Abstract
Aims
Recent studies have demonstrated that augmentation of lymphangiogenesis and tissue engineering hold promise as a treatment for lymphedema. The purpose of this study was to determine whether adipose-derived stem cells (ASCs) can be used in lymphatic tissue-engineering by altering the balance between pro- and anti-lymphangiogenic cytokines.
Materials & methods
ASCs were harvested and cultured in media with or without recombinant VEGF-C for 48 h. ASCs were then implanted in mice using Matrigel plugs. Additional groups of animals were implanted with ASCs transfected with a dominant-negative TGF-β1 receptor-II adenovirus with or without VEGF-C stimulation, since TGF-β1 has been shown to have potent antilymphangiogenic effects. Lymphangiogenesis, lymphatic differentiation and cellular proliferation were assessed.
Results
Stimulation of ASCs with VEGF-C in vitro significantly increased expression of VEGF-A, VEGF-C and Prox-1. ASCs stimulated with VEGF-C prior to implantation induced a significant (threefold increase) lymphangiogenic response as compared with control groups (unstimulated ASCs or empty Matrigel plugs; p < 0.01). This effect was significantly potentiated when TGF-β1 signaling was inhibited using the dominant-negative TGF-β1 receptor-II virus (4.5-fold increase; p < 0.01). Stimulation of ASCs with VEGF-C resulted in a marked increase in the number of donor ASCs (twofold; p < 0.01) and increased the number of proliferating cells (sevenfold; p < 0.01) surrounding the Matrigel. ASCs stimulated with VEGF-C expressed podoplanin, a lymphangiogenic cell marker, whereas unstimulated cells did not.
Conclusion
Short-term stimulation of ASCs with VEGF-C results in increased expression of VEGF-A, VEGF-C and Prox-1 in vitro and is associated with a marked increase lymphangiogenic response after in vivo implantation. This lymphangiogenic response is significantly potentiated by blocking TGF-β1 function. Furthermore, stimulation of ASCs with VEGF-C markedly increases cellular proliferation and cellular survival after in vivo implantation and stimulated cells express podoplanin, a lymphangiogenic cell marker.
Keywords: adipose-derived stem cells, antilymphangiogenic, lymphangiogenesis, TGF-β, tissue engineering, VEGF-C
Secondary lymphedema is a dreaded complication of cancer treatment resulting from injury to the lymphatic system [1]. Patients who suffer from lymphedema have severe, life-long and often progressive arm or leg swelling resulting in frequent infections, secondary malignancies, functional issues, such as heaviness, stiffness and pain, and decreased quality of life [2]. Unfortunately, lymphedema is very common and affects a large number of cancer survivors. It may seem surprising, for example, that lymphedema is more common than chemotherapy-induced cardiomyopathy or secondary tumors resulting from chemotherapy or radiation [1,3,4]. In fact, it is estimated that 20-40% of breast cancer patients who undergo axillary lymph node dissection go on to develop lymphedema [1,5]. Lymphedema is also a common complication of other solid tumors, with one recent meta-analysis reporting an overall rate of 16% in a variety of cancers [6].
Current treatment for lymphedema is palliative and designed to mechanically drain interstitial fluid from the affected extremity, and then prevent its re-accumulation by wrapping the limb in tight compressive garments. Recent experimental animal studies, however, have shown promise in curing lymphedema by transferring lymph nodes or lymphangiogenic cytokines to the area of injury [7-9]. Clinical application of these studies has been hampered, however, by the fact that harvesting even a few lymph nodes from another area can potentially cause lymphedema in the donor extremity [10]. In addition, delivery of lymphangiogenic cytokines alone has had limited success in some circumstances [11,12].
Our group and others have recently shown that lymphangiogenesis is a complex process and it is dependent not only on molecules that promote lymphangiogenesis, but also cytokines that prevent lymphangiogenesis [13-15]. This balance of pro- and anti-lymphangiogenic forces is critical and may be responsible for circumstances in which delivery of lymphangiogenic cytokines, such as VEGF-C, fail to induce lymphatic repair [11,12]. For example, we have shown that lymphatic fluid stasis during wound repair induces the expression of TGF-β1, an anti-lymphangiogenic growth factor that directly decreases lymphatic endothelial cell (LEC) proliferation, tubule formation and migration [13,15]. Increased TGF-β1 expression in this setting prevents lymphatic repair and results in markedly diminished lymphatic function despite high levels of VEGF-C expression. As a result, inhibition of TGF-β1 promotes lymphatic repair resulting in improved lymphatic function and restoration of homeostasis [14]. Collectively, these findings suggest that the regulation of lymphangiogenesis, similar to angiogenesis, is a complex and well-orchestrated balance between pro- and anti-lymphangiogenic mechanisms.
Tissue engineering may hold promise in the treatment of lymphedema, since this approach may obviate the need for harvesting lymphatic tissues from another region of the body, thereby removing the potential for lymphedema at the donor site. In this way, lymphatic vessels can be engineered in vitro or in vivo and used to bypass damaged lymphatic channels. In support of this concept, we have recently shown that acellular matrices can support lymphatic regeneration and that transferred lymphatic structures can be used to bypass damaged lymphatics [16,17]. Delivery of mesenchymal stem cells (MSCs) in these settings may be even more effective since recent studies have shown that these cells can improve angiogenesis and wound repair [18]. Adipose-derived stem cells (ASCs) hold particular promise since these cells are readily available with minimal donor site morbidity and share significant phenotypic similarity with other MSCs by retaining the capacity to differentiate into a number of adult cell types [18-20].
The purpose of the current study was to determine if ASCs can be used in lymphatic tissue engineering by altering the balance between pro- and anti-lymphangiogenic cytokines. We demonstrate that short-term stimulation of ASCs with VEGF-C or inhibition of TGF-β1 potently induces lymphangiogenesis. Concomitant stimulation with VEGF-C and blockade of TGF-β1 was even more effective. Furthermore, we show that exposure of ASCs to VEGF-C induces the expression of podoplanin, a lymphatic cell marker in transferred cells and significantly increases cellular proliferation of transferred cells.
Materials & methods
Harvest, culture & characterization of ASCs
Three-week-old green fluorescent protein (GFP) transgenic mice (C57BL/6-g[CAG-EGFP]1Osb/J; Jackson Laboratories, Bar Harbor, ME, USA) were euthanized via carbon dioxide asphyxiation. ASCs were isolated and cultured using a modification of our previously published methods [21]. Briefly, inguinal fat pads were excised and finely minced. Tissues were digested with 0.1% type II collagenase (Sigma, St Louis, MO, USA) dissolved in RPMI with 10 mM Hepes incubated in a shaking water bath for 45 min at 37 °C. The collagenase was then inactivated by adding an equal volume of media with 10% fetal calf serum. The digested fat was centrifuged at 350 g for 10 min. The supernatant was discarded and the pellet resuspended and filtered through a 100-μm cell strainer to remove undigested tissue fragments. Cells were resuspended and cultured in Mesencult media (Stemcell technologies, Vancouver, BC, Canada) supplemented with penicillin and streptomycin, in a humidified 5% CO2 incubator at 37°C. Nonadherent cells were discarded after 48 h and media was changed every 2-3 days. Only early passage cells (<5 passages) were used. All animal experiments were approved by the Institutional Animal Care and Use Committee at the Memorial Sloan-Kettering Cancer Center.
ASCs were characterized for expression of known MSC markers (Sca1, CD29, CD73 and CD105) and absence of hematopoietic cell markers (CD34, CD45 and CD31) using our previously described flow cytometry techniques [21,22]. Briefly, cells were trypsinized, washed and 1 × 106 cells were incubated with fluorescent-tagged primary antibodies (from eBioscience; catalogue numbers as follows: Sca1 17-5981–81, CD29 12-0291-81, CD73 12-0731-81, CD105 12-1051-81, CD34 12-0341-81, CD45 15-045-81 [San Diego, CA, USA]; and CD31 from Biolegend [San Diego, CA, USA]) for 30 min at 4°C. Cells were then washed and assayed using the FACSCalibur flow-cytometer (BD Biosciences, San Jose, CA, USA) and data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
In order to analyze the pluripotential differentiation capacity of harvested ASCs, we induced bone, fat and cartilage differentiation using our previously published techniques [22,23]. Briefly, bone differentiation was induced by exposing cells to MesenCult® stem cell media supplemented with 5 mM β-glycerophosphate, 10−8 M dexamethasone and 0.28 mM ascorbic acid (all from Sigma-Aldrich, St Louis, MO, USA) for 14 days. Von Kossa staining was then performed as previously described to stain mineralized bone nodules [22,23]. For fat differentiation, cells were initially cultured in MesenCult stem cell media supplemented with 10−6 M dexamethasone (Sigma), 1 mM insulin (Sigma) and 0.25 mM 3-isobutyl-1-methyl-xanthine for 4 days and then maintained in MesenCult supplemented with just insulin (1 mM) for 10 days. Cells were then stained with 0.18% Oil Red O (Sigma) to stain fat droplets. Finally, to promote chondrogenic differentiation, cells were cultured in serum-free μ-Minimal Essential Medium (Gibco BRL, Gaithersburg, MD, USA) with 10 ng/ml TGF-β3 and 0.2 mM ascorbic acid (Sigma) for 14 days. Cartilage cells were stained with Alcian blue (Sigma) dissolved in glacial acetic acid for 15 min.
Adenoviral transfection
In order to block TGF-β signaling, we transfected isolated ASCs with an adenovirus-expressing a soluble, defective type 2 TGF-β receptor (DN-RII) lacking the intracellular signaling domain of the endogenous receptor as described in detail in our previous publications [14,24]. Briefly, the transgene in this construct is expressed under the control of the constitutively active cytomegalovirus, and overexpression and secretion of the defective receptor by transfected cells competes with the endogenous TGF-β receptor, thereby acting in a dominant-negative manner to suppress TGF-β function. We have previously shown that secretion of the transgene by transfected cells results in regional blockade of TGF-β activity, and improves lymphangiogenesis by increasing LEC migration, proliferation and tubule formation [14]. An adenovirus with the identical genetic background expressing the bacterial B-Galactosidase gene (B-Gal) was used as a control for adenoviral transfection.
Adenoviruses were grown in 293 cells, purified and quantified using our previously published methods [25]. ASCs (1×106) were transfected with DN-RII or B-Gal viruses at a multiplicity of infection of 100 plaque forming units per cell. Cells were harvested 48 h later for evaluation and in vivo experimentation as outlined below.
In vivo lymphangiogenesis assay
We used Matrigel as a carrier to determine whether ASCs can be used in lymphatic tissue engineering and whether changing the balance of pro- and anti-lymphangiogenic cytokines affects this process. The use of Matrigel in this context was based on the fact that this material has been previously used successfully for lymphangiogenesis assays and also because Matrigel contains basement membrane proteins, thereby preventing fibrous tissue ingrowth and re-absorption of ASCs [15,26]. We divided the animals (n = 6-8 per group) into five groups and used adult female (8-12 weeks old) C57B6 mice (Jackson Labs). These mice have the same genetic background as the mice from which we harvested ASCs but do not express GFP, thereby enabling us to track implanted cells. Groups 1 and 2 served as controls and were subdermally implanted with 300 μl of growth factor-depleted Matrigel (BD Biosciences) containing no cells or unstimulated ASCs (5 × 105 cells). Group 3 animals also served as controls and were implanted with ASCs transfected with the B-Gal virus. Animals in groups 4-6 were experimental and were implanted with Matrigel plugs containing ASCs transfected with the DN-RII virus, ASCs cultured for 48 h prior to implantation in media supplemented with recombinant human VEGF-C (100 ng/ml; R&D Microsystems, Minneapolis, MN, USA), or ASCs transfected with DN-RII virus and 1 day following transfection cultured for 48 h prior to implantation in media supplemented with recombinant VEGF-C. Each group had six to eight animals and each animal underwent placement of two Matrigel plugs (one on either side of the upper, dorsal back). We specifically chose not to add VEGF-C to the Matrigel plugs as this intervention is known to promote lymphangiogenesis independent of ASCs [27].
Specimen preparation, histology & immunofluorescent staining
Matrigel plugs were harvested 2 weeks after implantation, fixed in 4% paraformaldehyde at room temperature overnight, embedded in paraffin and sectioned (5 μm). To ensure identical specimen harvest and fixation, plugs were harvested using a 5-mm punch to include the overlying skin, thus enabling us to use capillary lymphatics in the dermis as our positive control for immunohistochemistry. LECs were localized using antibodies directed against lymphatic-specific markers, podoplanin (syrian hamster monoclonal; Ab11936; Abcam, Cambridge, MA, USA) and LYVE-1 (AF413; mouse monoclonal; R&D Systems). To localize blood vessels and microvascular endothelial cells, sections were stained with antibodies against von Willebrand Factor ([vWF] rabbit polyclonal; Ab6994; Abcam). GFP-positive cells were identified with mouse monoclonal anti-GFP antibodies (Abcam). We chose to use immunofluorescence staining of GFP rather than endogenous florescence based on our preliminary studies demonstrating insufficient tissue section quality with frozen sections. Finally, proliferating cells were identified using mouse monoclonal antibodies against PCNA (Ab2426; Abcam, Cambridge, MA, USA).
Immunofluorescent secondary antibodies used were Alexa Fluor 594 and 647 (Molecular Probes®, Invitrogen, Carlsbad, CA, USA) and counterstaining was performed using Prolong mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). For immunohistochemical staining, secondary antibody from the Vectastain® ABC Kit (Vector Laboratories, Burlingame, CA, USA) was used and developed using diaminobenzidine with counterstaining performed using hematoxylin (Dako). Negative-control sections were incubated with secondary antibody only. Images were obtained using bright-field microscopy (Leica TCS, Buffalo Grove, IL, USA) for immunohistochemistry and confocal microscopy (Leica) for immunofluorescence. Cell and vessel counts were performed in three to five high power fields per section (n = 8-10 per group) by two blinded reviewers.
PCR
ASCs were cultured for 2 days in recombinant human VEGF-C (100 ng/ml; R&D Microsystems) or media alone followed by harvest and isolation of RNA using the QIAGEN Allprep RNA/DNA/Protein Isolation kit (Valencia, CA, USA) according to the manufacturer’s instructions. Reverse transcription was performed using TaqMan® Reverse Transcription reagents (Applied Biosystems, Foster City, CA, USA) followed by semiquantitative reverse transcriptase-PCR (RT-PCR) using TaqMan® Universal Mastermix (Applied Biosystems) and LightCycler® thermocycler (Roche Diagnostics, Indianapolis, IN, USA). Expression of Prox-1 (primer Mm00435969_ m1; Applied Biosystems), LYVE-1 (primer Mm00475056; Applied Biosystems), VEGFR3 (primer Mm0043337_m1; Applied Biosystems), VEGF-A (primer Mm00437308m1; Applied Biosystems) and VEGF-C (Mm01202432_m1; Applied Biosystems) was normalized to 18S (primer Mm03928990_g1; Applied Biosystems) measured concurrently. Experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, USA). Multigroup comparison was analyzed by one-way ANOVA with post-hoc Tukey-Kramer test. Mean values and standard deviations presented with p < 0.05 are considered significant unless otherwise noted.
Results
ASCs derived from GFP transgenic animals are multipotent & express GFP
Adipose-derived stem cells harvested from GFP transgenic animals strongly expressed GFP both in situ and in culture (Figure 1A & B). In addition, these cells maintained the potential to differentiate along the bone, fat and cartilage paths when exposed to appropriate environmental stimuli (Figure 1C-e). Flow cytometry analysis demonstrated that, although ASCs were comprised of a mixed cell population, the majority of cells expressed typical MSC markers (Sca1 [56% positive], CD29 [98.4% positive], CD105 [32.4% positive], CD73 [11.7% positive]) and lacked cell surface expression of hematopoietic stem cell markers (CD45, CD31, CD34; Figure 1F-L ). The LEC marker VEGFR3 was positive in 3.1% of ASCs (Figure 1M). As we have previously shown, transfection of ASCs in vitrowith the DN-RII or B-Gal virus was highly efficient (>75% of cells) and resulted in high-level transgene expression (not shown) [25,28].
Figure 1. Adipose-derived stem cells derived from green fluorescent protein transgenic animals are multipotent and express green fluorescent protein.
(A & B) Isolated epigastric fat pad (A) and cultured adipose-derived stem cells (ASCs) harvested from GFP transgenic mice. (C) Von Kossa staining of ASCs differentiated in bone differentiation media. (D) Oil red O staining of ASCs differentiated in adipogenic media. (E) Alcian Blue staining of ASCs differentiated in cartilage differentiation media. Scale bars represent 50 μm. (F-M) Flow cytometry analysis of isolated ASCs demonstrating expression of mesenchymal stem cell markers (F) Sca1, (G) CD29, (H) CD73 and (I) CD105 and lack of expression of hematopoietic cell and lymphatic endothelial cell markers (J) CD45, (K) CD34, (L) CD31 and (M) VEGFR3. GFP: Green fluorescent protein.
Short-term stimulation of ASCs with VEGF-C or inhibition of TGF-β increases lymphangiogenesis
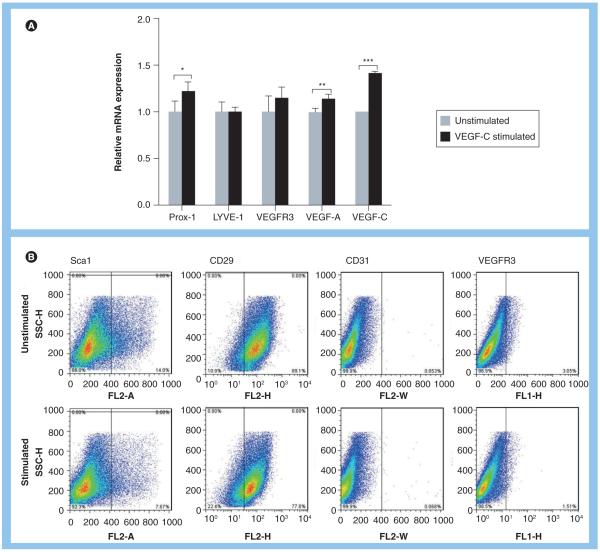
We sought to determine whether unstimulated ASCs or ASCs stimulated briefly in culture with VEGF-C can modulate lymphangiogenesis in vivo. In addition, we aimed to determine whether the balance of pro- and anti-lymphangiogenic stimuli can alter this response. First, we characterized phenotypic responses of ASCs after 48 h of culture with VEGF-C in vitro prior to implantation within Matrigel. PCR analysis revealed significant upregulation of Prox-1 (1.2-fold; p < 0.05), VEGF-C (1.4-fold; p < 0.001) and VEGF-A (1.13-fold; p < 0.01, Figure 2A) expression but no significant changes in LYVE-1 or VEGFR3 expression, suggesting that VEGF-C exposure introduced early but incomplete differentiation toward the LEC lineage. In support of this finding, flow cytometry analysis of cells before and after VEGF-C stimulation similarly revealed no changes in VEGFR3 expression (Figure 2B) but demonstrated a reduction in the expression of stem cell markers initially highly expressed by ASCs (46% reduction in Sca1 expression; 12.8% reduction in CD29; Figure 2B).
Figure 2. Adipose-derived stem cells stimulated with VEGF-C demonstrate early expression of lymphatic endothelial cell markers and loss of stem cell markers.
Podoplanin staining of tissues harvested from various groups after Matrigel implantation. (A) Relative mRNA expression by adipose-derived stem cells for Prox-1, LYVE-1, VEGF-C, VEGF-A and VEGFR-3 following 48-h exposure to VEGF-C in vitro. Baseline expression of these markers is set to one in unstimulated cells (*p < 0.05, **p < 0.01 and ***p < 0.001). (B) Flow cytometry analysis of isolated adipose-derived stem cells demonstrating expression of stem cell markers (Sca1, CD29) and endothelial and lymphatic endothelial cell markers (CD31, VEGFR3) before (upper panel) and after 48-h stimulation with VEGF-C (lower panel) in vitro.
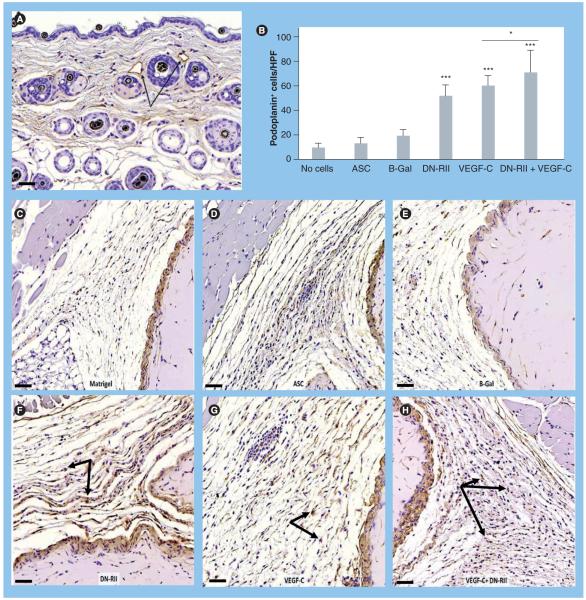
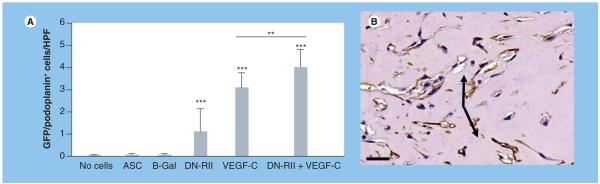
Localization of the lymphatic specific marker podoplanin demonstrated specific staining for capillary lymphatics in the dermis (Figure 3A). Cell counts of podoplanin-positive cells surrounding the Matrigel plug demonstrated no statistical differences between acellular Matrigel plugs, Matrigel plugs containing ASCs alone or Matrigel plugs containing ASCs transfected with B-Gal adenovirus (Figure 3B-e). By contrast, constructs containing ASCs transfected with DN-RII virus demonstrated a 4.5-fold increase in the number of podoplanin-positive cells/high-powered field (HPF) surrounding the Matrigel plug (p < 0.001; Figure 3B & F). Culturing ASCs in media containing VEGF-C for 48 h prior to implantation also significantly increased the number of podoplanin positive cells/HPF (p < 0.001; Figure 3B & g). Interestingly, ASCs transfected with the DN-RII virus and subsequently cultured for 48 h with VEGF-C prior to implantation resulted in an even more potent lymphangiogenic response than either the DN-RII virus alone or pretreatment with VEGF-C alone (p < 0.001; Figure 3B & H). Augmentation of lymphangiogenesis in the region surrounding the Matrigel plug was secondary to expansion of host cells rather than direct differentiation of donor ASCs since we did not observe GFP-positive cells in the regenerated lymphatic tissues (not shown).
Figure 3. Inhibition of TGF-β1 increases the number of podoplanin-positive cells in response to VEGF-C stimulation.
(A) Podoplanin staining of dermal capillary lymphatics (arrows) demonstrating specific staining (5× magnification). Scale bar represents 50 μm. (B) Cell counts of podoplanin-positive cells surrounding implanted Matrigel plugs in experimental and control groups (mean + standard deviation; each bar represents sections obtained from three to four HPF by two independent reviewers per animal with four to five animals per group). Note significant increase in podoplanin-positive cells in animals implanted with Matrigel plugs containing ASCs transfected with DN-RII virus or stimulated with VEGF-C for 48 h prior to implantation compared with Matrigel implanted with ASCs alone (***p < 0.001). Also note additive effect of DN-RII transfection and VEGF-C stimulation (*p < 0.05). (C-H) Representative figures of podoplanin staining in tissues harvested from animals implanted with Matrigel without cells (C), unstimulated ASCs (D) ASCs transfected with B-Gal virus (E), ASCs transfected with DN-RII virus (F), ASCs stimulated with recombinant VEGF-C for 48 h prior to implantation (G) and ASCs transfected with DN-RII virus and stimulated with VEGF-C prior to implantation (H) (20× magnification). Arrows show positively stained cells. Counterstain was performed with hematoxylin. Scale bars represent 50 μm. ASC: Adipose-derived stem cell; B-Gal: B-galactosidase; DN-RII: Dominant-negative TGF-β receptor II; FGFR3: FGF receptor 3; HPF: High-powered field.
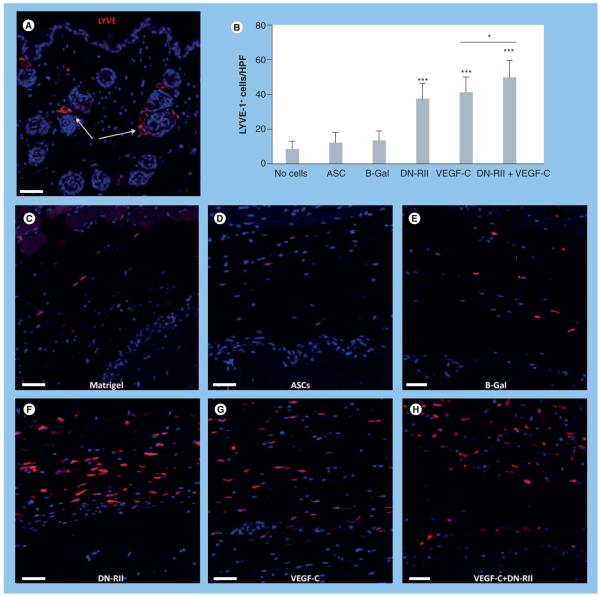
Examination of LYVE-1, another lymphatic specific marker, demonstrated similar patterns as those noted for podoplanin (Figure 4) . Once again, we noted specific staining of capillary lymphatics in the dermis (Figure 4A). In addition, similar to podoplanin staining, we noted no differences in the number of LYVE-1-positive cells/HPF when comparing Matrigel without cells, ASCs alone or ASCs transfected with the B-Gal adenovirus (Figure 4B-e). Analysis of constructs containing ASCs transfected with DN-RII or after pre-treatment with VEGF-C prior to implantation demonstrated a significant increase in the number of LYVE-1-positive cells/HPF (1.8-2-fold, respectively; p < 0.001; Figure 4B & F-H). Further, similar to our findings with podoplanin staining, we noted a significant increase in the number of LYVE-1-positive cells/HPF when concomitant blockade of TGF-β1 and pretreatment with VEGF-C was performed (p < 0.001 compared with DN-RII or VEGF-C alone). Taken together, these findings suggest that even a short-term exposure of ASCs to lymphangiogenic stimuli (VEGF-C) can induce lymphangiogenesis in the host tissues after transplantation. In addition, the balance of pro- and anti-lymphangiogenic cytokines is important since inhibition of antilymphangiogenic mechanisms in combination with short-term stimulation with prolymphangiogenic cytokines is even more effective than either therapy alone. Finally, our findings suggest that ASCs delivered without either of these stimuli do not inherently promote lymphangiogenesis.
Figure 4. Inhibition of TGF-β increases the number of LYVE-1-positive cells in response to VEGF-C stimulation.
Florescent LYVE-1 staining of tissues harvested from various groups after Matrigel implantation. (A) LYVE-1 staining of dermal capillary lymphatics (arrows) demonstrating specific staining (5× magnification). (B) Cell counts of LYVE-1-positive cells surrounding implanted Matrigel plugs in experimental and control groups (mean + standard deviation; each bar represents sections obtained from three to four high-powered fields by two independent reviewers per animal with four to five animals per group). Note significant increase in LYVE-1-positive cells in animals implanted with Matrigel plugs containing ASCs transfected with DN-RII virus or stimulated with VEGF-C for 48 h prior to implantation as compared with Matrigel implanted with ASCs alone (*p < 0.001). Also note the additive effect of DN-RII transfection and VEGF-C stimulation (***p < 0.001). (C-H) Representative figures of LYVE-1 staining in tissues harvested from animals implanted with Matrigel without cells (C), unstimulated ASCs (D), ASCs transfected with B-Gal virus (E), ASCs transfected with DN-RII virus (F), ASCs stimulated with recombinant VEGF-C for 48 h prior to implantation (G) and ASCs transfected with DN-RII virus and stimulated with VEGF-C prior to implantation (H) (20× magnification). All sections were counterstained with DAPI. Scale bars represent 50 μm. ASC: Adipose-derived stem cell; B-Gal: B-galactosidase; DN-RII: Dominant-negative TGF-β receptor II.
ASCs express podoplanin in vivo after stimulation with VEGF-C
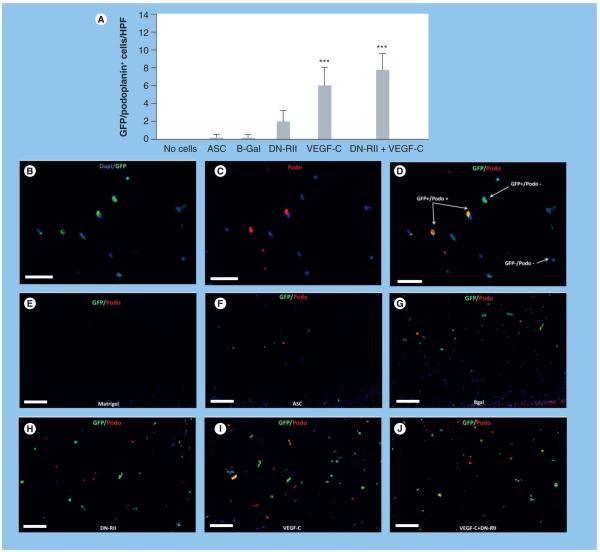
Mesenchymal cells and ASCs have been shown to differentiate into endothelial cells with appropriate environmental stimuli [19,29]. In addition, MSCs have been shown to express lymphatic markers after stimulation in vitro [30]. Therefore, in order to determine whether ASCs can express LEC markers in vivo, we analyzed ASCs within the Matrigel construct for expression of podoplanin and LYVE-1. We identified donor ASCs and their potential for expression of LEC markers by co-localizing GFP and podoplanin or LYVE-1. We found very few podoplanin-positive cells in unstimulated ASCs (received no VEGF-C prior to implantation [Figure 5A, F & g]). Transfection of ASCs with DN-RII resulted in a 92% increase in the number of GFP-positive/ podoplanin-positive cells per HPF; however, this change was not statistically significant (Figure 5A & H). By contrast, stimulation of ASCs with VEGF-C prior to implantation in Matrigel resulted in a significant increase in the number of double-positive cells (97% increase as compared with ASCs alone; p < 0.001; Figure 5A-D, i & J). Blockade of TGF-β in conjunction with pre-implantation exposure to VEGF-C further increased the number of GFP-positive/podo-/podoplanin-positive cells; however, this difference was not statistically significant when compared with VEGF-C stimulation alone.
Figure 5. Adipose-derived stem cells express podoplanin in vivo after stimulation with VEGF-C.
(A) Counts of GFP-positive/ podoplanin-positive cells/HPF in Matrigel plugs harvested from various groups (mean + standard deviation; each bar represents sections obtained from three to four HPFs by two independent reviewers per animal with four to five animals per group). Note the significant increase in number of double-positive cells in animals implanted with Matrigel plugs containing ASCs stimulated with VEGF-C as compared with plugs implanted with unstimulated ASCs (***p < 0.001). Transfection with DN-RII caused a modest increase in double-positive cells; however this change was not statistically significant (B-D). Representative florescent double staining of GFP (green; [B]), podoplanin (red; [C]) and overlay ([D] 40× magnification). Dapi stain (blue) was used to stain nuclei of live cells (E-J). Representative double immunofluorescent straining of GFP (green) and podoplanin (red) staining in the various experimental groups (as indicated; 20×). ASC: Adipose-derived stem cell; B-Gal: B-galactosidase; DN-RII: Dominant-negative TGF-β receptor II; GFP: Green fluorescent protein; HPF: High-powered field.
We found that some of the podoplanin-positive cells in the groups transfected with DN-RII or stimulated with VEGF-C had assumed a tubular, vessel-like structure in the Matrigel (Figure 6A & B). The number of these tubular structures was significantly increased by DN-RII treatment but even more so when cells were stimulated with VEGF-C prior to implantation (p < 0.001). By contrast to our podoplanin-positive cell counts, we found that combination of VEGF-C stimulation and DN-RII transfection was additive with significantly more podoplanin-positive vessels as compared with VEGF-C stimulation alone (p < 0.01). Interestingly, we found that ASCs did not express LYVE-1, another lymphatic cell marker, even when cells were stimulated with VEGF-C, in any of the experimental groups evaluated (not shown).
Figure 6. Adipose-derived stem cells form podoplanin-positive tubular structures following VEGF-C stimulation or TGF-β1 inhibition.
(A) Counts of podoplanin-positive vessels/HPF in Matrigel plugs harvested from various groups (left; mean + standard deviation) and (B) representative image of podoplanin-positive vessels (arrows). Note significant increase in number of podoplanin-positive vessels/HPF in groups treated with ASCs transfected with DN-RII virus or stimulated with VEGF-C prior to implantation (***p < 0.001). Also note the additive effect of DN-RII transfection (**p < 0.01). Scale bar represents 50 μm. ASC: Adipose-derived stem cell; B-Gal: B-galactosidase; DN-RII: Dominant-negative TGF-β receptor II; HPF: High-powered field.
Treatment with VEGF-C increases ASC proliferation
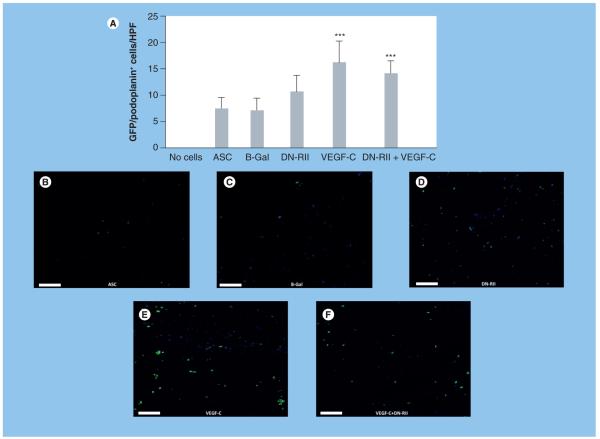
Analysis of GFP staining in the Matrigel plug demonstrated that constructs containing ASCs stimulated prior to implantation with VEGF-C had significantly more donor ASCs (GFP-positive cells) as compared with other groups (Figure 7A-F; p < 0.001). In fact, Matrigels implanted with VEGF-C-treated ASCs contained twofold more GFP-positive cells as compared with ASCs transferred without VEGF-C treatment (p < 0.001). TGF-β1 blockade also modestly increased the number of GFP-positive cells; however, this increase was not statistically significant (Figure 7A & D). Similarly, concomitant TGF-β1 blockade and preimplantation exposure to VEGF-C did not result in increased numbers of GFP+ cells as compared with VEGF-C stimulation alone. As expected, we found no GFP-positive cells in acellular Matrigel constructs (not shown).
Figure 7. VEGF-C treatment increases the number of adipose-derived stem cells.
(A) Counts of GFP-positive cells/HPF in various experimental groups. Note the increase in number of GFP-positive cells/HPF in animals implanted with Matrigel plugs containing ASCs stimulated with VEGF-C prior to implantation (***p < 0.001). (B-F) Representative florescent staining of GFP-positive cells (green) in various experimental groups as noted (20× magnification). Scale bars represent 50 μm. ASC: Adipose-derived stem cell; B-Gal: B-galactosidase; DN-RII: Dominant-negative TGF-β receptor II; GFP: Green fluorescent protein; HPF: High-powered field.
In order to determine how VEGF-C increased ASC numbers, we co-localized GFP with PCNA; Figure 8A-D. We have previously shown that this technique can be used to identify proliferating cells.[15] This analysis demonstrated a sevenfold increase in the number of proliferating ASCs when cells were stimulated with VEGF-C prior to implantation (GFP-positive/PCNA-positive; Figure 8A; p < 0.001). We also found a modest increase (twofold) in proliferating ASCs after TGF-β1 blockade (p < 0.01). However, similar to our GFP counts, we found no significant increase in the number of proliferating ASCs when TGF-β1 blockade was combined with VEGF-C stimulation. Taken together, these findings suggest that stimulation of ASCs with VEGF-C or blockade of TGF-β1 increase ASC proliferation in vivo, however, these effects, unlike the changes in lymphangiogenesis, are not additive.
Figure 8. VEGF-C increases ASC proliferation.
(A) Counts of GFP-positive/PCNA-positive cells/HPF in various experimental groups. Note significant increase in double-positive cells in animals implanted with Matrigel plugs containing ASCs stimulated with VEGF-C prior to implantation (***p < 0.001). (C-D) Fluorescent staining for GFP (green; [B]), PCNA (red; [C]) and overlay, demonstrating GFP-positive/PCNA-positive and GFP-positive/PCNA-negative cells (40× magnification [D]). Scale bars represent 50 μm. ASC: Adipose-derived stem cell; B-Gal: B-galactosidase; DN-RII: Dominant-negative TGF-β receptor II; GFP: Green fluorescent protein; HPF: High-powered field; PCNA: Proliferating cell nuclear antigen.
Inhibition of TGF-β1 signaling does not augment angiogenesis in response to VEGF-C
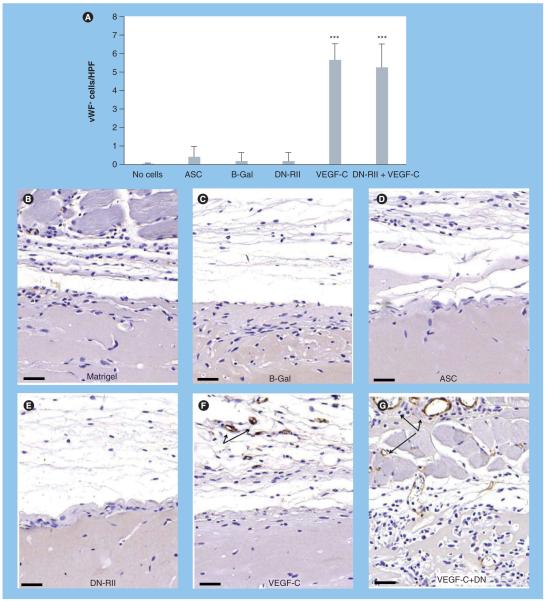
In order to determine whether blockade of TGF-β1 in conjunction with VEGF-C stimulation also has a synergistic effect on angiogenesis, we evaluated the expression of vWF in the regions surrounding the Matrigel plugs in our various groups (Figure 9). Similar to our findings with lymphangiogenesis, we found very few vWF-positive cells or blood vessels in animals treated with acellular Matrigel, Matrigel-containing unstimulated ASCs, or Matrigel plugs with B-Gal transfected ASCs (Figure 9A-D). By contrast, we found a significant (fivefold) increase in the number of vWF-positive cells in the regions surrounding Matrigel plugs containing ASCs stimulated with VEGF-C prior to implantation (p < 0.001; Figure 9A & F). In contrast to our lymphangiogenesis findings, however, we saw no significant effect of TGF-β1 blockade on vWF expression either when this treatment was performed in isolation or in combination with VEGF-C stimulation (Figure 9e & g). These findings suggest that TGF-β1-induced inhibition of lymphangiogenesis is independent of effects on microvascular endothelial cells.
Figure 9. Inhibition of TGF-β signaling does not augment angiogenesis in response to VEGF-C stimulation.
(A) Cell counts of vWF-positive cells/HPF in various experimental groups (mean + standard deviation). Note significant increase in cell counts only in groups stimulated with VEGF-C prior to implantation (***p < 0.001). (B-G) Representative figures (20× magnification) of vWF staining in various experimental groups as noted. Arrows show vWF-positive cells. Scale bars represent 50 μm. ASC: Adipose-derived stem cell; B-Gal: B-galactosidase; DN-RII: Dominant-negative TGF-β receptor II; HPF: High-powered field; vWF: von Willebrand factor.
Discussion
In this study we found that short-term (48 h) stimulation of ASCs with VEGF-C in culture can induce a potent lymphangiogenic response when the cells are subsequently implanted in vivo. Our findings are supported by and add to those of Hwang et al., who recently demonstrated that delivery of ASCs in hydrogels containing VEGF-C significantly increases lymphangiogenesis when implanted in a mouse hindlimb model [31]. One notable difference between our study and those reported by Hwang et al. is that we stimulated ASCs with VEGF-C prior to implantation in vivo (i.e., the Matrigel plug contained no additional VEGF-C). This difference is important and suggests that VEGF-C stimulated ASCs have an altered phenotype and can independently promote lymphangiogenesis. This hypothesis is supported by our data demonstrating that VEGF-C stimulation for 48 h leads to significant upregulation of Prox-1, VEGF-C and VEGF-A mRNA expression by ASCs. Furthermore, in response to VEGF-C stimulation, ASC stem cell marker expression, including Sca1 and CD29, was reduced, suggesting these cells begin to differentiate in response to VEGF-C. This finding is further supported by a recent study by Conrad and colleagues demonstrating that stimulation of peripheral blood MSCs with VEGF-C induces the expression of LEC markers [30]. Stimulation of stem cells with VEGF-C prior to implantation has important clinical implications since this approach avoids delivery of large amounts of VEGF-C, thereby potentially avoiding regeneration of hyperplastic but dysfunctional lymphatics as has been previously reported [11,12]. In addition, delivery of high concentrations of VEGF-C in cancer patients (the population at highest risk for developing secondary lymphedema in developed countries) may have the unwanted effect of stimulating cancer recurrence or metastasis, as this cytokine is a central regulator of tumor growth and spread in a wide variety of solid tumors [32-34].
An additional finding in our study that adds to the existing literature on stem cells and lymphangiogenesis is the observation that blockade of TGF-β1, an antilymphangiogenic cytokine, can also induce lymphangiogenesis and that concomitant blockade of TGF-β1 significantly potentiates the effects of VEGF-C. This finding is important because it may provide an alternative approach to circumstances in which augmentation of VEGF-C expression alone is insufficient to promote lymphatic regeneration. For example, using the mouse-tail model Rutkowski et al. have shown that lymphatic stasis increases VEGF-C expression, but that this response actually exacerbates edema due to formation of poorly functional, hyperplastic lymphatic vessels [12]. Similarly, Goldman et al. reported that overexpression of VEGF-C in this model failed to increase LEC migration or function despite regeneration of hyperplastic lymphatics [11]. Using a similar model, our group has shown that blockade of TGF-β1 markedly accelerates lymphatic regeneration in this scenario, and that this molecule has direct antilymphangiogenic effects in wound repair [14,15]. Thus, the balance between pro- and antilymphangiogenic factors may be a critical regulator of lymphatic regeneration and efforts directed at optimizing these forces are likely to be an important step in clinical application.
In the current study, we found that unstimulated ASCs did not induce lymphangiogenesis when implanted in vivo. This finding contrasts with to previous studies by Hwang et al. and Conrad et al. [30,31]. For example, Hwang found that ASCs alone, delivered via a hydrogel, led to an increased number of lymphatic vessels in a hindlimb lymphedema model in which the lymphatic vessels were ligated after a circumferential incision of the thigh. Similarly, Conrad et al. showed that intradermal injections of MSCs in a mouse-tail skin excision model were associated with improved lymphangiogenesis and lymphatic function in this model. One possible explanation for the differences noted between these reports and our current study is that the former experiments were confounded by the presence of a wound. Therefore, delivery of ASCs to wounds may have simply led to enhanced overall wound healing secondary to delivery of ASCs or MSCs, subsequently leading to enhanced lymphatic regeneration rather than direct stimulation of lymphangiogenesis by ASCs. This is supported by previous studies demonstrating improved wound repair after local or systemic delivery of ASCs [35-37]. It is also possible that differences in delivery (i.e., hydrogel or direct intradermal injection) had an effect on lymphangiogenesis. These concepts are interesting and require additional investigation.
We found that exposure of ASCs to VEGF-C resulted in expression of podoplanin in vivo. This is supported by the findings of Conrad et al. who showed that in vitro exposure of peripheral blood-derived MSCs to LEC conditioned media or VEGF-C resulted in upregulation of podoplanin expression [30]. In addition, we found that the number of podoplanin-positive vessels in Matrigel constructs containing ASCs stimulated with VEGF-C was potentiated by inhibition of TGF-β1, suggesting the combination of VEGF-C and blockade of antilymphangiogenic signals is more effective in inducing lymphatic differentiation. This is supported by the findings of Oka et al. and our group demonstrating that inhibition of TGF-β increases expression of LEC markers, including LYVE-1 and Prox-1, by isolated human dermal LECs [15,38].
Interestingly, we found that stimulation of ASCs with VEGF-C or inhibition of TGF-β1 did not result in expression of LYVE-1. This finding is similar to those of Lee et al., who found that stimulation of bone marrow derived mononuclear cells with VEGF-C in vitro results in only temporary expression of Prox-1 and LYVE-1; expression is present for the first 4 days in culture but is completely lost on culture day 7 or longer time periods [39]. By contrast, podoplanin expression is maintained even 10 days after initiation of VEGF-C stimulation. Similarly, Conrad et al. reported lymphatic differentiation of MSCs in response to in vitro stimulation with VEGF-C resulting in podoplanin expression; however, expression of Prox-1 or LYVE-1 was not analyzed [30]. Thus, it is possible that lymphatic differentiation of MSCs in response to VEGF-C is limited and requires additional stimuli to acquire the complete phenotype of mature LECs; however, this hypothesis warrants additional investigation.
Only one recent study has suggested that MSCs could differentiate into LECs, incorporating into newly formed lymphatics and expressing LYVE-1 after in vivo transfer into a wound [31]. However, in this study the authors used co-localization without z-stacking to evaluate coexpression of MSC markers and LYVE-1 making it difficult to conclude definitively that in vivo co-localization was due to expression in a single cell with a single nucleus rather than simply adjacent cells. In fact, it is possible that ASCs may contribute to supporting structures around collecting lymphatics since these vessels are known to have pericytes [9]. This hypothesis is supported by the findings of Sasaki et al., who showed that MSCs contribute to wound healing by differentiating into a number of skin cell types including endothelial cells and pericytes [37]. Similarly, Zannettino et al. have shown that ASCs can also differentiate into pericytes [40].
In the current study, we found that VEGF-C pre-treatment significantly increased ASC proliferation after implantation in vivo. In fact, exposure of ASCs to VEGF-C doubled the number of donor cells present in the Matrigel plug after 2 weeks and was associated with a sevenfold increase in the number of proliferating ASCs in vivo. These observations are supported by and add to those of Hwang et al. who demonstrated that stimulation of ASCs with VEGF-C increased cellular proliferation in vitro [31]. It is also possible that VEGF-C increased ASC numbers in our study by other mechanisms distinct from increased cellular proliferation alone. For example, VEGF-C treatment may inhibit programmed cell death or senescence as supported by the findings of Wang and colleagues on the effects of VEGF-C on LECs [41]. In addition, this hypothesis is supported by the findings of Pons and colleagues, who demonstrated that VEGF-A increases survival of bone marrow-derived MSCs in vitro and in vivo by inhibiting cellular senescence and decreasing expression of proapoptotic genes [42].
Finally, we found that VEGF-C pretreatment of ASCs significantly increased angiogenesis surrounding Matrigel plugs after in vivo implantation. This finding suggests that stimulated ASCs produce cytokines that promote both angiogenesis and lymphangiogenesis. Interestingly, we found that concomitant inhibition of TGF-β1, by contrast to lymphangiogenesis, had no effect on angiogenesis, suggesting that the antilymphangio genic effects of TGF-β1 are specific rather than global changes in cytokine expression patterns.
Conclusion
In conclusion, we have shown that short-term stimulation of ASCs with VEGF-C results in a marked increase lymphangiogenic response after in vivo implantation and that blocking TGF-β1 function potentiates this response. Furthermore, exposure of ASCs to VEGF-C markedly increases cellular proliferation and cellular survival after in vivo implantation and stimulated cells express the lymphangiogenic cell marker podoplanin.
Future perspective
Transfer of ASCs is a technique that holds promise for the reconstruction and regeneration of tissues, given the differentiation potential of these cell populations. Furthermore, the availability of the cells and ease in attaining them with minimal morbidity to the donor makes ASCs an attractive candidate for tissue reconstruction. This study, and others, have recently suggested that these cells have the potential to differentiate in response to lymphangiogenic signals, including VEGF-C, and promote lymphangiogenesis through direct or indirect mechanisms. Given these findings, the therapeutic potential of ASCs in the treatment and/or prevention of lymphedema is strong; however, future investigation is required to characterize the role of these cells in promoting lymphatic repair.
Executive summary.
Adipose-derived stem cells are responsive to VEGF-C stimulation & can promote lymphangiogenesis
-
■
In vitro stimulation of adipose-derived stem cells (ASCs) with VEGF-C increases VEGF-C expression in vitro and induces lymphangiogenesis after in vivo implantation.
-
■
Stimulation of ASCs with VEGF-C increases expression of podoplanin; a lymphatic endothelial cell marker.
-
■
VEGF-C markedly increases ASC survival after in vivo delivery.
-
■
VEGF-C stimulation in vitro increases ASC proliferation after in vivo implantation.
-
■
ASCs stimulated with VEGF-C in vitro may be useful for lymphatic tissue engineering.
Modulation of TGF-β1 function can alter lymphangiogenic response by stimulated ASCs
-
■
Blockade of TGF-β1 function potentiates lymphangiogenesis resulting from in vitro VEGF-C stimulation.
-
■
Inhibition of TGF-β1 function increases proliferation of stimulated ASCs after in vivo implantation.
Footnotes
Financial & competing interests disclosure
This manuscript was funded in part by a Plastic Surgery Education Foundation Grants to Tomer Avraham and BJ Mehrara. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J. Clin. Oncol. 2008;26(35):5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonneterre J, Roche H, Kerbrat P, et al. Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French adjuvant study group. J. Clin. Oncol. 2004;22(15):3070–3079. doi: 10.1200/JCO.2004.03.098. [DOI] [PubMed] [Google Scholar]
- 4.Fallahi B, Adabi K, Majidi M, et al. Incidence of second primary malignancies during a long-term surveillance of patients with differentiated thyroid carcinoma in relation to radioiodine treatment. Clin. Nucl. Med. 2011;36(4):277–282. doi: 10.1097/RLU.0b013e31820a9fe3. [DOI] [PubMed] [Google Scholar]
- 5.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J. Clin. Oncol. 2008;26(21):3536–3542. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 6.Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-ana lysis of cancer-related secondary lymphedema. Cancer. 2010;116(22):5138–5149. doi: 10.1002/cncr.25458. ■ Meta-analysis of up to 8000 patients demonstrating an overall risk of up to 16% for developing secondary lymphedema in patients treated for gynecologic, sarcoma, melanoma and head and neck cancers.
- 7.Baker A, Kim H, Semple JL, et al. Experimental assessment of pro-lymphangiogenic growth factors in the treatment of post-surgical lymphedema following lymphadenectomy. Breast Cancer Res. 2010;12(5):R70. doi: 10.1186/bcr2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahteenvuo M, Honkonen K, Tervala T, et al. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation. 2011;123(6):613–620. doi: 10.1161/CIRCULATIONAHA.110.965384. ■ First large-animal study demonstrating efficacy of VEGF-C treatment and autologous lymph node transfer in treating experimentally induced lymphedema.
- 9.Tammela T, Saaristo A, Holopainen T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 2007;13(12):1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 10.Mclaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J. Clin. Oncol. 2008;26(32):5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman J, Le TX, Skobe M, Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ. Res. 2005;96(11):1193–1199. doi: 10.1161/01.RES.0000168918.27576.78. ■ Mouse model of tail lymphedema was used to demonstrate that VEGF-C expression alone does not induce functional lymphatic repair during wound repair but rather promotes regeneration of hyperplastic and dysfunctional lymphatics.
- 12.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc. Res. 2006;72(3):161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avraham T, Clavin NW, Daluvoy SV, et al. Fibrosis is a key inhibitor of lymphatic regeneration. Plast. Reconstr. Surg. 2009;124(2):438–450. doi: 10.1097/PRS.0b013e3181adcf4b. [DOI] [PubMed] [Google Scholar]
- 14.Avraham T, Daluvoy S, Zampell J, et al. Blockade of transforming growth factor-{β}1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 2010;177(6):3202–3214. doi: 10.2353/ajpath.2010.100594. ■ Using the mouse-tail model of lymphedema, the authors demonstrated that local or systemic blockade of TGF-β1 markedly accelerates lymphatic regeneration, inhibits local inflammatory responses and decreases tissue changes associated with lymphatic fluid stasis.
- 15.Clavin NW, Avraham T, Fernandez J, et al. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am. J. Physiol. Heart Circ. Physiol. 2008;295(5):H2113–H2127. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- 16.Wong AK, Schonmeyr B, Singh P, Carlson DL, Li S, Mehrara BJ. Histologic ana lysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast. Reconstr. Surg. 2008;121(4):1144–1152. doi: 10.1097/01.prs.0000302505.43942.07. [DOI] [PubMed] [Google Scholar]
- 17.Yan A, Avraham T, Zampell J, Aschen S, Mehrara B. Mechanisms of lymphatic regeneration after tissue transfer. PLoS ONE. 2011;6(2):E17201. doi: 10.1371/journal.pone.0017201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meliga E, Strem BM, Duckers HJ, Serruys PW. Adipose-derived cells. Cell Transplant. 2007;16(9):963–970. doi: 10.3727/096368907783338190. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005;332(2):370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 20.Tapp H, Hanley EN, Jr, Patt JC, Gruber HE. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp. Biol. Med. (Maywood) 2009;234(1):1–9. doi: 10.3181/0805/MR-170. [DOI] [PubMed] [Google Scholar]
- 21.Schonmeyr B, Clavin N, Avraham T, Longo V, Mehrara BJ. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells. Tissue Eng. Part A. 2009;15(7):1833–1841. doi: 10.1089/ten.tea.2008.0446. [DOI] [PubMed] [Google Scholar]
- 22.Mehrara BJ, Avraham T, Soares M, et al. p21cip/WAF is a key regulator of long-term radiation damage in mesenchyme-derived tissues. FASEB J. 2010;24(12):4877–4888. doi: 10.1096/fj.10-155762. [DOI] [PubMed] [Google Scholar]
- 23.Clavin NW, Fernandez J, Schonmeyr BH, Soares MA, Mehrara BJ. Fractionated doses of ionizing radiation confer protection to mesenchymal stem cell pluripotency. Plast. Reconstr. Surg. 2008;122(3):739–748. doi: 10.1097/PRS.0b013e318180edaa. [DOI] [PubMed] [Google Scholar]
- 24.Haviv YS, Takayama K, Nagi PA, et al. Modulation of renal glomerular disease using remote delivery of adenoviral-encoded soluble type II TGF-β receptor fusion molecule. J. Gene Med. 2003;5(10):839–851. doi: 10.1002/jgm.428. [DOI] [PubMed] [Google Scholar]
- 25.Schonmeyr BH, Soares M, Avraham T, Clavin NW, Gewalli F, Mehrara BJ. Vascular endothelial growth factor inhibits bone morphogenetic protein 2 expression in rat mesenchymal stem cells. Tissue Eng. Part A. 2010;16(2):653–662. doi: 10.1089/ten.tea.2009.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinella F, Garrafa E, Di Castro V, et al. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69(6):2669–2676. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- 27.Yoon CM, Hong BS, Moon HG, et al. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/ PLC/Ca2+ signaling pathways. Blood. 2008;112(4):1129–1138. doi: 10.1182/blood-2007-11-125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehrara BJ, Saadeh PB, Steinbrech DS, et al. Adenovirus-mediated gene therapy of osteoblasts in vitro and in vivo. J. Bone Miner. Res. 1999;14(8):1290–1301. doi: 10.1359/jbmr.1999.14.8.1290. [DOI] [PubMed] [Google Scholar]
- 29.Vodyanik MA, Yu J, Zhang X, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7(6):718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conrad C, Niess H, Huss R, et al. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation. 2008;119(2):281–289. doi: 10.1161/CIRCULATIONAHA.108.793208. ■ ■ Demonstrated that treatment of peripheral blood-derived mesenchymal cells with VEGF-C results in expression of podoplanin in a subpopulation of cells and that injection of stem cells into the wound improves lymphedema in a mouse-tail model.
- 31.Hwang JH, Kim IG, Lee JY, et al. Therapeutic lymphangiogenesis using stem cell and VEGF-C hydrogel. Biomaterials. 2011;32(19):4415–4423. doi: 10.1016/j.biomaterials.2011.02.051. ■ ■ Reports the use of hydrogels to deliver VEGF-C and adipose-derived stem cells to a mouse-hindlimb model of lymphatic injury. Animals treated with VEGF-C and cells had decreased edema and augmented lymphangiogenesis.
- 32.Karpanen T, Egeblad M, Karkkainen MJ, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61(5):1786–1790. [PubMed] [Google Scholar]
- 33.Kondo K, Kaneko T, Baba M, Konno H. VEGF-C and VEGF-A synergistically enhance lymph node metastasis of gastric cancer. Biol. Pharm. Bull. 2007;30(4):633–637. doi: 10.1248/bpb.30.633. [DOI] [PubMed] [Google Scholar]
- 34.Sugiura T, Inoue Y, Matsuki R, et al. VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: implications for use as a prognostic marker. Int. J. Oncol. 2009;34(3):673–680. doi: 10.3892/ijo_00000193. [DOI] [PubMed] [Google Scholar]
- 35.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 38.Oka M, Iwata C, Suzuki HI, et al. Inhibition of endogenous TGF-{β} signaling enhances lymphangiogenesis. Blood. 2008;111(9):4571–4579. doi: 10.1182/blood-2007-10-120337. ■ Using inflammatory models of lymphangiogenesis, the authors demonstrated that TGF-β1 has potent and direct antilymphangiogenic effects.
- 39.Lee JY, Park C, Cho YP, et al. Podoplanin- expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122(14):1413–1425. doi: 10.1161/CIRCULATIONAHA.110.941468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J. Cell Physiol. 2008;214(2):413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 41.Wang JF, Zhang X, Groopman JE. Activation of vascular endothelial growth factor receptor-3 and its downstream signaling promote cell survival under oxidative stress. J. Biol. Chem. 2004;279(26):27088–27097. doi: 10.1074/jbc.M314015200. [DOI] [PubMed] [Google Scholar]
- 42.Pons J, Huang Y, Arakawa-Hoyt J, et al. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem. Biophys. Res. Commun. 2008;376(2):419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]