Abstract
INTRODUCTION
We previously developed a multi-assay algorithm (MAA) to identify recent HIV infection that includes the BED-Capture Enzyme Immunoassay, an avidity assay based on the Genetic Systems HIV-1/HIV-2+O Enzyme Immunoassay, CD4 cell count, and HIV viral load. We used this MAA to evaluate the association between recent maternal HIV infection and in utero transmission of HIV.
METHODS
Plasma samples were collected at delivery from 2,561 HIV-infected women in the PEPI-Malawi trial. The MAA described above was used to identify women with recent HIV infection. Logistic regression models assessed association between recent HIV infection and in utero HIV transmission (defined as a positive infant HIV DNA test at birth).
RESULTS
Seventy-three women were identified as recently infected using the MAA. Those women were younger and had lower parity than women who were identified as not recently infected using the MAA (P<0.0001 for age and parity). The frequency of in utero HIV transmission was 17.8% among women identified as recently infected, compared to 6.7% among women identified as not recently infected (13/73 vs. 166/2488, P=0.001). In a multivariate model, three factors were independently associated with in utero HIV transmission: recent infection (adjusted odds ratio [AOR]: 2.49, 95% CI: 1.30–4.78, P=0.006), log10 HIV viral load at delivery (AOR: 2.01, 95% CI: 1.60–2.51, P<0.0001), and younger age (per 10 year increase, AOR: 0.66, 95% CI: 0.43–0.93, P=0.02).
CONCLUSIONS
Results obtained using a MAA suggest that recent maternal HIV acquisition is strongly associated with in utero HIV transmission, independent of HIV viral load at delivery.
Keywords: HIV, incidence, multiassay algorithm, mother-to-child transmission, Malawi
INTRODUCTION
In sub-Saharan Africa, prevalence of HIV infection among young women attending antenatal clinics often exceeds 20% [1]. Studies have shown that women who acquire HIV infection in the post-partum period are at increased risk for post-natal mother-to-child transmission (MTCT) of HIV [2–4]. Women are also at risk of acquiring HIV infection during pregnancy. In a South African study, incident HIV infection was four times more frequent in pregnant women than in non-pregnant women [5]. In that study, 3% of women acquired HIV infection during pregnancy, testing positive approximately 24 weeks after an initial negative test [5]. Because HIV viral loads are often very high near the time of HIV infection [6–7], and because high HIV viral load is one of the factors most strongly associated with mother-to-child transmission of HIV [8–11], acquisition of HIV infection by women shortly before the onset of pregnancy or during pregnancy is likely to be associated with an increased risk of in utero HIV transmission.
Despite recent successes in counseling and testing in sub-Saharan Africa, some barriers exist to achieving universal coverage. Potential barriers to wider coverage in Malawi include structural limitations of the health care system [12]. In Malawi, women are frequently first tested for HIV infection near the time of delivery. Without a previous HIV test result, it is not possible to identify women who are likely to have acquired HIV infection shortly before or during pregnancy. Women with acute (pre-seroconversion) HIV infection can be identified at the time of delivery using methods such as pooled HIV RNA testing [13] (i.e., by detecting HIV RNA in samples from women who do not have detectable HIV antibody). However, because the duration of acute HIV infection is short (e.g., 1–2 weeks), that testing approach would only identify women who acquired HIV infection very close to the time of testing. Even if a large number of women are tested and the incidence rate in pregnant women is high, relatively few women with acute HIV infection are likely to be identified using that approach. Using a pooled HIV RNA testing strategy, a study in Malawi documented acute HIV infection in 5 (0.21%) of 2.327 women tested in the third trimester [14], and a study in South Africa documented acute HIV infection in 4 (0.9%) of 467 women tested at the first antenatal visit [15].
Another approach to identifying women who acquired HIV infection shortly before or during pregnancy is to test samples collected at the time of delivery using laboratory methods developed for cross-sectional HIV incidence determination. Serologic (antibody-based) assays have been developed for cross-sectional analysis of HIV incidence, based on the premise that the anti-HIV antibody response matures during the course of HIV infection [16–18]. Those assays measure different characteristics of the HIV antibody response (e.g., antibody avidity, titer, isotype, specificity, or the proportion of the antibody response that is HIV-specific) [16–18]. Unfortunately, the serologic response to HIV infection is quite variable. Some HIV-infected individuals never attain a mature HIV-antibody response; in others, factors such as advanced HIV disease and viral suppression (natural or antiretroviral drug-induced) can weaken the anti-HIV antibody response [19–20]. For these reasons, individuals with long-standing HIV infection may be misclassified as recently infected using a single serologic assay [21–23].
We previously developed a multi-assay algorithm (MAA) for determination of HIV incidence using cross-sectional samples, as part of the HIV Prevention Trials Network (HPTN) Network Laboratory’s HIV Incidence Initiative. This MAA uses two serologic assays (the BED-Capture enzyme immunoassay (BED) [21] and an avidity assay based on the Genetic Systems HIV-1/HIV-2 + O EIA [24]) as well as non-serologic biomarkers (CD4 cell count and HIV viral load) to assess HIV incidence [18, 25]. These two serologic assays use different HIV antigens to detect and characterize antibodies. The BED assay includes antigens from subtypes B and D, as well as CRF01_AE, while the avidity assay includes antigens from a broader spectrum of HIV strains. These two assays also measure different characteristics of the anti-HIV antibody response. The BED assay measures the proportion of antibody that is HIV-specific, while the avidity assay measures the strength with which anti-HIV antibodies bind to target antigens [21, 23–24]. Each of these assays when used alone is known to misclassify some individuals with long-standing HIV infection as recently infected [21–23]. Our MAA uses modified cut-offs for the BED and avidity assays (BED result > 1 normalized optical density unit, OD-n; avidity result >80%), and combines results from these assays with results from HIV viral load and CD4 cell count testing (see Methods) [19, 22, 26]; this approach reduces the rate of misclassification of individuals with long-standing infection as recently infected [19, 22, 26]. In this study, we used the MAA to identify Malawian women who were likely to have been infected with HIV shortly before or during pregnancy, and evaluated the relationship between recent maternal HIV infection and in utero HIV transmission. We hypothesized that the high HIV viral loads associated with early HIV infection would contribute to higher levels of in utero HIV transmission.
METHODS
Source of samples that were used for analysis of mother-to-child transmission of HIV
Samples were obtained from HIV-infected women enrolled at the time of delivery in the Post-Exposure Prophylaxis of Infants in Malawi (PEPI-Malawi) trial (conducted in 2004–2009, NCT00115648) [27]. Approximately 70% of the women enrolled in the PEPI-Malawi trial presented >4 hours before anticipated delivery; those women were described as early presenters and received a single intra-partum dose of nevirapine (sdNVP) for prevention of MTCT of HIV. In contrast, women with unknown HIV status who presented <4 hours before anticipated delivery were described as late presenters and did not receive intra-partum sdNVP. Infants of the enrolled women were randomized at birth to receive one of three regimens to prevent post-natal HIV transmission, as described previously [27]. Maternal blood samples used to measure CD4 cell count and HIV viral load were collected at delivery. In utero HIV transmission was defined as a positive infant HIV DNA test at birth [27]. This study included 2,561 (76.8%) of the 3,335 women enrolled in the PEPI-Malawi trial; 774 women were excluded from analysis for the following reasons: 470 had no HIV viral load or no CD4 cell count result from the delivery visit, 33 had infants whose HIV infection status was undetermined, 271 did not have an available sample collected at delivery. Nine (0.35%) of the 2,561 women started antiretroviral therapy prior to delivery.
Source of samples that were used to evaluate the performance of the multi-assay algorithm
The performance of the MAA was assessed by analyzing samples from adults with known duration of HIV who were likely to be infected with subtype C HIV infection (individuals enrolled in three clinical cohorts: the Hormonal Contraception and HIV Acquisition (HC-HIV) Study [28], the Orange Farm Male Circumcision Trial (ANRS-1265) [29–30], and the Partners in Prevention HSV/HIV Transmission Study [31]. This sample set included samples collected from 87 individuals (58 men from South Africa [29–30] and 29 women from Zimbabwe [28]) <180 days after a negative HIV test. Data for CD4 cell count and HIV viral load were not available for those individuals, but were assumed to be >200 cells/mm3 and >400 copies/ml in these recently infected individuals; none of these individuals were receiving antiretroviral drugs at the time of sample collection. The sample set also included samples from 529 men and women in South Africa and Botswana who were known to have been HIV-infected for at least two years [31].
Laboratory analysis of recent HIV infection
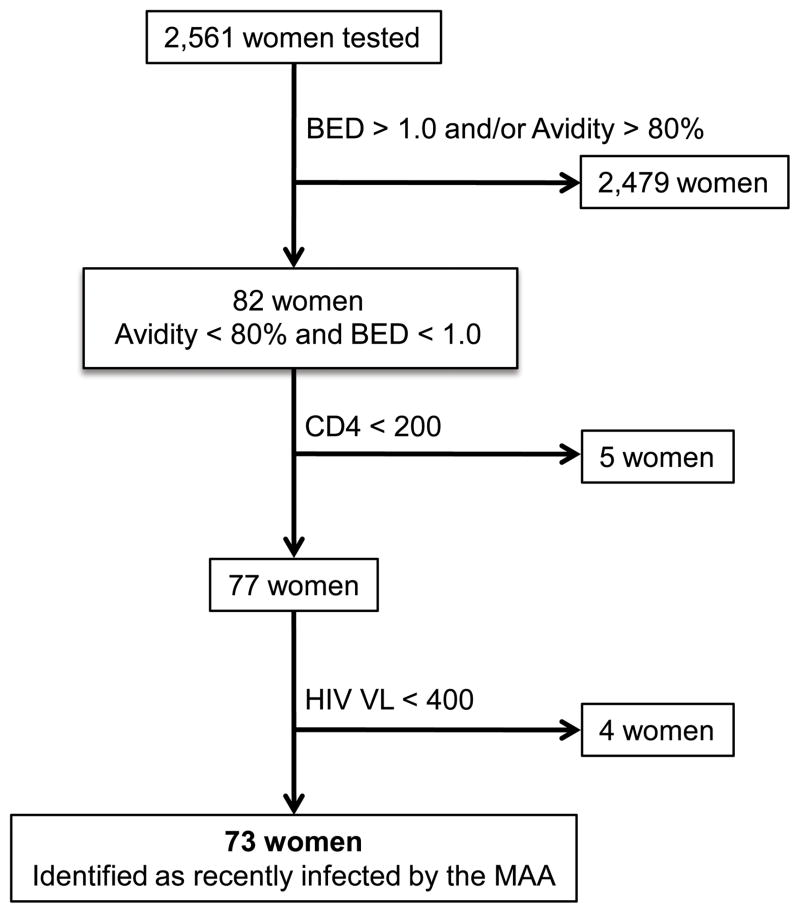
The BED assay was performed according to the manufacturer’s instructions (Calypte Biomedical Corporation, Lake Oswego, OR, USA) [21], except that an assay cut-off of 1.0 OD-n was used to screen for recent HIV infection (rather than the standard cut-off of 0.8 OD-n). The avidity assay is a modified version of the Genetic Systems HIV-1/HIV-2 + O EIA (enzyme linked immunoassay, Bio-Rad Laboratories, Redmond, WA). The avidity assay was performed as previously described [19, 24], except that an assay cut-off of 80% was used to screen for recent infection (rather than the standard cut-off of 40%). Women were identified as recently infected using the MAA described previously [13]: BED <1.0 OD-n + avidity <80% + CD4 >200 cells/mm3 + HIV viral load >400 copies/ml (Figure 1).
Figure 1. Use of a multi-assay algorithm (MAA) to identify women with recent HIV infection.
A multi-assay algorithm (MAA) was used to identify women in the PEPI-Malawi trial who were recently infected with HIV at the time of delivery. In the MAA, women are classified as recently infected if they have the following test results: BED result < 1.0 optical density units (OD-n) + avidity result < 80% + CD4 cell count > 200 cells/mm3 + HIV viral load > 400 copies/ml. The assay cut-offs used for BED and avidity assays in this MAA (1.0 OD-n and 80%, respectively) are higher than the assay cut-offs that are typically used when each these assays is used alone for cross-sectional incidence testing (see below). Most of the women tested (2479/2561=96.8%) were classified as not recently infected based on results from the BED and avidity assays; those women either had a BED assay result >1.0 (N=175), an avidity assay result >80% (N=44) or both (N=2,260). The remaining 82 women had BED and avidity test results below the assay cut-offs, but were classified as not recently infected because they had either a CD4 cell count < 200 cells/mm3 (N=5) or an HIV viral load < 400 copies/ml (N=4). The remaining 73 women (2.9 % of the 2,561 women tested) were classified as recently infected using the MAA.
Statistical analysis
For unadjusted comparisons between groups, Fishers exact tests compared categorical variables and Wilcoxon tests compared continuous variables. Univariate and multivariate logistic regression models were used to obtain unadjusted and adjusted odds ratios for the association of recent infection with in utero HIV transmission. Statistical significance was considered for two-sided P≤0.05. All analyses were performed using SAS Software Version 9.1 (Cary, North Carolina).
Ethical considerations
Written informed consent was obtained from all women for participation in the PEPI-Malawi trial. The PEPI-Malawi trial was approved by Institutional Review Boards in Malawi and the U.S. as described [27], including the U.S. Centers for Disease Control and Prevention.
RESULTS
This sub-study included 2,561 women enrolled in the PEPI-Malawi trial. There was no significant difference in the frequency of in utero HIV transmission among the 2,561 women included in the study and the 774 women who were not included in the study (7.0% vs. 6.2%, respectively, P=0.48, Fisher’s exact test). Fifty-four of the 2,561 women included in the study were previously diagnosed with HIV infection in the Nevirapine and Zidovudine (NVAZ) trial, which was conducted in 2000–2004 at the same study sites as the PEPI-Malawi trial [32–33]. Therefore, those 54 women were known not to have recent HIV infection when they subsequently enrolled in the PEPI-Malawi trial. The median duration of time between their enrollment in the NVAZ trial and their subsequent enrollment in the PEPI-Malawi trial was 4.3 years (range: 2.3–6.1 years). Overall, 179 (7.0%) of the 2,561 women had infants who were diagnosed with HIV infection at the time of birth (in utero HIV infection).
Maternal samples collected at delivery from the 2,561 women were analyzed using the BED and avidity assays. Results from those assays were combined with results from CD4 cell count and HIV viral load testing to identify women with recent HIV infection (Figure 1). The performance of the MAA in individuals likely to be infected with subtype C HIV was evaluated by analyzing samples from individuals from subtype C endemic areas who had known duration of infection (see Methods). In those individuals, the positive predictive value of the MAA for identifying individuals with recent HIV infection (defined as <180 days) was 93.9%; that result can be compared to the result obtained by testing the same sample set with the avidity assay alone using a standard assay cut-off of 40% (positive predictive value: 90.9%), and to the result obtained by testing the same sample set with the BED assay alone using a standard assay cut-off of 0.8 OD-n (positive predictive value: 71.7%).
Using the MAA described above, 73 (2.9%) of the 2,561 women were identified as recently infected; the remaining 2,488 women were identified as not recently infected. All but nine of the 2,488 women were identified as not recently infected based on results of serologic testing (BED>1.0 OD-n and/or avidity >80%, Figure 1). Those nine women had low BED and/or low avidity test results. Four of those nine women had CD4 cell counts <200 cells/ul and five of those women had viral loads <400 c/ml (Figure 1). Individuals who have low CD4 cell counts and low HIV viral loads are unlikely to be recently infected; furthermore, those factors have been associated with misclassification of individuals with non-recent HIV infection as recently infected using serologic HIV incidence assays [20, 34–35].
None of the 54 women who had known non-recent HIV infection when they enrolled in the PEPI-Malawi trial were misclassified as recently infected using the MAA (0/54 vs. 73/2507, P=0.40, Fishers exact test). In addition, nine women (0.4% of the 2,561 women included in this study) were receiving antiretroviral therapy at the time of enrollment into the PEPI-Malawi trial (a co-formulated regimen of stavudine, lamivudine and nevirapine; Triommune, Cipla, India); none of those women were identified as recently infected using the MAA and none had an infant who was HIV-infected in utero. The median time between antiretroviral treatment initiation and enrollment in the PEPI-Malawi trial for those nine women was 56 days (range: 3–263, interquartile range: 25–97 days). They had a median CD4 cell count of 180 (range: 33–701 cells/mm3, five had a CD4 cell count <200 cells/mm3) and a median log10 HIV viral load of 2.73 (range: 2.30–4.36 copies/ml) at enrollment.
Table 1 shows demographic, clinical, and laboratory characteristics of the 73 women who were identified as recently infected using the MAA and the 2,488 women identified as not recently infected by the MAA. Women who were identified as recently infected using the MAA were younger and had lower parity than the women who were identified as not recently infected (median age: 23 vs. 26 years, P<0.0001, Wilcoxon test; median parity: two vs. three pregnancies, P<0.0001, Wilcoxon test). Women identified as recently infected by the MAA also had significantly higher CD4 cell counts at delivery; this is partly explained by the fact that the MAA excludes women with CD4 cell counts below 200 cells/mm3 from the recently infected group. There was a significant association between recent maternal HIV infection (defined using the MAA) and in utero HIV transmission. The frequency of in utero HIV transmission was 17.8% in the recently infected group, compared to only 6.7% in the not recently infected group (13/73 vs. 166/2488, P=0.001, Table 1). There was no significant association between identification of women as recently infected using the MAA and other demographic, clinical, and laboratory variables (Table 1).
Table 1.
Characteristics of women identified as recently infected and as not recently infected using a multi-assay algorithm (PEPI-Malawi trial, Malawi, 2004–2009)*.
| Characteristic | Recently infected Value (IQRa) | Not recently infected Value (IQRa) | P value |
|---|---|---|---|
| Have electricity in the house (yes, %) | 30.1% | 32.3% | 0.80c |
| Have running water in the house (yes, %) | 26.0% | 22.4% | 0.48c |
| Body mass index (median, kg/m2) | 22.7 (20.9–24.7) | 23.2 (21.5–25.4) | 0.13b |
| Age (median, years) | 23 (20–25) | 26 (23–29) | <.0001b |
| Parity (median) | 2 (2–3) | 3 (2–4) | <.0001b |
| CD4 cell count (median, cells/mm3) | 482 (372–618) | 391 (256–567) | <.0001b |
| Log10 HIV viral load (median) | 4.1 (3.7–4.8) | 4.1 (3.5–4.7) | 0.25b |
| Infant gender (male, %) | 45.2% | 51.0% | 0.34c |
| Infant birth weight (median, kilograms) | 3 (2.8–3.3) | 3 (2.8–3.3) | 0.86b |
| Presentation at delivery (early presenter, yes, %) d | 98.6% | 99.7% | 0.25c |
| Infant prophylaxis regimen (control, %) e | 28.8% | 33.8% | 0.45c |
| In utero HIV transmission (yes, %) f | 17.8% | 6.7% | 0.001c |
Statistically significant results are shown in bold font. All analyses included 73 women identified as recently infected using the multi-assay algorithm (MAA). All analyses included 2488 women identified as not recently infected using the MAA, with the following exceptions: the analysis of body mass index included 2,486 women, the analysis of intra-partum nevirapine receipt included 2, 481 women, the analysis of infant birth weight included 2,487 women/infants.
IQR: Interquartile range for median values.
Wilcoxon’s rank sum test was used for comparison of median values.
Fisher’s exact test was used for comparison of proportions.
Women who presented early in labor delivery received single dose nevirapine (sdNVP) prophylaxis, while women who presented late in labor did not receive sdNVP (see Methods).
Infants were randomized at birth to one of three antiretroviral regimens for prevention of HIV mother-to-child transmission: single dose nevirapine (sdNVP) plus one week of zidovudine (ZDV, control), control plus up to 14 weeks of daily infant NVP, or control plus up to 14 weeks of daily infant NVP plus daily infant ZDV. Prophylaxis was stopped at the time that infant HIV infection was confirmed.
In utero HIV infection transmission was defined as a positive HIV DNA test at birth.
We considered the possibility that some women who were identified as not recently infected based on CD4 cell count or HIV viral load data alone might have been misclassified; among the 2,488 women identified as not recently infected, only nine had a BED result <1.0 OD-n and an avidity result <80% (four with a CD4 cell count <200 cells/ul and five with a viral load <400 copies/ml). Even if all nine of those women were included in the recently infected group, recent infection would still have been significantly associated with an increased risk of in utero HIV infection (P=0.0062).
Logistic regression analysis was performed to assess the association between in utero HIV infection and identification of women as recently infected using the MAA, controlling for other factors (Table 2). In a multivariate model (which excluded parity because it was correlated with age), increased risk of in utero transmission was significantly associated with recent HIV infection (as defined by the MAA, adjusted odds ratio [AOR]: 2.49, 95% CI: 1.30–4.78, P=0.006), HIV viral load at delivery (per log10 increase, AOR: 2.01, 95% CI: 1.60–2.51, P<0.0001), and younger age (per 10 year increase, AOR: 0.66, 95% CI: 0.43–0.93, P=0.02), but not with CD4 cell count at delivery, or other factors (Table 2).
Table 2.
Association of clinical and laboratory factors with in utero HIV transmission (PEPI-Malawi trial, Malawi, 2004–2009)*
| Factor | Univariate models | Multivariate model | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Identified as recently infected using the multi-assay algorithm (yes vs. no) | 3.03 | 1.63, 5.63 | 0.0005 | 2.49 | 1.30, 4.78 | 0.006 |
|
| ||||||
| Age (per 10 year increase) | 0.63 | 0.45–0.89 | 0.009 | 0.66 | 0.43–0.93 | 0.02 |
|
| ||||||
| Parity (per pregnancy) a | 0.91 | 0.82, 1.01 | 0.09 | -- | -- | -- |
|
| ||||||
| CD4 cell count (per 100 cell increase) | 0.94 | 0.88, 1.00 | 0.04 | 0.98 | 0.92, 1.06 | 0.66 |
|
| ||||||
| Log10 HIV viral load (per log increase) | 2.03 | 1.65, 2.51 | <0.0001 | 2.01 | 1.60, 2.51 | <0.0001 |
|
| ||||||
| Infant gender (female vs. male) | 1.30 | 0.96, 1.77 | 0.09 | 1.28 | 0.94, 1.74 | 0.12 |
|
| ||||||
| Presentation at delivery (late vs. early presenter) b | 1.66 | 0.21, 13.36 | 0.63 | 1.84 | 0.22, 15.25 | 0.57 |
|
| ||||||
| Infant prophylaxis regimen (control vs. extended prophylaxis) c | 1.22 | 0.88, 1.70 | 0.24 | 1.17 | 0.84, 1.64 | 0.35 |
Logistic regression analysis. Statistically significant results are shown in bold. OR: odds ratios; CI: confidence intervals. All univariate models included 2,561 women except for the model for presenter status; presenter status was only available for 2,554 women; women who presented early in labor delivery received single dose nevirapine (sdNVP) prophylaxis, while women who presented late in labor did not receive sdNVP (see Methods). The multivariate model included 2,554 women. In utero transmission was defined as a positive HIV DNA test at birth. The association of variables such as the availability of running water and electricity, maternal body mass index (BMI) and infant birth weight with identification of women as recently infected using the multi-assay algorithm was not statistically significant (see Table 1). Those variables were also not significantly associated with in utero transmission of HIV when included in the multivariate models shown in Table 2 (data not shown).
All of the variables included in the univariate models were also included in the multivariate logistic regression model, with one exception: parity was not included in the multivariable model because it was correlated with age.
Women who presented early in labor delivery received single dose nevirapine (sdNVP) prophylaxis, while women who presented late in labor did not receive sdNVP (see Methods).
In this analysis, results from the extended nevirapine and the extended nevirapine plus zidovudine study arms were combined.
DISCUSSION
We used a multi-assay algorithm (MAA) to identify women in the PEPI-Malawi trial who were likely to have had recent HIV infection at the time of delivery. The frequency of in utero HIV transmission was significantly higher among women who were identified as recently infected using the MAA compared to women who were identified as not recently infected (17.8% vs. 6.7%, P=0.001). The high frequency of in utero HIV transmission in women with recent HIV infection is consistent with the dynamics of HIV infection, since those women were likely to have been acutely infected during pregnancy. HIV viral load is typically very high during the acute stage of HIV infection [6–7], and high HIV viral load is one of the factors most strongly associated with an increased risk of MTCT [8–11]. In this study, identification of women as recently infected using the MAA and maternal HIV viral load at delivery were independently associated with in utero HIV transmission.
The independence of HIV viral load at delivery and recent HIV infection (as defined by the MAA) suggests that other factors associated with recent HIV infection may increase the risk of in utero HIV transmission. This hypothesis is consistent with data from studies of heterosexual HIV transmission. In heterosexual transmission, adults with recent HIV infection are more likely to transmit HIV than adults with chronic (non-recent) HIV infection [36]. This is not explained by differences in HIV viral load alone [37] and suggests that some viral strains present early in infection may be more fit for transmission [38–39]. Adults with recent HIV infection are also more likely to have immature anti-HIV antibody responses (e.g., low anti-HIV antibody titer or avidity, low proportion of IgG anti-HIV antibody). During pregnancy, women who have immature anti-HIV antibody responses might have lower proportions of antibody-associated or neutralized HIV. Previous studies have found that women who transmit HIV to their infants in utero are less likely to have neutralizing antibodies than are women who transmit HIV to their infants at or after delivery [40–41]. Further studies are needed to evaluate the relative contributions of high HIV viral load, viral transmission phenotype, and the quality or level of the HIV antibody response to in utero HIV transmission in women with recent HIV infection.
There are some limitations of this study. Because we cannot determine the actual time of infection for women in this study, we do not know what proportion of women who were identified as recently infected using the MAA were infected during pregnancy, rather than in the months preceding pregnancy. We also recognize that some women who acquired HIV infection during pregnancy may not have been identified using the MAA, and that the MAA may also have classified some women as recently infected who had chronic HIV infection. Several observations from this report provide indirect evidence supporting use of the MAA. First, the MAA identified 2.9% of the women as recently infected; that result is consistent with findings from a South African study, where 3% of women acquired HIV infection during pregnancy based on longitudinal HIV serological testing [5]. Second, none of 54 women with known long-standing HIV infection and none of the nine women on antiretroviral therapy were misclassified as recently infected using the MAA. Third, women identified as recently infected using the MAA were younger than other women in the cohort; younger age has been associated with HIV acquisition in sub-Saharan Africa [42–43]. We also note that BED and avidity test results are not significantly affected by pregnancy; therefore, pregnancy is not likely to impact the performance of the MAA) [36].
This study suggests that women who acquire HIV near the onset of pregnancy or during pregnancy have significantly higher risk of transmitting HIV to their infants in utero than do women with chronic HIV infection. Unfortunately, identification of women with recent HIV infection is difficult unless there is a prior history of a recent negative HIV antibody test, because use of methods such as the MAA to detect recent HIV infection is not feasible in large scale in resource-limited countries. In high-prevalence settings, retesting during the third trimester of women who tested HIV-negative shortly before pregnancy or earlier in pregnancy would allow identification of women with recent HIV infection [5]. In that subset of women, triple antiretroviral drug prophylaxis for prevention of MTCT of HIV [1] may be preferable given the increased risk of HIV transmission associated with recent HIV infection. However, it is important to recognize that in utero infection might have already occurred by the time that maternal infection would have been diagnosed using this strategy. For these reasons, the results of our study emphasize that prevention of HIV acquisition in women during pregnancy is a critical step in preventing in utero HIV transmission and reducing the global epidemic of pediatric HIV/AIDS.
Acknowledgments
The authors thank the women and infants who participated in the PEPI-Malawi trial, and the PEPI-Malawi study team in Malawi. The authors also thank the laboratory staff at the College of Medicine, University of Malawi-Johns Hopkins University Research Project in Blantyre, Malawi for their assistance with sample processing and shipping for the PEPI-Malawi study. The authors thank the study teams and participants of the following three studies for providing samples and data used to evaluate the performance of the multi-assay algorithm: Hormonal Contraception and HIV Acquisition (HC-HIV) Study, the Orange Farm Male Circumcision Trial (ANRS-1265), and the Partners in Prevention HSV/HIV Transmission Study. The authors thank Shauna Wolf for help with sample and data management, and Kevin Eaton for assistance with sample testing.
SOURCES OF SUPPORT
International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group, American Recovery and Reinvestment Act (ARRA) award (Cooperative Agreement 3U01AI068632-05S3).
The HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the Office of AIDS Research, of the National Institutes of Health (NIH), Dept. of Health and Human Services (DHHS) (U01AI068613).
The Centers for Disease Control and Prevention (Cooperative Agreement U50/CCU022061).
The International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) Group (U01-AI068633).
The Partners in Prevention HSV/HIV Transmission Study (grant #26469, Bill and Melinda Gates Foundation)
the Hormonal Contraception and HIV Acquisition (HC-HIV) Study (contract N01-HD-0-3310 through Family Health International, The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH, DHHS).
The Division of Intramural Research, NIAID, NIH.
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention (CDC) or the National Institutes of Health (NIH). Use of trade names is for identification purposes only and does not constitute endorsement by the U.S. CDC or the NIH.
Note: A portion of this work was presented at the 18th Conference on Retroviruses and Opportunistic Infections (Feb, 2011; Boston, MA).
Conflict of Interest Statement
None of the authors has a commercial or other association that might pose a conflict of interest.
Authors’ contributions: All of the authors reviewed the manuscript and provided input into the manuscript prior to submission. In addition, individual authors had the following contributions:
TE Taha: Contributed to study design, data analysis, data interpretation, and manuscript preparation; U.S. Principal Investigator (PI) for the PEPI-Malawi trial.
MM James: Coordinated laboratory studies, analyzed laboratory data, contributed to manuscript preparation.
DR Hoover: Senior statistician for this study; statistician for the PEPI-Malawi trial, contributed to manuscript preparation.
J Sun: Data analyst for this study.
O Laeyendecker: Responsible for overseeing BED and avidity testing and reviewing BED and avidity test results, contributed to manuscript preparation.
CE Mullis: Performed BED and avidity testing.
JJ Kumwenda: Clinician in charge of clinical care for participants in the PEPI-Malawi trial.
J Lingappa: Provided samples and data used to analyze the performance of the multi-assay algorithm.
B Auvert: Provided samples and data used to analyze the performance of the multi-assay algorithm.
CS Morrison: Provided samples and data used to analyze the performance of the multi-assay algorithm.
LM Mofensen: NICHD Medical Officer for the PEPI-Malawi trial; involved with the original design of the PEPI-Malawi trial and with monitoring of adverse events during the trial, and contributed to manuscript revisions.
A Taylor: CDC Medical Officer for the PEPI-Malawi trial.
MG Fowler: Former CDC Medical Officer for the PEPI-Malawi trial; involved with the original design of the PEPI Malawi trial and with monitoring of adverse events during the trial, and contributed to manuscript preparation.
NI Kumenda: Malawi PI for the PEPI-Malawi trial; involved with the original design of the PEPI-Malawi trial and with monitoring of adverse events during the trial.
SH Eshleman: Conceived of the study, coordinated the study, responsible for study design, data interpretation, and manuscript preparation.
References
- 1.Interagency Task Team on Prevention of HIV Infection in Pregnant Women M, and their Children. [Accessed October 2010];Guidance on global scale-up of the prevention of mother-to-child transmission of HIV. 2007 http://www.who.int/hiv/pub/guidelines/pmtct_scaleup2007/en/index.html.
- 2.Dunn DN, ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet. 1992;340:585–588. doi: 10.1016/0140-6736(92)92115-v. [DOI] [PubMed] [Google Scholar]
- 3.Liang K, Gui X, Zhang YZ, Zhuang K, Meyers K, Ho DD. A case series of 104 women infected with HIV-1 via blood transfusion postnatally: high rate of HIV-1 transmission to infants through breast-feeding. J Infect Dis. 2009;200(5):682–6. doi: 10.1086/605123. [DOI] [PubMed] [Google Scholar]
- 4.Lockman S, Creek T. Acute maternal HIV infection during pregnancy and breast-feeding: substantial risk to infants. J Infect Dis. 2009;200(5):667–9. doi: 10.1086/605124. [DOI] [PubMed] [Google Scholar]
- 5.Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23(10):1255–9. doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- 6.Lindback S, Karlsson AC, Mittler J, Blaxhult A, Carlsson M, Briheim G, et al. Viral dynamics in primary HIV-1 infection. Karolinska Institutet Primary HIV Infection Study Group. AIDS. 2000;14(15):2283–91. doi: 10.1097/00002030-200010200-00009. [DOI] [PubMed] [Google Scholar]
- 7.Soogoor M, Daar ES. Primary human immunodeficiency virus type 1 infection. Curr HIV/AIDS Rep. 2005;2(2):55–60. doi: 10.1007/s11904-005-0019-1. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Krogstad P, Korber BT, Koup RA, Muldoon M, Macken C, et al. Maternal HIV-1 viral load and vertical transmission of infection: the Ariel Project for the prevention of HIV transmission from mother to infant. Nat Med. 1997;3(5):549–52. doi: 10.1038/nm0597-549. [DOI] [PubMed] [Google Scholar]
- 9.Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341(6):394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 10.John GC, Nduati R, Mbori-Ngacha D. Correlataes of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;(183):206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 11.Mmiro FA, Aizire J, Mwatha AK, Eshleman SH, Donnell D, Fowler MG, et al. Predictors of early and late mother-to-child transmission of HIV in a breastfeeding population: HIV Network for Prevention Trials 012 experience, Kampala, Uganda. J Acquir Immune Defic Syndr. 2009;52(1):32–9. doi: 10.1097/QAI.0b013e3181afd352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumwenda J, Matchere F, Mataya R, Chen S, Mipando L, Li Q, et al. Coverage of highly active antiretroviral therapy among post-partum women in Malawi. International Journal of STDs and AIDS. 2011 doi: 10.1258/ijsa.2011.010359. [In Press] [DOI] [PubMed] [Google Scholar]
- 13.Quinn TC, Brookmeyer R, Kline R, Shepherd M, Paranjape R, Mehendale S, et al. Feasibility of pooling sera for HIV-1 viral RNA to diagnose acute primary HIV-1 infection and estimate HIV incidence. AIDS. 2000;14(17):2751–7. doi: 10.1097/00002030-200012010-00015. [DOI] [PubMed] [Google Scholar]
- 14.Gay CL, Mwapasa V, Murdoch DM, Kwiek JJ, Fiscus SA, Meshnick SR, et al. Acute HIV infection among pregnant women in Malawi. Diagn Microbiol Infect Dis. 2010;66(4):356–60. doi: 10.1016/j.diagmicrobio.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharsany AB, Hancock N, Frohlich JA, Humphries HR, Abdool Karim SS, Abdool Karim Q. Screening for ‘window-period’ acute HIV infection among pregnant women in rural South Africa. HIV Med. 2010;11(10):661–5. doi: 10.1111/j.1468-1293.2010.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy G, Parry JV. Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill. 2008;13(36) [PubMed] [Google Scholar]
- 17.Guy R, Gold J, Calleja JM, Kim AA, Parekh B, Busch M, et al. Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: a systematic review. Lancet Infect Dis. 2009;9(12):747–59. doi: 10.1016/S1473-3099(09)70300-7. [DOI] [PubMed] [Google Scholar]
- 18.Mastro TD, Kim AA, Hallett T, Rehle T, Welte A, Laeyendecker O, et al. Estimating HIV incidence in populations: issues, challenges and the way forward. Journal of HIV/AIDS Surveillance and Epidemiology. 2010;2(1):7. [PMC free article] [PubMed] [Google Scholar]
- 19.Laeyendecker O, Rothman RE, Henson C, Horne BJ, Ketlogetswe KS, Kraus CK, et al. The effect of viral suppression on cross-sectional incidence testing in the Johns Hopkins Hospital emergency department. J Acquir Immune Defic Syndr. 2008;48(2):211–5. doi: 10.1097/QAI.0b013e3181743980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinda ET, Hargrove J, Preiser W, Slabbert H, van Zyl G, Levin J, et al. Significantly diminished long-term specificity of the BED capture enzyme immunoassay among patients with HIV-1 with very low CD4 counts and those on antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;53(4):496–9. doi: 10.1097/qai.0b013e3181b61938. [DOI] [PubMed] [Google Scholar]
- 21.Dobbs T, Kennedy S, Pau CP, McDougal JS, Parekh BS. Performance characteristics of the immunoglobulin G-capture BED-enzyme immunoassay, an assay to detect recent human immunodeficiency virus type 1 seroconversion. J Clin Microbiol. 2004;42(6):2623–8. doi: 10.1128/JCM.42.6.2623-2628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laeyendecker O, Oliver A, Neal J, Gamiel J, Krauss C, Eshleman SH, et al. Decreasing HIV incidence and prevalence at the Johns Hopkins emergency department with a concurrent increase of virally suppressed HIV-infected individuals. 16th Conf on Retroviruses and Opportunistic Infections; San Francisco, CA. 2009. p. Abstract # 1045. [Google Scholar]
- 23.Busch MP, Pilcher CD, Mastro TD, Kaldor J, Vercauteren G, Rodriguez W, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS. 2010 doi: 10.1097/QAD.0b013e32833f1142. [DOI] [PubMed] [Google Scholar]
- 24.Masciotra S, Dobbs T, Candal D, Hanson D, Delaney K, Rudolph D, et al. Antibody avidity-based assay for identifying recent HIV-1 infections based on Genetic Systems TM 1/2 plus O EIA. 17th Conf on Retroviruses and Opportunistics Infections; 2010; San Francisco, CA. 2010. p. Abstract #937. [Google Scholar]
- 25.FHI. Development of assays to measure HIV incidence: Meeting Proceedings; Chapel Hill, NC. May 13–14, 2009; 2009. [Accessed Nov 2010]. http://www.fhi.org/en/HIVAIDS/pub/meeting_reports/HIV_inc_assays.htm. [Google Scholar]
- 26.Laeyendecker O, Oliver A, Astemborski J, Owen M, Kirk G, Mehta S, et al. Improved precision of cross-sectional HIV incidence testing using a multi-assay algorithm that includes BED and an avidity assay with modified assay cut-offs. 17th Conf. on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. p. Abstract #935. [Google Scholar]
- 27.Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359(2):119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 28.Morrison CS, Richardson BA, Mmiro F, Chipato T, Celentano DD, Luoto J, et al. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21(1):85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 29.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiamma A, Lissouba P, Amy OE, Singh B, Laeyendecker O, Quinn TC, et al. Can HIV incidence testing be used for evaluating HIV intervention programs? A reanalysis of the Orange Farm male circumcision trial (ANRS-1265) BMC Infect Dis. 2010;10:137. doi: 10.1186/1471-2334-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375(9717):824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taha TE, Kumwenda NI, Gibbons A, Broadhead RL, Fiscus S, Lema V, et al. Short postexposure prophylaxis in newborn babies to reduce mother-to-child transmission of HIV-1: NVAZ randomised clinical trial. Lancet. 2003;362(9391):1171–7. doi: 10.1016/S0140-6736(03)14538-2. [DOI] [PubMed] [Google Scholar]
- 33.Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, Nkhoma C, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA. 2004;292(2):202–9. doi: 10.1001/jama.292.2.202. [DOI] [PubMed] [Google Scholar]
- 34.Hladik W, Olara D, Mermin J, Moore D, Were W, Alexander L, et al. Effect of CD4(+) T Cell Count and Antiretroviral Treatment on Two Serological HIV Incidence Assays. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/AID.2010.0347. [DOI] [PubMed] [Google Scholar]
- 35.Karita E, Price M, Hunter E, Chomba E, Allen S, Fei L, et al. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS. 2007;21(4):403–8. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- 36.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 37.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198(5):687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 38.Sagar M, Laeyendecker O, Lee S, Gamiel J, Wawer MJ, Gray RH, et al. Selection of HIV variants with signature genotypic characteristics during heterosexual transmission. J Infect Dis. 2009;199(4):580–9. doi: 10.1086/596557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagar M. HIV-1 transmission biology: selection and characteristics of infecting viruses. J Infect Dis. 2010;202 (Suppl 2):S289–96. doi: 10.1086/655656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray GE, Tiemessen CT, de Bruyn G. Immune-based prevention of mother-to-child HIV-1 transmission. Curr Opin Mol Ther. 2007;9(2):168–75. [PubMed] [Google Scholar]
- 41.Lehman DA, Farquhar C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev Med Virol. 2007;17(6):381–403. doi: 10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- 42.Braunstein SL, van de Wijgert JH, Nash D. HIV incidence in sub-Saharan Africa: a review of available data with implications for surveillance and prevention planning. AIDS Rev. 2009;11(3):140–56. [PubMed] [Google Scholar]
- 43.Bulterys M, Chao A, Habimana P, Dushimimana A, Nawrocki P, Saah A. Incident HIV-1 infection in a cohort of young women in Butare, Rwanda. AIDS. 1994;8(11):1585–91. doi: 10.1097/00002030-199411000-00010. [DOI] [PubMed] [Google Scholar]