Abstract
OBJECTIVE
C-reactive protein (CRP) is associated with the risk of cardiovascular disease (CVD); whether the effects are modified by diabetes status still is unclear. This study investigated these issues and assessed the added value of CRP to predictions.
RESEARCH DESIGN AND METHODS
Participants were drawn from representative samples of adults living in England and Scotland. Cox proportional hazards regression models were used to relate baseline plasma CRP with all-cause and CVD mortality during follow-up in men and women with and without diabetes. The added value of CRP to the predictions was assessed through c-statistic comparison and relative integrated discrimination improvement.
RESULTS
A total of 25,979 participants (4.9% with diabetes) were followed for a median of 93 months, during which period there were 2,767 deaths (957 from CVD). CRP (per SD loge) was associated with a 53% (95% CI 43–64) and 43% (38–49) higher risk of cardiovascular and all-cause mortality, respectively. These associations were log linear and did not differ according to diabetes status (both P ≥ 0.08 for interaction), sex, and other risk factors. Adding CRP to conventional risk factors improved predictions overall and separately by diabetes status but not for CVD mortality, although such improvements only were marginal based on several discrimination statistics.
CONCLUSIONS
The association between CRP and CVD was similar across diabetes status, and the effects are broadly similar across levels of other conventional risk factors.
With classic risk factors failing to fully explain the variance in cardiovascular disease (CVD), investigators have sought to identify new risk indices (1–3). This effort has implicated several biomarkers, potentially reflecting different metabolic pathways, in the etiology of CVD (3). C-reactive protein (CRP), an inflammatory biomarker, is one of the most well-documented emerging CVD risk factors (4,5). Concentrations of CRP in the upper part of the distribution within the normal range and above are associated with the long-term risk of CVD and all-cause mortality in different populations (6,7).
There is a suggestion that the association of CRP with CVD is modified by diabetes status (8); however, few such studies exist. In the present population-based cohort studies among individuals with and without diabetes, we investigated the associations of baseline plasma CRP levels with cardiovascular and all-cause mortality. In doing so, we also took the opportunity to investigate whether these associations were modified by sex and other conventional cardiovascular risk factors. In addition, we examined whether the knowledge of CRP can improve CVD risk prediction beyond conventional risk factors alone.
RESEARCH DESIGN AND METHODS
Participants were 25,979 individuals with data available on diabetes status (history of doctor-diagnosed or newly diagnosed diabetes based on an HbA1c ≥6.5%) and CRP at baseline. They were drawn from four prospective U.K. studies comprising either Scottish health surveys (1998 and 2003) or the health surveys for England (1998 and 2003) (9). All cohorts were representative of the general population, sampling individuals living in households in each country. Study participants gave full informed consent, and ethical approval was obtained from the London Research Ethics Council.
The full study protocol has been described in detail elsewhere (10,11). In brief, study members were visited twice in their homes. During the first of these meetings, trained interviewers collected data on demographics and health behaviors, including socioeconomic status (as indexed by occupational social class) and self-reported smoking, alcohol use (frequency per week), and physical activity (frequency of moderate to vigorous sessions per week). Interviewers also collected information about existing physician-diagnosed CVD (stroke, ischemic heart disease, and angina symptoms), other medical conditions (hypertension and diabetes), and antihypertension medications (β-blockers, ACE inhibitors, diuretics, and calcium blockers). During the second visit, conducted within a few days of the first, nurses gathered clinical data. In the seated position, systolic and diastolic blood pressures were measured on three occasions using an Omron HEM-907, with a 5-min rest between each reading; an average of the second and third readings was used in the present analyses.
Biochemical measures
Peripheral blood samples were collected in serum tubes and centrifuged at room temperature. All serum samples were frozen at −70°C until assay. CRP concentrations were analyzed from serum using the N Latex high-sensitivity CRP (hsCRP) mono immunoassay on the Behring Nephelometer II analyzer. The limit of detection was 0.17 mg/L, and the coefficient of variation was <6% for this assay. The analysis of HbA1c levels from plasma was performed using the Tosoh G7 analyzer (Tosoh Bioscience, Worcestershire, U.K.), with a coefficient of variation <2.5%.
Ascertainment of disease-specific mortality
Consenting study members were linked to the National Health Service mortality records, from which a death certificate was located. Classification of the underlying cause of death was based on information on the death certificate together with any additional observations made by the certifying physician. Diagnoses for primary cause of death were made using the ICD-9 and ICD-10, 390–459, denoting CVD deaths.
Statistical methods
Normal distribution was obtained with the natural logarithm (loge) of the positively skewed CRP. Cox proportional hazards regression models were used to compute the hazard ratio and accompanying 95% CI for a 1-SD increase in loge CRP in relation to all-cause and CVD mortality. The proportional hazards assumption was tested with the use of the cumulative sums of Martingale-based residuals methods (12) and found not to be violated. Cox models were also used to compare participants across quintiles of CRP and across three subgroups defined by CRP <1, 1–3, and >3 mg/L (13), with 95% CIs in both analyses derived from the floating absolute-risk methods (14). The log linearity of the association was assessed by fitting a continuous predictor across quintiles of CRP. Interaction between diabetes and CRP was assessed by adding an interaction term to models that included the main effect of diabetes and loge CRP. The heterogeneity of the association also was assessed within sex and other subgroups of participants defined by the level of classical risk factors (above vs. below the median for continuous variables). Heterogeneity across subgroups was assessed through three-way interaction tests.
The predictive utility of the models was assessed overall and separately for participants with and without diabetes by computing the area under the receiver operating characteristic curve (AUC). AUC comparisons used nonparametric methods (15). The relative integrated discrimination improvement (RIDI%), which measures the percentage improvement in discrimination when an extra variable is added to a prediction model (16), was computed. The 95% CIs for the RIDI% were derived with the use of the nonparametric bootstrap percentiles CI method, based on 1,000 replications. We also calculated 1) the likelihood ratio χ2, which compares the adequacy of a model with covariates fitted to a set of data to that of the null model (without covariates) fitted to the same dataset; and 2) the Akaike information criterion (AIC), which allows for comparisons between models (nested or not); the smaller the value of the statistic, the better the model fits the data (17). Finally, we assessed the closeness between predicted and observed outcome rates using the Hosmer and Lemeshow calibration test (18). The basic model included age, sex, smoking, systolic blood pressure, BMI, waist circumference, physical activity, and total cholesterol. Additional models were constructed by adding CRP to the basic model as well as interaction terms of CRP with diabetes status and sex. The incremental value of CRP was further assessed by refitting the Framingham Anderson general equation for the prediction of cardiovascular mortality (without electrocardiographic left ventricular hypertrophy, a missing predictor in our sample) data with and without CRP (19). Comparisons were extended with the computation of the net reclassification improvement (16) based on four categories of 5-year predicted probability (i.e., 0 to <2.5, 2.5 to <5, 5 to <7.5, and ≥7.5%).
The main analyses included all participants with valid data. Sensitivity analyses also were conducted to account for the possible effects of infections and other factors on baseline levels of CRP, because these could distort the association of CRP with outcomes. This was performed by restricting the analyses to those participants with baseline levels of CRP ≤10 mg/L. All data analysis used SAS/STAT version 9.1 for Windows (SAS Institute, Cary, NC).
RESULTS
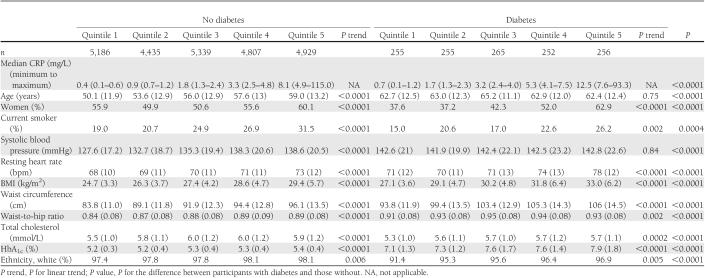
Of 25,979 participants included, 1,283 (4.9%) had diabetes. Participants mainly were white (97.7%), with ethnic minorities (2.4%) comprising three distinct groups (black: 144 [0.6%], Asians: 321 [1.2%], and others: 126 [0.5%]). The median CRP was 1.8 mg/L (interquartile range 0.8–4.1) overall; 1.7 mg/L (0.8–3.6) in men and 2 mg/L (0.8–4.5) in women (Wilcoxon test, P < 0.0001); and 3.2 mg/L (1.5–6.2) and 1.8 mg/L (0.8–4.0), respectively, in participants with and without diabetes (P < 0.0001). The relationships between quintiles of CRP and study covariates are depicted in Table 1 and Supplementary Table 1 based on three subgroups of CRP (i.e., <1, 1–3, and >3 mg/L). The least favorable levels of conventional risk factors were apparent in the higher CRP groups. Most of these relationships were stepwise across the CRP quintiles.
Table 1.
Baseline characteristics across quintiles of CRP according to diabetes status
Fatal outcomes
A median follow-up of 93 months (25th to 75th percentiles 56–118) gave rise to 2,767 deaths (cumulative incidence 10.6%) recorded (including 1,466 [53%] deaths in men and 1,301 [47%] in women), of which 957 (cumulative incidence 3.7%) were of cardiovascular origin (including 535 [56%] cardiovascular deaths in men and 422 [44%] in women). During this period, 305 (23.8%) deaths, including 134 cardiovascular deaths (cumulative frequency 10.4%), were recorded in participants with diabetes. The equivalent in those without diabetes was 2,462 (10%) all-cause deaths and 823 (3.3%) cardiovascular deaths.
CRP and outcomes overall and by diabetes status
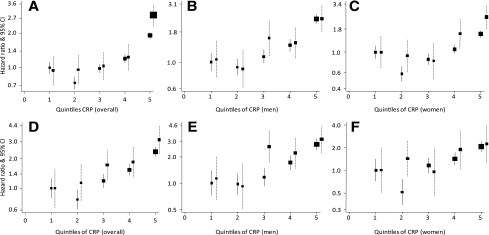
CRP was positively and continuously associated with CVD and all-cause and cardiovascular mortality, with an SD higher loge CRP conferring a 53% (95% CI 43–64) higher risk of cardiovascular death and a 43% (38–49) higher risk of death from any cause. In people with and without diabetes, an SD higher loge CRP was associated with 54% (28–85) and 52% (41–63), respectively, greater risk of cardiovascular death and 53% (35–72) and 41% (35–47), respectively, greater risk of all-cause mortality. There was little evidence of heterogeneity by diabetes status for those associations (both P ≥ 0.08 for interaction). Across quintiles of CRP distribution, there also was a graded association between CRP and mortality overall and in participants with and without diabetes. These associations were log linear for both all-cause and cardiovascular mortality (all P < 0.0001 for log linearity) (Fig. 1). Across subgroups of participants based on three categories of CRP (i.e., <1, 1–3, and >3 mg/L), there also was a graded association between CRP and outcomes, except in people with diabetes, for whom a higher risk of all-cause mortality was observed only in those within the upper stratum (Supplementary Table 2).
Figure 1.
Hazard ratios and 95% CIs for the association between CRP and all-cause (upper panels) and CVD (lower panels) mortality, overall (left column) and in men (middle column) and women (right column) with and without diabetes. Boxes (■) represent the effect estimates (hazard ratio) and the vertical bars represent the 95% CIs (from floating absolute-risk methods) within quintiles of CRP, separately for people with diabetes (broken lines) and those without (solid lines). Cox models are stratified by cohort and adjusted for sex and age. A–F: The hazard ratio for an SD higher loge CRP for people with diabetes versus people without diabetes, the accompanying P value for log linearity of the association (P trend), and the P value for the interaction between diabetes and CRP (P interaction) are all-cause mortality overall 1.53 (95% CI 1.35–1.72; P trend <0.0001) and 1.41 (1.35–1.47; P trend <0.0001; P interaction = 0.08) (A); all-cause mortality in men 1.27 (1.21–1.34; P trend <0.0001) and 1.46 (1.38–1.54; P trend <0.0001; P interaction = 0.56) (B); all-cause mortality in women 1.53 (91.27–1.83; P trend = 0.006) and 1.64 (1.35–1.98; P trend <0.0001; P interaction = 0.06) (C); CVD mortality overall 1.54 (1.28–1.85; P trend <0.0001) and 1.52 (1.42–1.63; P trend <0.0001; P interaction = 0.80) (D); CVD mortality in men 1.41 (1.29–1.54; P trend = 0.0001) and 1.51 (1.38–1.66; P trend <0.0001; P interaction = 0.61) (E); and CVD mortality in women 1.44 (1.07–1.93; P trend = 0.47) and 1.81 (1.36–2.39; P trend <0.0001; P interaction = 0.98) (F).
CRP and outcomes by sex and other conventional risk factors
In men and women with and without diabetes, CRP also was continuously associated with the risk of all-cause and cardiovascular mortality, again with little evidence of statistical interaction (all P ≥ 0.06). For the two outcomes, point estimates for the associations with CRP in women with and without diabetes always were higher than those in their male counterparts. For instance, the hazard ratios for an SD higher loge CRP, diabetes versus no diabetes, were 1.41 (95% CI 1.29–1.54) and 1.51 (1.38–1.66) for cardiovascular death in men and 1.27 (1.21–1.34) and 1.46 (1.38–1.54) for all-cause mortality. The equivalent figures in women were 1.44 (1.07–1.93) and 1.81 (1.36–2.39) for cardiovascular death and 1.53 (1.27–1.83) and 1.64 (1.35–1.98) for all-cause mortality. The higher-order interaction tests always confirmed that associations of CRP with cardiovascular (three-way interaction P = 0.80) and all-cause (three-way interaction P = 0.08) mortality were similar in people with and without diabetes for both sexes. Across quintiles of CRP distribution, graded associations of CRP with both outcomes were observed in men and women with and without diabetes (Fig. 1). Associations mostly were log linear in subgroups defined by sex and diabetes status. Based on the three subgroups of CRP (i.e., <1, 1–3, and >3 mg/L), there also was a graded association of CRP with both outcomes in men and with CVD mortality in women, with a significant high risk of all-cause mortality observed only within the upper strata of CRP (Supplementary Table 2).
The association of CRP with mortality in other subgroups of participants according to diabetes status is summarized in Supplementary Table 3. Within subgroups, the association of CRP with cardiovascular mortality was similar in participants with and without diabetes (all P value ≥17 for interaction). Associations also were broadly similar within subgroups for all-cause mortality but with two exceptions. For this outcome, significant heterogeneity was apparent in those below the median of BMI (P = 0.01 for interaction) and physical activity frequency (P = 0.04), largely driven by the small number of participants with diabetes within those subgroups. Across complementary subgroups, associations of CRP with cardiovascular mortality by diabetes status always were similar (all P ≥ 0.09 for the three-way interaction tests).
Added value of CRP to CVD risk prediction
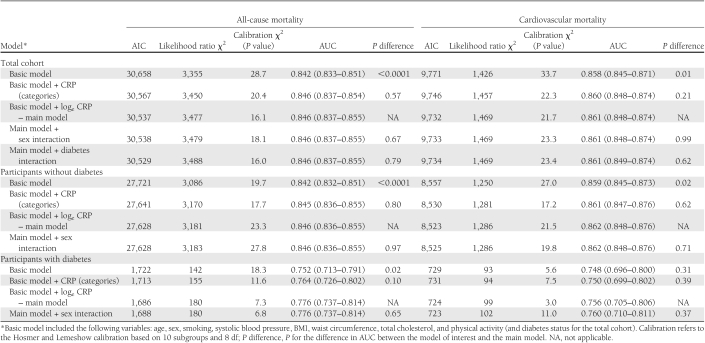
Measures of model performance are summarized in Table 2 for the total cohort and participants with and without diabetes separately. The basic model (without CRP) had acceptable to good discriminatory power in predicting cardiovascular and all-cause mortality, with an AUC ranging from 0.748, for cardiovascular mortality in people with diabetes, to 0.859 for the same outcome in participants without diabetes. Adding CRP to the basic model improved both the model goodness of fit and its discriminatory power. In participants with diabetes, however, there was no evidence for improvements in the AUC for the prediction of cardiovascular death (P = 0.31 for the difference in AUC). Despite some attenuation in the effect sizes, CRP always was positively associated with the outcomes in all multivariable models. Fit statistics for models with continuous loge CRP were better than those for the equivalent models with three categories of CRP (i.e., <1, 1–3, and >3 mg/L). For instance, at the total cohort level, the AIC and Δlikelihood ratio χ2 (main vs. basic models) for CVD mortality were 9,732 and 46, respectively, for models with loge CRP and 9,746 and 31, respectively, for models with three categories for CRP (Table 2). Adding the interaction terms of sex × CRP or diabetes × CRP (applicable only to the total cohort) did not improve the performance of the model (Table 2).
Table 2.
AUCs (95% CIs), AIC, likelihood ratio χ2, and calibration χ2 for the prediction of all-cause and CVD mortality in participants with and without diabetes
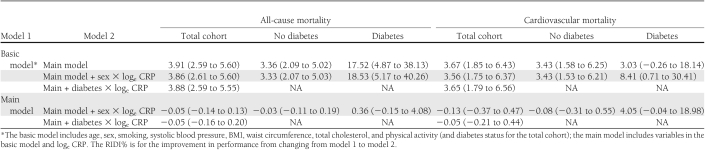
Based on RIDI% estimates, adding CRP to the basic model conferred similar levels of improvement for cardiovascular mortality prediction in the total cohort and in participants with and without diabetes taken separately (Table 3). For the prediction of all-cause mortality, the magnitude of the improvement was greater for participants with diabetes (RIDI% 17.52 [95% CI 4.87–38.13]), although the CI was large and always overlapped with other estimates. For the prediction of CVD mortality in diabetic participants, RIDI% was not significant when CRP was added to the basic model (RIDI% 3.03 [−0.26 to 18.14]).
Table 3.
RIDI% statistics (95% CI) for nested model comparisons
In multivariable Cox models that incorporated components of the Framingham Anderson CVD mortality risk score, CRP was significantly associated with CVD mortality during follow-up (hazard ratio per SD loge 1.36 [95% CI 1.24–1.48]). Change in likelihood ratio χ2 with the addition of loge CRP to this model was significant (change in χ2 = 47.5, P < 0.0001). The AUC for the prediction of 5-year CVD mortality was 0.873 (95% CI 0.856–0.890) for the multivariable model only and 0.877 (0.860–0.894) with the addition of loge CRP (P = 0.02 for the difference in AUC). The net reclassification improvement was −0.4% (P = 0.49).
Sensitivity analysis
CRP level was >10 mg/L in 1,973 participants (including 170 with diabetes) who were excluded in secondary analyses. In the subcohort with CRP ≤10 mg/L (24,006 participants [4.6% with diabetes]), 2,273 deaths (236 in participants with diabetes), of which 786 (112 in participants with diabetes) were cardiovascular deaths, were recorded. In this subcohort, CRP also was continuously associated with cardiovascular and total mortality, similarly among participants with diabetes and those without. The hazard ratios for a loge SD higher CRP in participants with diabetes and those without diabetes were 1.46 (95% CI 1.18–1.81) and 1.44 (1.32–1.58) for CVD death (P ≥ 0.69 for interaction) and 1.21 (1.05–1.38) and 1.26 (1.19–1.32) for all-cause mortality (P ≥ 0.83 for interaction).
CONCLUSIONS
Findings from this study confirm that CRP is an independent risk factor for CVD and all-cause mortality. The associations of CRP with these two outcomes mostly are log linear and seem to be similar in participants with and without diabetes. The magnitude of the relationship of CRP with CVD and all-cause mortality did not seem to vary markedly by diabetes status, sex, and other conventional cardiovascular risk factors. Knowledge from CRP significantly adds to all-cause mortality prediction beyond the performance achieved with the use of conventional risk factors alone but not to CVD mortality prediction in people with diabetes.
Previous studies
In the Hoorn Study (20), it was suggested that the magnitude of the association of CRP with cardiovascular death was similar in participants with type 2 diabetes and those without. This study, however, was largely underpowered, with only 24 cardiovascular deaths in the overall population of 610 participants (169 with diabetes), and, as a result, associations of CRP with cardiovascular mortality were nonsignificant in the total cohort and subgroups (20). In the Strong Heart Study (21), Honolulu Heart Program (22), and the study by Biasucci et al. (23), CRP was a significant predictor of CVD only among participants without diabetes, and point estimates, when provided, were lower in participants with diabetes but with a substantial overlap of the confidence interval about point estimates in those with and without diabetes (21–23). In those three studies, statistical interaction, if any, was not formally tested. In the Emerging Risk Factors Collaboration (ERFC) overview, where statistical interaction was tested, CRP was a similar determinant of coronary heart disease in participants with diabetes and those without (7).
Mechanisms of effect
Several mechanistic pathways have been suggested to explain the accelerated atherothrombotic process in people with diabetes. Those related to chronic hyperglycemia include oxidative stress, advanced glycation end products, endothelial dysfunction, acute-phase response, and procoagulant states. Based on these mechanisms, differences in the effects of CRP on CVD risk could be hypothetically observed in people with and without diabetes (with a less favorable risk for the former) as the result of differences in the pathological processes responsible for increased CRP in people with and without diabetes. The absence of any significant heterogeneity in our study and the large ERFC overview (7) argues against such a hypothesis. Some have instead suggested that other cardiovascular risk factors that frequently displayed less favorable levels in people with diabetes compared with those without can mask the association between CRP and CVD and make it a less stronger determinant in people with diabetes (24). However, our data, and other adequately powered studies (7), have shown that despite attenuation in effect estimates after adjustment for multiple cardiovascular risk factors, the association of CRP with CVD does not differ by diabetes status.
Sex differentials
In the ERFC overview (7), there was a significant heterogeneity between men and women in the association of CRP with coronary heart disease, with the magnitude of the association being less important in women. Others instead have found significant interactions in both people with and without diabetes, with greater effect estimates always recorded in women (8). We found that CRP affected the risk of mortality in similar ways in men and women regardless of their diabetes status. Some apparent differences by sex in the effect estimates were a reflection of the low statistical power in some subgroups, as indicated by wider CI about estimates and the lack of any significant interaction by diabetes status within and across sex subgroups.
Incremental predictive utility of CRP
The incremental value of CRP to CVD prediction has been largely assessed in the general population (25,26) but less in people with diabetes (27). Results in the general population have been inconsistent, with some showing marginal to sizable improvement and others no added value at all (25,26). However, methods for assessing improvement in model performance in many of those studies have been largely criticized (25,26). One study in people with diabetes found that adding CRP to established risk factors had meaningless effects on the performance of models in predicting cardiovascular mortality (27). This is consistent with our findings of nonsignificant improvement in the goodness-of-fit statistics and discriminatory power subsequent to adding CRP in people with diabetes. However, such improvement was more apparent in people with diabetes for the prediction of all-cause mortality and among participants without diabetes and the total cohort for both outcomes. However, the clinical and public health relevance of the range of improvement found has to be questioned.
Study limitations and strengths
The current analyses were based on a single measurement of CRP, and there was no possibility to adjust for regression dilution bias (7). Our results, however, are similar to those from studies that have performed such adjustment (7). In addition, it has been demonstrated that CRP was stable enough for use in long-term predictions (3). The percentage of people with diabetes in our population was low as a result of the use of self-reported diagnosis without testing of blood glucose levels. Characteristics of diabetes, such as etiologic types and use of insulin were poorly defined, and data were not available on the duration of diagnosed diabetes and urinary albumin excretion, both powerful determinants of cardiovascular risk in people with diabetes. Therefore, we were unable to account for their possible effects in regression analyses. Our study also has major strengths, including its large sample size and number of deaths recorded, our ability to directly compare the effects of CRP on mortality risk in people with and without diabetes, and our ability to assess the possible effects of sex and other cardiovascular risk factors on the observed relationships.
Based on available evidence (4,28,29), a causal role for CRP in CVD is less certain. Therefore, CRP may not be a target for CVD prevention (2,30). It is expected that trials of specific CRP antagonists and low-dose methotrexate will clarify this in the future (31). The JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) has shown that CRP was useful for targeting statin therapy in healthy individuals with normal-range LDL cholesterol (32). Other studies have suggested inconsistent effects of CRP on CVD risk prediction beyond traditional predictors (25,26). Our study suggests that CRP levels convey prediction information on the risk of CVD that is complementary to that provided by conventional cardiovascular risk factors and that improvement in the predictions is likely similar in people with diabetes and those without but is not affected by sex. Such improvements, however, remain modest and do not lend strong support to any recommendation of routinely measuring CRP for the purpose of enhancing the prediction of future risk of major outcomes. Our study also extends previous reports that traditional risk factors were similar determinants of the risk of CVD regardless of diabetes status (33–35).
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
A.P.K. conceived of the design of the study, analyzed and interpreted data, drafted the manuscript, and performed critical revision of the manuscript for important intellectual content. G.D.B. and S.C. conceived of the design of the study, analyzed and interpreted data, and performed critical revision of the manuscript for important intellectual content. M.H. performed critical revision of the manuscript for important intellectual content. E.S. acquired data, analyzed and interpreted data, performed critical revision of the manuscript for important intellectual content, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1588/-/DC1.
References
- 1.Dent TH. Predicting the risk of coronary heart disease: II: the role of novel molecular biomarkers and genetics in estimating risk, and the future of risk prediction. Atherosclerosis 2010;213:352–362 [DOI] [PubMed] [Google Scholar]
- 2.Hingorani AD, Shah T, Casas JP, Humphries SE, Talmud PJ. C-reactive protein and coronary heart disease: predictive test or therapeutic target? Clin Chem 2009;55:239–255 [DOI] [PubMed] [Google Scholar]
- 3.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387–1397 [DOI] [PubMed] [Google Scholar]
- 4.Keavney B. C reactive protein and the risk of cardiovascular disease. BMJ 2011;342:d144. [DOI] [PubMed] [Google Scholar]
- 5.Fortmann SP, Ford E, Criqui MH, et al. ; Centers for Disease Control and Prevention; American Heart Association CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: report from the population science discussion group. Circulation 2004;110:e554–e559 [DOI] [PubMed] [Google Scholar]
- 6.Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. C-reactive protein and coronary heart disease: a critical review. J Intern Med 2008;264:295–314 [DOI] [PubMed] [Google Scholar]
- 7.Kaptoge S, Di Angelantonio E, Lowe G, et al. ; Emerging Risk Factors Collaboration C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qasim AN, Budharaju V, Mehta NN, et al. Gender differences in the association of C-reactive protein with coronary artery calcium in type-2 diabetes. Clin Endocrinol (Oxford) 2011;74:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk? Evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev 2011;12:680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Scottish Government Statistics. Scottish Health Survey Publications. Available from http://www.scotland.gov.uk/Topics/Statistics/Browse/Health/scottish-healthsurvey/Publications. Accessed November 2007
- 11.Joint Health Surveys Unit. Health Survey for England 1998. Cardiovascular Disease. Volume 2: Methodology and Documentation. London, The Stationery Office, 1999.
- 12.Lin DY, Wei LJ, Yin Z. Checking the Cox model with cumulative sums of Martingales-based residuals. Biometrika 1993;80:557–572 [Google Scholar]
- 13.Pearson TA, Mensah GA, Hong Y, Smith SC, Jr; Centers for Disease Control and Prevention; American Heart Association CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: overview. Circulation 2004;110:e543–e544 [DOI] [PubMed] [Google Scholar]
- 14.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med 1991;10:1025–1035 [DOI] [PubMed] [Google Scholar]
- 15.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845 [PubMed] [Google Scholar]
- 16.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 17.Collett D. Modelling Survival Data in Medical Research. 2nd ed. New York, Chapman & Hall/CRC, 2003 [Google Scholar]
- 18.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997;16:965–980 [DOI] [PubMed] [Google Scholar]
- 19.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991;121:293–298 [DOI] [PubMed] [Google Scholar]
- 20.Jager A, van Hinsbergh VW, Kostense PJ, et al. von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc Biol 1999;19:3071–3078 [DOI] [PubMed] [Google Scholar]
- 21.Best LG, Zhang Y, Lee ET, et al. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation 2005;112:1289–1295 [DOI] [PubMed] [Google Scholar]
- 22.Sakkinen P, Abbott RD, Curb JD, Rodriguez BL, Yano K, Tracy RP. C-reactive protein and myocardial infarction. J Clin Epidemiol 2002;55:445–451 [DOI] [PubMed] [Google Scholar]
- 23.Biasucci LM, Liuzzo G, Della Bona R, et al. Different apparent prognostic value of hsCRP in type 2 diabetic and nondiabetic patients with acute coronary syndromes. Clin Chem 2009;55:365–368 [DOI] [PubMed] [Google Scholar]
- 24.Soinio M, Marniemi J, Laakso M, Lehto S, Rönnemaa T. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care 2006;29:329–333 [DOI] [PubMed] [Google Scholar]
- 25.Tzoulaki I, Liberopoulos G, Ioannidis JP. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA 2009;302:2345–2352 [DOI] [PubMed] [Google Scholar]
- 26.Shah T, Casas JP, Cooper JA, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 2009;38:217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruno G, Fornengo P, Novelli G, et al. C-reactive protein and 5-year survival in type 2 diabetes: the Casale Monferrato Study. Diabetes 2009;58:926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wensley F, Gao P, Burgess S, et al. ; C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC) Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ 2011;342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009;302:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 2006;440:1217–1221 [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the Cardiovascular Inflammation Reduction Trial (CIRT). J Thromb Haemost 2009;7(Suppl.1):332–339 [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Danielson E, Fonseca FA, et al. ; JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207 [DOI] [PubMed] [Google Scholar]
- 33.Kengne AP, Patel A, Barzi F, et al. ; Asia Pacific Cohort Studies Collaboration Systolic blood pressure, diabetes and the risk of cardiovascular diseases in the Asia-Pacific region. J Hypertens 2007;25:1205–1213 [DOI] [PubMed] [Google Scholar]
- 34.Asia Pacific Cohort Studies Collaboration Cholesterol, diabetes and major cardiovascular diseases in the Asia-Pacific region. Diabetologia 2007;50:2289–2297 [DOI] [PubMed] [Google Scholar]
- 35.Asia Pacific Cohort Studies Collaboration Smoking, diabetes and cardiovascular diseases in men in the Asia-Pacific Region. J Diabetes 2009;1:173–181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.