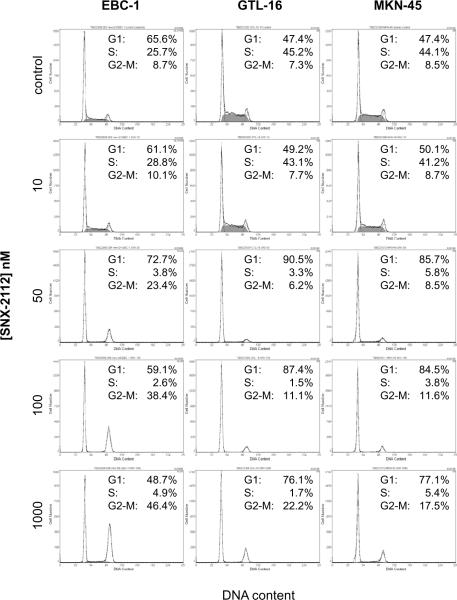
SNX-2112 induces cell cycle arrest in tumor cells with MET amplification
Figure 2 shows the cell cycle data obtained after 24h of SNX-2112 treatment: Compatible with the cell proliferation results, SNX-2112 induced G1-arrest at a concentration of 50 nmol/l in all cell lines, while at higher concentrations (100 and 1000 nmol/l), an increasing proportion of the cells accumulated in G2.
Figure 2. Cell cycle analysis.
Cells were treated for 24 h with increasing doses of SNX-2112, followed by flow cytometric analysis of the cell cycle distribution.
Efficacy of HSP-90 inhibition in a MET-amplified xenograft model
To study the antitumor efficacy of HSP-90 inhibition in MET-amplified tumors in vivo, nude mice bearing xenografts of GTL-16 and EBC-1 tumor cells were treated with increasing doses of SNX-5542, an orally bioavailable prodrug of SNX-2112. As shown in Figure 3, treatment of mice with 50 mg/kg of SNX-5542 using a Monday – Wednesday – Friday schedule significantly inhibited growth of GTL-16 and EBC-1 xenografts as compared with the control group (p<0.0001 and p=0.0003, respectively) and was well tolerated without measurable toxicity. Importantly, increasing the dose of SNX-5542 to 75 mg/kg led to a further significant enhancement of the growth-inhibitory effect with partial regression of GTL-16 xenografts (p<0.0001). However, consistent with a previous report by Chandarlapaty et al., SNX-5542 at the higher dose of 75 mg/kg also induced an average weight loss of 21.3% with 2/5 animal deaths by day 24 (25). Consequently, the maximum SNX-5542 dose was reduced to 60 mg/kg for further experiments, albeit no significant enhancement of the growth-inhibitory effect as compared with the 50 mg/kg was noted (p<0.7115) (Figure 3, lower graph).
Figure 3. Treatment with SNX-5542 induces inhibition of tumor growth in GTL-16 and EBC-1 xenograft models.
Four to 6-week old athymic nu/nu BALB/c mice with established GTL-16 and EBC-1 xenografts (5 per group) were randomized to treatment with SNX-5542 or the control group. Mice were treated with SNX-5542, an orally bioavailable prodrug of SNX-2112, by oral gavage on a Monday – Wednesday – Friday schedule. The tumor volume was measured as described in Materials and Methods. Data are depicted as mean ± 68% CI to account for right-skewed data distribution. This would correspond to a depiction as mean ± SE in the case of normal distribution. P-values are from the regression model. The control arm of the EBC-1 xenograft model had to be discontinued after day 22 because of excessive tumor growth and ulceration of the tumors.
Creation of MET kinase inhibitor-resistant GTL-16 tumor cells
To assess whether HSP-90 inhibition maintains its antitumor efficacy in MET-amplified tumor cells with acquired resistance to a selective MET kinase inhibitor, we created GTL-16 cells resistant to the highly selective MET kinase inhibitor PHA-665752. Cells were cultured in vitro in increasing concentrations of PHA-665752 over a 6-month period, resulting in a resistant cell line (PHA-resistant [PR]-GTL-16) which displayed uninhibited growth and cell cycle distribution even in the presence of 800 nM PHA-665752 (Figures 4A and B).
Figure 4. Characterization of PHA-665752-resistant (PR) GTL-16 tumor cells.
(A) GTL-16 and PR-GTL-16 tumor cells were treated with PHA-665752 as indicated. After 24 h, a BrdU cell proliferation assay was performed. (B) Cells were treated with 400 nM PHA-665752 for 24 h. Thereafter, flow cytometric analysis of the cell cycle distribution was performed. (C) RT-PCR analysis for 18s, MET and FGF-3 RNA of PR-GTL-16 as compared with parental GTL-16 tumor cells. CT = cycle threshold. * = in parental GTL-16 cells, no CT value could be determined, indicating that FGF-3 is not expressed in these cells. Data are depicted as mean ± SE.
PR-GTL-16 cells display acquired expression of fibroblast growth factor-3 (FGF-3) which is not detected in parental GTL-16 cells
Microarray analysis of PR-GTL-16 cells using the HG-U133A2.0 GeneChip revealed that 170 genes were differentially regulated with a fold-change cutoff of plus or minus 2 and a p-value cutoff of p<0.005. Thirty-six of these genes were regulated at least 4-fold. The most highly regulated gene was FGF-3, which displayed a 64-fold increase of expression in PR-GTL-16 as compared with parental GTL-16 cells (Supplementary Table 1), making it the most likely candidate for the resistant phenotype of PR-GTL-16 cells although other changes (e.g. >4-fold upregulation of cyclin D2) should not be overlooked. The microarray data were confirmed by RT-PCR analysis, where FGF-3 mRNA was readily detectable in PR-GTL-16 cells, while it was below the detection limit in parental GTL-16 cells. Importantly, MET mRNA expression did not differ significantly between the cells, indicating that the resistance mechanism of PR-GTL-16 cells does not involve a change of MET expression as compared with the parental line (Figure 4C).
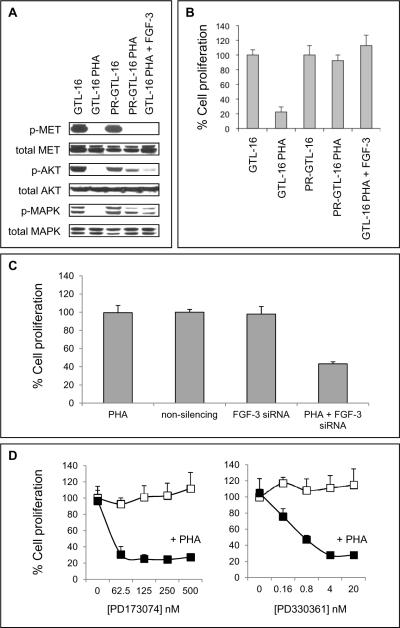
FGF-3 confers resistance to MET inhibition through reactivation of AKT and MAPK signaling
While parental GTL-16 cells displayed complete dephosphorylation of MET, AKT and MAPK following PHA-665752 treatment, MET but not AKT and MAPK were dephosphorylated in PR-GTL-16 cells (Figure 5A). Based on the microarray and RT-PCR results, we hypothesized that autocrine FGF-3 signaling could be responsible for sustained AKT and MAPK activation in PR-GTL-16 cells. This was supported by the observation that exogenous FGF-3 led to rephosphorylation of AKT and MAPK in parental GTL-16 cells and was able to rescue them from PHA-665752-mediated growth inhibition (Figures 5A and 5B, lanes 5). Furthermore, siRNA-mediated knockdown of FGF-3 in combination with PHA-665752 (but neither treatment alone) significantly inhibited proliferation of PR-GTL-16 cells (Figure 5C) and induced complete dephosphorylation of AKT and MAPK (data not shown). Finally, combined blockade of MET and FGF receptor (FGFR) signaling using PHA-665752 together with either of two selective FGFR inhibitors (PD173074 or PD330361) led to a dose-dependent inhibition of PR-GTL-16 proliferation, further illustrating FGF-3/FGFR-mediated signaling as the central mechanism of resistance to MET kinase inhibition in PR-GTL-16 cells (Figure 5D). To assess in more detail which FGFRs mediate the FGF-3 effect in PR-GTL-16 cells, the expression of FGFR-1 and -2 (the receptors with the highest affinity for FGF-3 (29)) was determined using quantitative RT-PCR: Importantly, PR-GTL-16 cells were found to express both FGFR-1 and -2 mRNA, which strongly suggests that both receptors contribute to the FGF-3 rescue effect in these cells. This was further confirmed by FGFR-1/-2 gene silencing using shRNA constructs (data not shown).
Figure 5. FGF-3 stimulates AKT and MAPK phosphorylation and confers resistance to PHA-665752-mediated growth inhibition.
(A) GTL-16 and PR-GTL-16 cells were treated for 24 h with 400 nM PHA-665752 with or without addition of 0.1 μM recombinant FGF-3, followed by Western Blot analysis. (B) Cells were treated as in (A), followed by a BrdU cell proliferation assay. Data are depicted as mean ± SE. (C) PR-GTL-16 cells were transfected with FGF-3 siRNA and concomitantly treated with 400 nM PHA-665752 for 48 h. Thereafter, cell proliferation was measured using a BrdU cell proliferation assay. For control, cells were transfected with non-silencing siRNA or left untransfected. Data are depicted as mean ± SE. (D) PR-GTL-16 cells were treated with increasing doses of the selective FGF receptor (FGFR) inhibitor PD173074, alone or in combination with 400 nM PHA-665752. In a second set of experiments, PD173074 was replaced by PD330361, another selective small molecule FGFR inhibitor. After 24 h, cell proliferation was measured using a BrdU cell proliferation assay. Open squares = FGFR inhibitor alone, filled squares = FGFR inhibitor in combination with PHA-665752. Data are depicted as mean ± SE.
HSP-90 inhibition maintains its antitumor efficacy in MET-amplified tumor cells with acquired resistance to a MET kinase inhibitor
Having established a MET-amplified tumor cell line with acquired resistance to MET kinase inhibition, we next sought to determine whether inhibition of HSP-90 maintains its antitumor efficacy in the resistant cells. Figure 6A illustrates that treatment of PR-GTL-16 cells with SNX-2112 led to degradation of MET, AKT, EGFR and HER-2 as in parental GTL-16 cells. SNX-2112 also induced dephosphorylation of MET, AKT and MAPK together with loss of cyclin D1 expression, cell cycle arrest and induction of apoptosis (Figures 6B and 6D). The sensitivity of PR-GTL-16 cells towards SNX-2112 was comparable albeit slightly lower than that of parental GTL-16 cells (IC50: 57.5 nmol/l [PR-GTL-16] vs. 35.6 nmol/l [parental GTL-16]; Figures 6B and 6C). We also tested the in vivo efficacy of SNX-5542 in nude mice bearing xenografts of PR-GTL-16 cells. As shown in Figure 6E, SNX-5542 significantly inhibited growth of PR-GTL-16 xenografts, similar to its effect in parental GTL-16 xenografts.
Figure 6. HSP-90 inhibition maintains its in vitro and in vivo antitumor efficacy in PR-GTL-16 cells.
(A) PR-GTL-16 cells were treated with SNX-2112 at the indicated doses. After 24 h, Western Blot analysis was performed. (B) Cells were treated as in (A), followed by cell cycle analysis. (C) PR-GTL-16 and GTL-16 cells for comparison were treated with increasing doses of SNX-2112 for 24 h, followed by a BrdU cell proliferation assay. (D) GTL-16 and PR-GTL-16 cells were treated with increasing doses of SNX-2112 as indicated, followed by Annexin V/PI staining and flow cytometric analysis. Apoptotic cells: cells staining positive for Annexin V (including early apoptotic cells [Annexin V+/PI−] and late apoptotic cells [Annexin V+/PI+]). Necrotic cells: cells staining positive for PI without staining for Annexin V [Annexin V−/PI+]. Viable cells: cells staining negative for both Annexin V and PI [Annexin V−/PI−]. (E) Nude mice (5 per group) with established PR-GTL-16 xenografts were treated with the indicated doses of SNX-5542 on a Monday – Wednesday – Friday schedule. The tumor volume was measured as described in Materials and Methods. Data are depicted as mean ± 68% CI to account for right-skewed data distribution. This would correspond to a depiction as mean ± SE in the case of normal distribution. P-values are from the regression model.
Efficacy of HSP-90 inhibition in MET-amplified NCI-H820 cells
Recently, McDermott et al. have shown that secondary activation of the EGFR signaling pathway is another mechanism of resistance towards MET kinase inhibition in MET-amplified tumor cells (30). To assess whether HSP-90 inhibition would also be effective in such MET-amplified tumor cells with secondary activation of EGFR signaling, SNX-2112 was tested in lung cancer-derived NCI-H820 cells which harbor both MET amplification and an activating T790M EGFR mutation. As shown in Supplementary Figure 2, neither gefitinib (a selective EGFR inhibitor) nor PHA-665752 had an effect on cell proliferation in these cells while SNX-2112 led to a >50% inhibition of growth after 24 h of treatment.
DISCUSSION
Aim of the present study was to assess the effects of HSP-90 inhibition using the novel synthetic HSP-90 inhibitor SNX-2112 in tumor cells with MET amplification. We have found that HSP-90 inhibition induces degradation of MET, inhibition of MET and downstream PI3K/AKT and Ras/Raf/MEK/MAPK signaling as well as cell cycle arrest in 3 different MET-amplified tumor cell lines (EBC-1, GTL-16 and MKN-45). We were also able to demonstrate that SNX-5542, an orally bioavailable prodrug of SNX-2112, has significant antitumor efficacy in vivo in nude mice bearing MET-amplified tumor xenografts. Notably, HSP-90 inhibition maintained its in vitro and in vivo efficacy against MET-amplified tumor cells resistant to the highly specific MET kinase inhibitor PHA-665752, suggesting that HSP-90 inhibition could be a particularly valuable strategy in MET-amplified tumors with acquired resistance to selective MET kinase inhibition.
Tumor cells harboring amplification of the MET oncogene are largely dependent upon constitutive activation of the MET kinase for proliferation and survival, which renders them highly sensitive towards targeted MET inhibition (6, 7, 19). Several small molecule MET inhibitors have been developed and have recently entered early-stage clinical trials (31, 32). However, as with other highly selective kinase inhibitors (e.g. gefitinib, imatinib) development of acquired resistance is likely. Here, we show that de novo FGF-3 expression is a possible mechanism of resistance to the selective MET kinase inhibitor PHA-665752 in MET-amplified GTL-16 tumor cells. In the resistant cell line (PR-GTL-16), FGF-3, which displays tumorigenic potential in murine breast and colorectal cancer models (33–37), maintains AKT and MAPK phosphorylation even when MET is completely inhibited, while knockdown of FGF-3 or selective inhibition of FGFR signaling using either of two selective small molecule FGFR inhibitors (PD173074 or PD330361) largely restores PHA-665752-sensitivity. Our data add to the growing body of evidence that primary or secondary activation of parallel growth factor receptor signaling resulting in sustained PI3K/AKT and/or Ras/Raf/MEK/MAPK pathway activation is a mechanism of resistance to selective tyrosine kinase inhibition in oncogene-addicted cancer cells: Secondary amplification of MET confers resistance to the EGFR inhibitor gefitinib in EGFR-mutant non small-cell lung cancer cells (38), loss of IGF binding protein-3 expression with consecutive activation of the IGF receptor pathway mediates gefitinib resistance in A431 cells with EGFR amplification (39), and an autocrine FGF-2 signaling loop is associated with resistance to targeted EGFR inhibition in a subset of non small-cell lung cancer cells (40). Furthermore, it has recently been demonstrated by our group that HER-kinase activation confers resistance to targeted MET inhibition in MET-amplified gastric cancer cells (19).
Since oncogene-addicted tumor cells may readily overcome the effects of highly selective kinase inhibition through activation of redundant survival pathways, solitary inhibition of the oncogenically activated kinase is unlikely to exhibit long-term antitumor efficacy in these malignancies. Instead, the therapeutic strategy should take into account both oncogenic kinase activation as well as potential signaling pathway redundancy, i.e. by simultaneously inhibiting multiple key signaling molecules. Conceivably, such an approach would decrease the likelihood of resistance as well as maintain antitumor efficacy in tumors that have become resistant to selective kinase inhibitor treatment.
A large number of oncogenic receptor tyrosine kinases and downstream signaling molecules require HSP-90 for conformational stability, including HER-2, EGFR, v-Src, Raf1, cyclin-dependent kinase-4 and AKT (10–17). Given the important roles played by these HSP-90 clients in signal transduction, proliferation and survival, inhibition of HSP-90 has emerged as a potential antitumor treatment strategy (18). Its underlying mechanism involves proteasomal degradation of HSP-90 client proteins leading to disruption of the tumor cell signaling network – both at the receptor tyrosine kinase and downstream signaling level. HSP-90 inhibition therefore exemplifies the above-mentioned concept of multi-targeted signaling pathway inhibition, making it a highly attractive strategy for the treatment of oncogene-addicted malignancies (41). Indeed, it could be demonstrated that oncogene-addicted tumors – e.g. HER-2-amplified breast cancers or tumors with mutational activation of the EGFR – are particularly sensitive to HSP-90 inhibition both in vitro and in vivo (12, 15, 25).
In MET-amplified cells, a large number of HSP-90 clients (e.g. Raf-1, AKT, EGFR) are activated by MET either through MET-dependent downstream signaling or receptor cross-talk (5–7, 19, 38). Moreover, recent studies have indicated that MET itself is an HSP-90 client protein (20–24). We therefore hypothesized that MET oncogene-addicted tumors may be equally amenable to the effects of HSP-90 inhibition. Indeed, our study demonstrates that MET-amplified tumor cells are highly susceptible towards the antitumor effects of HSP-90 inhibition, with a sensitivity that is largely identical to that of HER-2-amplified breast cancer cells.
Of note, HSP-90 inhibition maintained its in vitro and in vivo antitumor efficacy in PR-GTL-16 cells with acquired resistance to the selective MET kinase inhibitor PHA-665752. In these cells, FGF-3 (through its receptors FGFR-1 and -2) induces sustained activation of the PI3K/AKT and Ras/Raf/MEK/MAPK pathways even when MET is completely inhibited, suggesting that combinatorial signal transduction blockade is required for antitumor activity. Due to its multi-targeted effect including up- (e.g. MET) and downstream (e.g. PI3K/AKT and Ras/Raf/MEK/MAPK) signaling molecules which are also targeted by FGF-3, HSP-90 inhibition was able to simultaneously abrogate MET, PI3K/AKT and Ras/Raf/MEK/MAPK signaling in the resistant cells, inducing growth inhibition both in vitro and in vivo at levels largely comparable to parental cells.
Recently, it could be demonstrated that secondary activation of EGFR signaling is another mechanism of resistance to targeted MET inhibition in MET-amplified tumor cells (30). To assess whether HSP-90 inhibition using SNX-2112 would also be effective in such tumor cells which display both MET amplification as well as secondary activation of EGFR signaling, MET-amplified NCI-H820 cells which also harbor an activating T790M EGFR mutation were used as an in vitro model (42): As shown in Supplementary Figure 2, these cells are resistant against both EGFR and MET inhibition, while a >50% inhibition of growth is observed upon tretament with SNX-2112. Importantly, these data not only suggest that HSP-90 inhibition could be a promising therapeutic strategy to overcome different mechanisms of MET kinase inhibitor resistance (e.g. secondary activation of FGFR or EGFR signaling) but also to treat EGFR-mutant non small-cell lung cancers which have become resistant to EGFR inhibition through secondary amplification of MET (38).
In conclusion, our study provides a strong rationale for the use of HSP-90 inhibition in tumors that have become resistant to selective tyrosine kinase inhibition and further illustrates that combinatorial signal transduction blockade offers significant advantages over highly selective kinase inhibition in oncogene-addicted malignancies.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Amplification of the MET oncogene is observed in approximately 15% of gastric cancers and has recently attracted wide interest as a mechanism of acquired resistance to selective EGFR inhibition in EGFR-mutant non small-cell lung cancer. Several selective MET small molecule MET inhibitors have been developed but these have so far failed to demonstrate significant in vivo activity in patients, suggesting a need for therapeutic alternatives. In this study, we demonstrate that inhibition of heat shock protein-90 (HSP-90) has strong efficacy against MET-amplified tumor cells both in vitro and in vivo. Of particular importance, HSP-90 inhibition maintains its in vitro and in vivo antitumor efficacy even in MET-amplified tumor cells which have become resistant to highly selective MET kinase inhibition. We conclude that HSP-90 inhibition is a promising therapeutic strategy for MET-amplified tumors, particularly if secondary resistance against selective MET inhibition has emerged.
Acknowledgments
Financial Support: This work was supported by a grant from the American Society of Colon and Rectal Surgeons. Thomas Bachleitner-Hofmann was supported by postdoctoral research grants from the Max Kade Foundation, New York and the Austrian Surgical Society. Martin R. Weiser was supported by a Career Development Award from the American Society for Clinical Oncology.
REFERENCES
- 1.Giordano S, Ponzetto C, Di Renzo MF, Cooper CS, Comoglio PM. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989;339:155–6. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- 2.Park M, Dean M, Kaul K, Braun MJ, Gonda MA, Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci U S A. 1987;84:6379–83. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 4.Francone TD, Landmann RG, Chen CT, et al. Novel xenograft model expressing human hepatocyte growth factor shows ligand-dependent growth of c-Met-expressing tumors. Mol Cancer Ther. 2007;6:1460–6. doi: 10.1158/1535-7163.MCT-06-0466. [DOI] [PubMed] [Google Scholar]
- 5.Lutterbach B, Zeng Q, Davis LJ, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res. 2007;67:2081–8. doi: 10.1158/0008-5472.CAN-06-3495. [DOI] [PubMed] [Google Scholar]
- 6.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103:2316–21. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen JG, Schreck R, Burrows J, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–55. [PubMed] [Google Scholar]
- 8.Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 9.Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–17. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 10.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–66. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 11.Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–66. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawai A, Chandarlapaty S, Greulich H, et al. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res. 2008;68:589–96. doi: 10.1158/0008-5472.CAN-07-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulte TW, Blagosklonny MV, Romanova L, et al. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16:5839–45. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimamura T, Li D, Ji H, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res. 2008;68:5827–38. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimamura T, Lowell AM, Engelman JA, Shapiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycins. Cancer Res. 2005;65:6401–8. doi: 10.1158/0008-5472.CAN-05-0933. [DOI] [PubMed] [Google Scholar]
- 16.Solit DB, Zheng FF, Drobnjak M, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93. [PubMed] [Google Scholar]
- 17.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerji U. Heat shock protein 90 as a drug target: some like it hot. Clin Cancer Res. 2009;15:9–14. doi: 10.1158/1078-0432.CCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 19.Bachleitner-Hofmann T, Sun MY, Chen CT, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther. 2008;7:3499–508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 20.Koga F, Tsutsumi S, Neckers LM. Low dose geldanamycin inhibits hepatocyte growth factor and hypoxia-stimulated invasion of cancer cells. Cell Cycle. 2007;6:1393–402. doi: 10.4161/cc.6.11.4296. [DOI] [PubMed] [Google Scholar]
- 21.Shen Y, Xie Q, Norberg M, Sausville E, Woude GV, Wenkert D. Geldanamycin derivative inhibition of HGF/SF-mediated Met tyrosine kinase receptor-dependent urokinase-plasminogen activation. Bioorg Med Chem. 2005;13:4960–71. doi: 10.1016/j.bmc.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 22.Webb CP, Hose CD, Koochekpour S, et al. The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-met-urokinase plasminogen activator-plasmin proteolytic network. Cancer Res. 2000;60:342–9. [PubMed] [Google Scholar]
- 23.Xie Q, Gao CF, Shinomiya N, et al. Geldanamycins exquisitely inhibit HGF/SF-mediated tumor cell invasion. Oncogene. 2005;24:3697–707. doi: 10.1038/sj.onc.1208499. [DOI] [PubMed] [Google Scholar]
- 24.Rice JW, Veal JM, Barabasz A, et al. Targeting of multiple signaling pathways by the Hsp90 inhibitor SNX-2112 in EGFR resistance models as a single agent or in combination with erlotinib. Oncol Res. 2009;18:229–42. doi: 10.3727/096504009x12596189659240. [DOI] [PubMed] [Google Scholar]
- 25.Chandarlapaty S, Sawai A, Ye Q, et al. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res. 2008;14:240–8. doi: 10.1158/1078-0432.CCR-07-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin L, Xiao CL, Lu CH, et al. Transcriptomic and proteomic approach to studying SNX-2112-induced K562 cells apoptosis and anti-leukemia activity in K562-NOD/SCID mice. FEBS Lett. 2009;583:1859–66. doi: 10.1016/j.febslet.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 27.Okawa Y, Hideshima T, Steed P, et al. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood. 2009;113:846–55. doi: 10.1182/blood-2008-04-151928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kammula US, Kuntz EJ, Francone TD, et al. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007;248:219–28. doi: 10.1016/j.canlet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Ornitz DM, Xu J, Colvin JS, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–7. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 30.McDermott U, Pusapati RV, Christensen JG, Gray NS, Settleman J. Acquired resistance of non-small cell lung cancer cells to MET kinase inhibition is mediated by a switch to epidermal growth factor receptor dependency. Cancer Res. 70:1625–34. doi: 10.1158/0008-5472.CAN-09-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jhawer M, Kindler HL, Wainberg Z, et al. Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): Interim results of a multicenter phase II study. J Clin Oncol (Meeting Abstracts) 2009;27:4502. [Google Scholar]
- 32.Kwak EL, Camidge DR, Clark J, et al. Clinical activity observed in a phase I dose escalation trial of an oral c-met and ALK inhibitor, PF-02341066. J Clin Oncol (Meeting Abstracts) 2009;27:3509. [Google Scholar]
- 33.Galdemard C, Brison O, Lavialle C. The proto-oncogene FGF-3 is constitutively expressed in tumorigenic, but not in non-tumorigenic, clones of a human colon carcinoma cell line. Oncogene. 1995;10:2331–42. [PubMed] [Google Scholar]
- 34.Galdemard C, Yamagata H, Brison O, Lavialle C. Regulation of FGF-3 gene expression in tumorigenic and non-tumorigenic clones of a human colon carcinoma cell line. J Biol Chem. 2000;275:17364–73. doi: 10.1074/jbc.M909316199. [DOI] [PubMed] [Google Scholar]
- 35.Hajitou A, Baramova EN, Bajou K, et al. FGF-3 and FGF-4 elicit distinct oncogenic properties in mouse mammary myoepithelial cells. Oncogene. 1998;17:2059–71. doi: 10.1038/sj.onc.1202126. [DOI] [PubMed] [Google Scholar]
- 36.Hajitou A, Calberg-Bacq CM. Fibroblast growth factor 3 is tumorigenic for mouse mammary cells orthotopically implanted in nude mice. Int J Cancer. 1995;63:702–9. doi: 10.1002/ijc.2910630516. [DOI] [PubMed] [Google Scholar]
- 37.Hajitou A, Deroanne C, Noel A, et al. Progression in MCF-7 breast cancer cell tumorigenicity: compared effect of FGF-3 and FGF-4. Breast Cancer Res Treat. 2000;60:15–28. doi: 10.1023/a:1006302602261. [DOI] [PubMed] [Google Scholar]
- 38.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 39.Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marek L, Ware KE, Fritzsche A, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol. 2009;75:196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–16. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 42.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.