Abstract
The pituitary gland is composed of the adenohypophysis and neurohypophysis. The adenohypophysis contains endocrine cells, folliculo-stellate (FS) cells, and marginal layer cells, whereas the neurohypophysis mainly comprises axons and pituicytes. To understand the molecular nature of water transfer in the pituitary gland, we examined the immunohistochemical localization of the membrane water channels aquaporin-4 (AQP4) and AQP5 in rat tissue. Double immunofluorescence analysis of AQP4 and S100 protein, a known marker for FS cells, marginal layer cells, and pituicytes, clearly revealed that FS cells and marginal layer cells in the adenohypophysis and the pituicytes in pars nervosa are positive for AQP4. AQP5 was found to be localized at the apical membrane in some marginal layer cells surrounding the Rathke’s residual pouch, in which AQP4 was observed to be localized on the basolateral membranes. These results suggest the following possibilities: 1) FS cells especially require water for their functions and 2) transepithelial water transfer could occur between the lumen of Rathke’s residual pouch and the interstitial fluid in the adenohypophysis through the AQP4 and AQP5 channels in the marginal layer cells.
Keywords: aquaporin, rat, pituitary gland, folliculo-stellate (FS) cell
I. Introduction
The pituitary gland is composed of the adenohypophysis and neurohypophysis. The adenohypophysis is further divided into three parts: the pars tuberalis, pars distalis and pars intermedia, whereas the neurohypophysis is divided into the infundibulum and pars nervosa. The adenohypophysis is also composed of several types of granular cells, which are the endocrine cells that produce pituitary hormones, and agranular cells. Folliculo-stellate (FS) cells are agranular stellate-shaped follicle-forming cells located in the parenchyma of the pars tuberalis and pars distalis [4]. These cells were first distinguished as chromophobes by electron microscopic observations and subsequently termed “folliculo-stellate” cells by Vila-Porcile [30] in accordance with their morphological characteristics [4]. FS cells encircle neighboring endocrine cells via their cytoplasmic processes and establish intra-pituitary communication via gap junctions [4, 9, 25]. Although it is well known that FS cells have scavenger activity by engulfing degenerated cells and produce various growth factors and cytokines [10], their function is not completely understood.
Aquaporins are integral membrane proteins that facilitate the efficient transfer of water across the membrane. In mammalian cells, 13 isoforms of aquaporins (AQP0–AQP12) have been identified to date [19, 29]. The expression of these isoforms varies according to the cell and tissue types involved in the fluid transport [15, 28]. Kuwahara et al. [11] have reported previously via RT-PCR analysis that AQP1, AQP3, AQP4, and AQP5 are expressed in the rat pituitary gland. These researchers also examined the immunohistochemical localization of these aquaporin isoforms by the use of commercial antibodies in a subsequent study [12]. We have raised antibodies against each aquaporin isoform in our own laboratory and previously examined the tissue distribution of these isoforms in detail [1, 13, 17–19]. In our present study, we have performed detailed immunolocalization analysis of AQP4 and AQP5 in the rat pituitary gland using our own antibodies, because we have found that some commercially available aquaporin antibodies, including an anti-AQP4 antibody, sometimes produce non-specific immunoreactions (Matsuzaki et al., unpublished data). We present some new findings in our current study that add to the body of evidence reported in earlier studies [11, 12].
II. Materials and Methods
Antibodies
The AQP1, AQP3, and AQP4 antibodies used in this study were AffRaTM31 [16], AffRaTM5 [14], and AffRaTM13 [1], respectively. The AQP5 antibodies used were AffRaTM14 [13], AffRaTM41 [17], and AffGPTM41 [17]. The mouse anti-S100 protein (beta-subunit) antibody was purchased from Sigma-Aldrich (S2532; Sigma-Aldrich, St. Louis, MO).
Animals and tissue preparation
All animal experiments were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Experimentation Committee, Nippon Medical School (admission no. 21-053) and Gunma University (admission no. 10-028). Eight-week-old male Wistar rats (n=5) were used. Under deep pentobarbital anesthesia (60 mg/kg), the animals were perfused through the left ventricle of the heart with physiological saline, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4. The pituitary glands were removed and fixed overnight at 4°C in the same fixative solution. To prepare paraffin sections, tissues were dehydrated through a graded ethanol series and embedded in paraffin. To prepare cryostat sections, tissues were immersed in 30% sucrose in phosphate-buffered saline (PBS) for cryo-protection, embedded in Tissue-Tek OCT compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan), frozen in liquid nitrogen, and stored at −80°C until use.
Immunohistochemistry
Immunohistochemistry was carried out on paraffin sections or cryostat sections. Paraffin sections were cut, mounted on MAS-coated glass slides (Matsunami, Osaka, Japan), deparaffinized, and then rehydrated. To retrieve the antigen, slides were placed in 20 mM Tris-HCl buffer (pH 9.0) and heated in a microwave oven (MI-77, AZUMAYA, Tokyo, Japan) for 30 min at 97°C [18]. Cryostat sections, which were either mounted on MAS-coated glass slides or unmounted, were processed for the immunolabeling without antigen retrieval.
For immunoperoxidase labeling, endogenous peroxidase activity was blocked by 0.5% H2O2 in methanol for 30 min at room temperature. The non-specific binding of antibodies was blocked by incubation with PBS containing 1% bovine serum albumin (BSA). The samples were then sequentially incubated with primary antibodies diluted in PBS containing 1% BSA overnight at 4°C followed by horseradish peroxidase-coupled goat anti-rabbit antibodies (P0448, diluted in 1:100; DAKO, Glostrup, Denmark). Diaminobenzidine (DAB) reactions were performed using a liquid DAB+ system (K3467, DAKO), after which the sections were stained with hematoxylin, washed with running water, dehydrated in a graded series of ethanol and xylene, and then mounted with Permount (Fisher Scientific, Fair Lawn, NJ).
For immunofluorescence labeling, the non-specific binding of antibodies was blocked by incubation with PBS containing 5% normal donkey serum. The specimens were then sequentially incubated with primary antibodies diluted in PBS containing 5% normal donkey serum overnight at 4°C and then incubated with fluorescently labeled secondary antibodies for 90 min at room temperature. The fluorescently labeled secondary antibodies [27] used were as follows: 1) Rhodamine Red-X-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA); 2) Rhodamine Red-X-conjugated donkey anti-guinea pig IgG (Jackson Immunoresearch); 3) AlexaFluor 488-conjugated donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA); 4) fluorescein isothiocyanate (FITC)-conjugated donkey anti-guinea pig IgG (Jackson Immunoresearch); and 5) AlexaFluor 488-conjugated donkey anti-goat IgG (Invitrogen). For nuclear counterstaining, 4',6-diamidino-2-phenylindole (DAPI) was added to the mixture of secondary antibodies. Specimens were mounted with Vectashield (Vector Laboratories, Burlingame, CA).
Peroxidase-labeled specimens were observed under an AX-80 microscope (Olympus, Tokyo, Japan). Fluorescently labeled specimens were observed with a BX-62 or AX-80 microscope equipped with Nomarski differential interference-contrast and epifluorescence optics (Olympus), or with a LSM710 laser confocal microscope (Carl Zeiss, Gottingen, Germany).
Histochemical controls
To examine the specificity of the immunostaining for aquaporins, sections were incubated with either a solution without antibodies or a solution with antibodies preabsorbed with the antigen peptide (0.03 mg/ml) rather than the primary antibody.
III. Results
We surveyed the expression of AQP1, AQP3, AQP4, and AQP5 in the rat pituitary gland. AQP1 was positive for capillary endothelial cells similar to other tissues (data not shown). AQP3 was not detected. Both AQP4 and AQP5 were positive and detailed localization was examined as follows.
Immunolocalization of AQP4
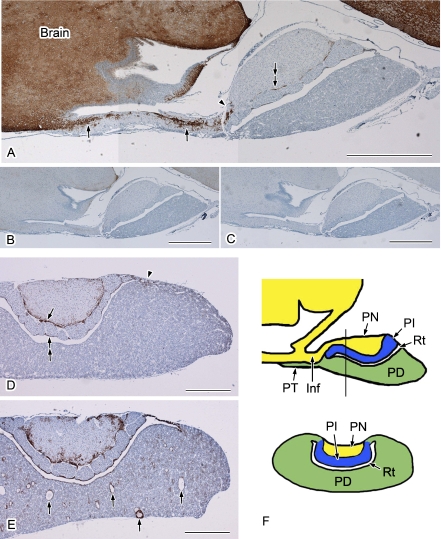
The localization of AQP4 in the rat pituitary gland was examined using immunoperoxidase (Figs. 1 and 2) and immunofluorescence (Fig. 3) staining. There were no differences found between these two methods in terms of the immunostaining patterns of AQP4. No immunostaining was observed using histochemical controls (Fig. 1B, C), which confirmed the specificity of the rabbit anti-AQP4 antibody used in this study. To survey the distribution of AQP4, sagittal sections of the rat pituitary gland connected to the brain via the stalk (Fig. 1A) and coronal sections (Fig. 1D, E) were prepared and examined by immunoperoxidase staining. Upon examination of these sagittal sections at lower magnification, labeling for AQP4 was widely evident in the brain. According to a previous study [21] and our own observations (data not shown), the AQP4-positive cells in the brain are astrocytes. In addition to the brain, strong labeling for AQP4 was observed in the infundibulum extending to the pars nervosa (Fig. 1A). Based on this labeling pattern and following double labeling with astrocyte markers such as glial fibrillary acidic protein (GFAP) and S100 protein (data not shown), the AQP4-positive cells also appeared to be astrocytes. In the area closer to the pars nervosa, the strong AQP4 signals were abruptly diminished (Fig. 1A). Upon examination of coronal sections, clear labeling for AQP4 was evident in the periphery of the pars nervosa (Fig. 1D) and in the marginal layer of Rathke’s residual pouch (Fig. 1D). There was also some labeling scattered in the pars distalis and pars nervosa (Fig. 1D). Strongly labeled AQP4-positive cells were often observed in the transitional area between the pars distalis and pars intermedia (Fig. 1D). The expression level of AQP4 differed from animal to animal, although the distribution pattern was similar (Fig. 1D, E). Figure 1E shows an example of higher expression of AQP4 in the pituitary gland, in which cyst-like structures were found in the pars distalis as described previously (Fig. 1E) [22].
Fig. 1.
Distribution of AQP4 in the rat pituitary gland. Paraffin sections were immunolabeled and visualized using a DAB reaction. Nuclei were stained with hematoxylin. Sagittal sections (A–C) and coronal sections (D, E) are shown. A: Strong AQP4 signals are evident in the infundibulum (arrows) as well as in the brain. This labeling is abruptly diminished in the transitional area between the infundibulum and pars nervosa (arrowhead). The periphery of pars nervosa is also positively stained for AQP4 (double-arrow). B, C: Histochemical controls. Anti-AQP4 antibodies were preabsorbed with an antigen peptide (B) or the antibody was omitted (C). D, E: Examples of average (D) and higher (E) expression of AQP4 from different animals. D: Apparent AQP4 labeling is evident in the periphery of the pars nervosa (arrow), marginal layer (double-arrow), and in the transitional area between the pars distalis and pars intermedia (arrowhead). E: The AQP4 staining pattern is similar to that in D, except for the strong labeling in the region surrounding the cyst-like structures (arrows). F: Schematic drawing of the sagittal section (upper) and coronal section (lower) cut along the line shown in the sagittal section. Inf, infundibulum; PN, pars nervosa; PT, pars tuberalis; PD, pars distalis; PI, pars intermedia; Rt, Rathke’s residual pouch. Bars=1 mm (A–C); 500 µm (D and E).
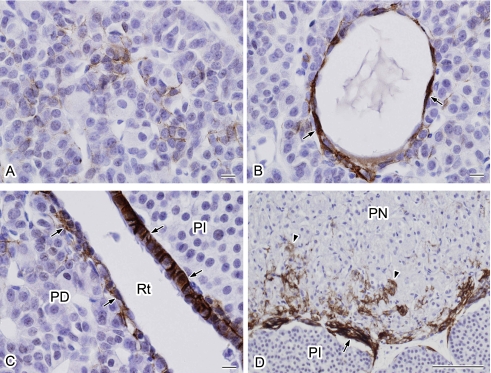
Fig. 2.
Higher magnification views of the localization of AQP4 in the rat pituitary gland. The specimen shown in Figure 1E was examined at a higher magnification. A: AQP4-positive cells are stellate in shape and surround the endocrine cells. B: Cells forming a cyst-like structure are positive for AQP4 (arrows). C: Marginal layer cells covering both pars distalis and pars intermedia are positive for AQP4 (arrows). D: Strong AQP4 signals are localized in the periphery of the pars nervosa (arrow) and in the pars nervosa (arrowheads). PD, pars distalis; PI, pars intermedia; Rt, Rathke’s residual pouch; PN, pars nervosa. Bars=10 µm (A–C); 100 µm (D).
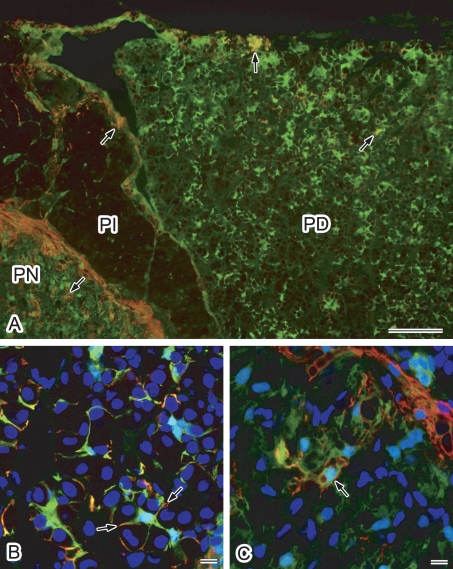
Fig. 3.
Double immunofluorescence localization of AQP4 and S100 protein in the rat pituitary gland. Cryostat sections were labeled for AQP4 (red) and S100 protein (green). Nuclei were stained with DAPI (blue in B and C). A: Conventional fluorescence microscopy at lower magnification. AQP4-positive cells were found to be consistently positive for S100 protein (arrows). Some S100 protein-positive cells were positive for AQP4. B, C: Higher magnification views via laser confocal microscopy. Projection images of consecutive 3-slice confocal images at 1-µm intervals are shown. B: Pars distalis. Labeling for AQP4 accumulates in the processes of S100 protein-positive folliculo-stellate cells (arrows). C: Pars nervosa. Some S100 protein-positive pituicytes are positive for AQP4 (arrow). PD, pars distalis; PI, pars intermedia; PN, pars nervosa. Bars=100 µm (A); 10 µm (B and C).
Figure 2 shows higher magnification views of the AQP4 localization pattern in the pituitary gland. AQP4-positive cells in the pars distalis were found to be stellate in shape and to surround the endocrine cells with long cytoplasmic processes (Fig. 2A). These cells resemble folliculo-stellate cells (FS cells) based on previously documented morphological characteristics [11]. In addition, AQP4-positive cells were detected in the area surrounding the cyst-like structures (Fig. 2B). Marginal layer cells of the Rathke’s residual pouch were labeled on the basolateral side (Fig. 2C). In the pars nervosa, the AQP4-positive cells along the surface (Fig. 2D) appeared to be a glial limiting membrane similar to the brain surface, where AQP4 is also highly expressed in a glial limiting membrane (Fig. 1A). In addition, scattered AQP4 signals were observed in the pars nervosa (Fig. 2D), which seemed to represent pituicytes based on the morphological features of the positively-stained cells.
Double immunofluorescence staining for aquaporin 4 and S100 protein
To identify the AQP4-immunopositive cell types, rat pituitary gland was double stained for AQP4 and S100 protein, a known marker for FS cells, marginal layer cells, and pituicytes [3, 20]. S100 protein-immunoreactive cells were found to be widely scattered in the pars distalis and pars nervosa. Some of the S100-positive cells were positive for AQP4 (Fig. 3A) in both the pars distalis and pars nervosa. Upon careful examination, AQP4-immunopositive cells in the pars distalis were observed to be consistently positive for S100 protein (Fig. 3A). These results confirmed that the AQP4-positive cells are FS cells. Examinations at a higher magnification with a laser confocal microscope revealed that AQP4 accumulates in the processes of S100 protein-positive FS cells. In the pars nervosa, some S100-positive pituicytes were also found to be immunopositive for AQP4 in addition to the cells located in the peripheral area (Fig. 3A, C), confirming that some pituicytes are also AQP4-positive cells.
Immunolocalization of AQP5 in marginal layer cells
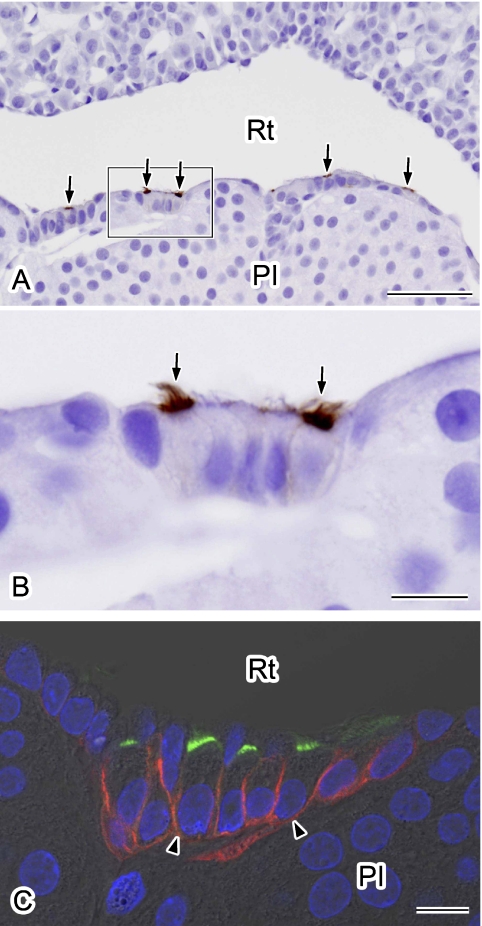
Kuwahara et al. [11] have reported previously that AQP5 is present in some marginal layer cells, although a more detailed localization analysis was not performed. We thus carefully examined the localization of AQP5 in our present experiments. AQP5 staining was confined to the luminal surface of some marginal layer cells lining Rathke’s residual pouch, particularly on the pars intermedia side (Fig. 4A). Upon examination at higher magnification, we found that AQP5 densely accumulates at the apical surface of ciliated marginal cells (Fig. 4B). Double immunofluorescence staining for AQP4 and AQP5 clearly showed that AQP5 is also localized on the apical membranes in some ciliated marginal layer cells in which AQP4 is localized on the basolateral side (Fig. 4C).
Fig. 4.
Immunolocalization of AQP5 in marginal layer cells. Paraffin sections were immunolabeled for AQP5 using an immunoperoxidase method (A, B). Nuclei were stained with hematoxylin. A: Labeling for AQP5 is evident in the apical surface of the marginal layer cells (arrows). B: A higher magnification view of the outlined area in A. AQP5 is localized at the apical membrane of some ciliated cells (arrows). C: Double-immunofluorescence staining for AQP5 (green) and AQP4 (red). Nuclei were stained with DAPI (blue). A single confocal image overlaid with differential interference-contrast image is shown. AQP5-positive cells are positive for AQP4 in their basolateral membranes (arrowheads). PI, pars intermedia; Rt, Rathke’s residual pouch. Bars=50 µm (A); 10 µm (B and C).
IV. Discussion
In our current study, we have surveyed the distribution of AQP1, AQP3, AQP4, and AQP5 in the rat pituitary gland using antibodies raised in our own laboratory. Our results are basically consistent with previously reported findings [11, 12] with some new observations.
Possible function of AQP4 in FS cells
FS cells are located in the parenchymal tissue of the adenohypophysis. They have a characteristic star-like morphology and could form follicles. Their long slender cytoplasmic processes intermingle in a fashion that produces a three-dimensional network [for review, see Ref. 4]. Although there are various views regarding the functional properties of FS cells, they remain almost unknown. Our results showing the expression of AQP4 in FS cells suggest that FS cells may have some function in which much water is required. The question is why AQP4-positive cells are restricted to some FS cells. Although each animal showed individual rate of AQP4-positive cells and expression level, it seems true that AQP4 is abundant in the transitional area between the pars distalis and pars intermedia. FS cells in this area might have more important roles in water handling. It is also true that neighboring FS cells are interconnected via gap junctions throughout the adenohypophysis except for the pars intermedia, suggesting the presence of the gap junctional network throughout the FS cell population [5, 9, 24, 26]. Since gap junctions allow water to pass through [2], FS cells, even if some of them do not have AQP4, could share water via the gap junctional network. In addition, we observed cyst-like structures in the pars distalis in our present experiments, as reported also in several previous studies [6, 7, 22]. Gon [6] and Gon et al. [7] have reported that FS cells are related to cyst-like structure formations. In our present study, we also confirmed that epithelial cells of the cyst-like structure were positive for S100 protein (data not shown). Moreover, we show herein for the first time that cyst-like structures are positive for AQP4. These observations suggest that the cyst-like structures may also contribute to water handling in the pars distalis, and/or that AQP4 influences the formation of cyst-like structures, although the reason why they are formed is unknown.
AQP4 and AQP5 in marginal layer cells
In addition to the presence of AQP4 in basolateral membranes, AQP5 was found to be localized on the apical membrane in some marginal layer cells. This finding suggested that transepithelial water transfer can occur between the lumen of Rathke’s residual pouch and the interstitial fluid in the adenohypophysis. It is also of interest that AQP5 is confined to the apical membrane of ciliated cells, because we have never observed ciliated epithelial cells bearing AQP5 or any other aquaporins in their apical surface. Indeed, AQP5 has been shown to be localized to the apical membrane of non-ciliated cells in the mouse respiratory epithelium [18]. AQP5 is usually localized to the apical membranes of exocrine acinar cells and related to the secretion of water [13]. The reason why AQP5 is specifically localized to the ciliated cells remains a mystery.
Possible function of AQP4 in the pars nervosa
AQP4 was found to be highly expressed in cells located in the periphery of the pars nervosa. In addition, AQP4 was observed to localize on the surface membrane of some S100-positive pituicytes. Virard et al. [31] suggested the presence of some different populations in pituicytes in their previous study. These results raised the possibility that only some cell populations are positive for AQP4. The physiological role of AQP4 in pituicytes is also a fundamental question. AQP4 is also expressed in brain astrocytes and is highly accumulated in the endfeet of the astrocytes facing the capillaries and is involved in water homeostasis in the brain [21]. In contrast, AQP4 in the pituicytes does not seem to accumulate at the perivascular area, suggesting a different function from that in brain astrocytes. Pituicytes show remarkable morphological changes in response to physiological stimuli such as dehydration [8, 23]. Under hypotonic conditions on the other hand, it has been suggested that pituicytes sense hypotonicity, causing taurine release which inhibits vasopressin release from the axon terminal [23]. Hence, pituicytes appear to play an important role as osmotic sensors [23] and AQP4 might be necessary for pituicytes to sense hypotonic or hypertonic conditions. Future analysis of the expression of AQP4 under hypertonic or hypotonic conditions might therefore provide some beneficial new insights into the physiological role of AQP4 in pituicytes.
V. Acknowledgments
We wish to thank K. Matsumoto, E. Furukawa, M. Ichikawa, Y. Takahashi-Tajika, and M. Shimoda for their assistance. This work was supported in part by Grants-in-Aid for Scientific Research (S0801035, 22590230 and 22790191) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
VI. References
- 1.Ablimit A., Matsuzaki T., Tajika Y., Aoki T., Hagiwara H. Immunolocalization of water channel aquaporins in the nasal olfactory mucosa. Arch. Histol. Cytol. 2006;69:1–12. doi: 10.1679/aohc.69.1. [DOI] [PubMed] [Google Scholar]
- 2.Bruzzone R., White T. W., Paul D. L. Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 3.Cocchia D., Miani N. Immunocytochemical localization of the brain-specific S-100 protein in the pituitary gland of adult rat. J. Neurocytol. 1980;9:771–782. doi: 10.1007/BF01205018. [DOI] [PubMed] [Google Scholar]
- 4.Devnath S., Inoue K. An insight to pituitary folliculo-stellate cells. J. Neuroendocrinol. 2008;20:687–691. doi: 10.1111/j.1365-2826.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher W. H., Anderson N. C., Everett J. W. Intercellular communication in the rat anterior pituitary gland. An in vivo and in vitro study. J. Cell Biol. 1975;67:469–476. doi: 10.1083/jcb.67.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gon G. The origin of ciliated cell cysts of the anterior pituitary. An experimental study in the rat. Virchows Arch. A Pathol. Anat. Histopathol. 1987;412:1–9. doi: 10.1007/BF00750723. [DOI] [PubMed] [Google Scholar]
- 7.Gon G., Shirasawa N., Ishikawa H. Appearance of the cyst- or ductile-like structures and their role in the restoration of the rat pituitary autograft. Anat. Rec. 1987;217:371–378. doi: 10.1002/ar.1092170408. [DOI] [PubMed] [Google Scholar]
- 8.Hatton G. I. Pituicytes, glia and control of terminal secretion. J. Exp. Biol. 1988;139:67–79. doi: 10.1242/jeb.139.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Horiguchi K., Fujiwara K., Kouki T., Kikuchi M., Yashiro T. Immunohistochemistry of connexin 43 throughout anterior pituitary gland in a transgenic rat with green fluorescent protein-expressing folliculo-stellate cells. Anat. Sci. Int. 2008;83:256–260. doi: 10.1111/j.1447-073X.2008.00239.x. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K., Couch E. F., Takano K., Ogawa S. The structure and function of folliculo-stellate cells in the anterior pituitary gland. Arch. Histol. Cytol. 1999;62:205–218. doi: 10.1679/aohc.62.205. [DOI] [PubMed] [Google Scholar]
- 11.Kuwahara S., Maeda S., Tanaka K., Hayakawa T., Seki M. Expression of aquaporin water channels in the rat pituitary gland. J. Vet. Med. Sci. 2007;69:1175–1178. doi: 10.1292/jvms.69.1175. [DOI] [PubMed] [Google Scholar]
- 12.Kuwahara S., Maeda S., Ardiles Y., Jun J. G., Tanaka K., Hayakawa T., Seki M. Immunohistochemical localization of aquaporin-4 in the rat pituitary gland. J. Vet. Med. Sci. 2010;72:1307–1312. doi: 10.1292/jvms.10-0087. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki T., Suzuki T., Koyama H., Tanaka S., Takata K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999;295:513–521. doi: 10.1007/s004410051257. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki T., Suzuki T., Koyama H., Tanaka S., Takata K. Water channel protein AQP3 is present in epithelia exposed to the environment of possible water loss. J. Histochem. Cytochem. 1999;47:1275–1286. doi: 10.1177/002215549904701007. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki T., Tajika Y., Tserentsoodol N., Suzuki T., Aoki T., Hagiwara H., Takata K. Aquaporins—a water channel family. Anat. Sci. Int. 2002;77:85–93. doi: 10.1046/j.0022-7722.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki T., Machida N., Tajika Y., Ablimit A., Suzuki T., Aoki T., Hagiwara H., and Takata K. Expression and immunolocalization of water-channel aquaporins in the rat and mouse mammary gland. Histochem. Cell Biol. 2005;123:501–512. doi: 10.1007/s00418-005-0753-x. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki T., Ablimit A., Suzuki T., Aoki T., Hagiwara H., Takata K. Changes of aquaporin 5-distribution during release and reaccumulation of secretory granules in isoproterenol-treated mouse parotid gland. J. Electron Microsc. (Tokyo) 2006;55:183–189. doi: 10.1093/jmicro/dfl023. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki T., Hata H., Ozawa H., Takata K. Immunohistochemical localization of the aquaporins AQP1, AQP3, AQP4, and AQP5 in the mouse respiratory system. Acta Histochem. Cytochem. 2009;42:159–169. doi: 10.1267/ahc.09023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morishita Y., Matsuzaki T., Hara-Chikuma M., Andoo A., Shimono M., Matsuki A., Kobayashi K., Ikeda M., Yamamoto T., Verkman A., Kusano E., Ookawara S., Takata K., Sasaki S., Ishibashi K. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005;25:7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima T., Yamaguchi H., Takahashi K. S100 protein in folliculostellate cells of the rat pituitary anterior lobe. Brain Res. 1980;191:523–531. doi: 10.1016/0006-8993(80)91300-1. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen S., Negelhus E. A., Amiry-Moghaddam M., Bourque C., Agre P., Ottersen O. P. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opper L. Incidence and morphology of epithelial cysts in the anterior lobe of the hypophysis of the rat. Anat. Rec. 1940;76:135–143. [Google Scholar]
- 23.Rosso L., Mienville J. M. Pituicytes modulation of neurohormone output. Glia. 2009;57:235–243. doi: 10.1002/glia.20760. [DOI] [PubMed] [Google Scholar]
- 24.Shirasawa N., Mabuchi Y., Sakuma E., Horiuchi O., Yashiro T., Kikuchi M., Hashimoto Y., Tsuruo Y., Herbert D. C., Soji T. Intercellular communication within the rat anterior pituitary gland: X. Immunohistocytochemistry of S-100 and connexin 43 of folliculo-stellate cells in the rat anterior pituitary gland. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004;278:462–473. doi: 10.1002/ar.a.20040. [DOI] [PubMed] [Google Scholar]
- 25.Shirasawa N., Sakuma E., Wada I., Naito A., Horiuchi O., Mabuchi Y., Kanai M., Herbert D. C., Soji T. Intercellular communication within the rat anterior pituitary: XIV electron microscopic and immunohistochemical study on the relationship between the agranular cells and GnRH Neurons in the dorsal pars tuberalis of the pituitary gland. Anat. Rec. 2007;290:1388–1398. doi: 10.1002/ar.20596. [DOI] [PubMed] [Google Scholar]
- 26.Soji T., Herbert D. C. Intercellular communication between rat anterior pituitary cells. Anat. Rec. 1989;224:523–533. doi: 10.1002/ar.1092240410. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T., Matsuzaki T., Hagiwara H., Aoki T., Takata K. Recent advances in fluorescent labeling techniques for fluorescence microscopy. Acta Histochem. Cytochem. 2007;40:131–137. doi: 10.1267/ahc.07023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takata K., Matsuzaki T., Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog. Histochem. Cytochem. 2004;39:1–83. doi: 10.1016/j.proghi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Verkman A. S. More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 30.Vila-Porcile E. Le reseau des cellules folliculo-stellaires et les les follicules de l’adenohypophyse du rat (Pars distalis). [The network of the folliculo-stellate cells and the follicles of the adenohypophysis in the rat (pars distalis)] Z. Zellforsch. Mikrosk. Anat. 1972;129:328–369. (in French) [PubMed] [Google Scholar]
- 31.Virard I., Gubkina O., Alfonsi F., Durbec P. Characterization of heterogeneous glial cell populations involved in dehydration-induced proliferation in the adult rat neurohypophysis. Neurosci. 2008;151:82–91. doi: 10.1016/j.neuroscience.2007.10.035. [DOI] [PubMed] [Google Scholar]