Abstract
OBJECTIVE
The association between pioglitazone and bladder cancer has not been investigated in Asians. We aimed to investigate this association.
RESEARCH DESIGN AND METHODS
A total of 1,000,000 individuals were randomly sampled from the National Health Insurance database, and incident cases of bladder cancer during the period from 1 January 2006 to 31 December 2009 were analyzed among 54,928 patients with type 2 diabetes and without previous bladder cancer.
RESULTS
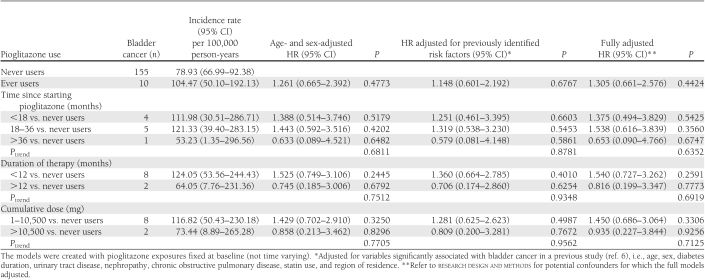
Among 165 incident case subjects, 10 (0.39%) were ever users and 155 (0.30%) were never users of pioglitazone (adjusted hazard ratio in full model 1.305 [95% CI 0.661–2.576]). All bladder cancer in ever users occurred within a duration of therapy <24 months, suggesting an early effect of pioglitazone on bladder cancer or late use of pioglitazone in high-risk patients.
CONCLUSIONS
The association between pioglitazone and bladder cancer was not significant. However, confirmation of this finding is required because of the possible lack of statistical power owing to the small number of events.
Clinical trials have suggested an association between pioglitazone and bladder cancer (1,2). A reporting system indicated an odds ratio of 4.30 (95% CI 2.82–6.52) (3), and an analysis of the Kaiser Permanente Northern California (KPNC) registry found a 40–50% higher risk for duration of use >2 years and cumulative dose >28,000 mg (4). This association was evaluated here using databases from the Bureau of National Health Insurance (NHI) in Taiwan.
RESEARCH DESIGN AND METHODS
Reimbursement records from 1996 through 2009 were retrieved from a random sample of 1,000,000 individuals in NHI databases in 2000 (5–8). The diagnostic codes, based on the ICD-9, were 250.1–250.9 for diabetes and 188 for bladder cancer.
The entry date of 1 January 2006 was selected because it was midway between the start of pioglitazone marketing in Taiwan (2002) and the end date of the databases (2009) and provided a maximum exposure of 4 years and a maximum follow-up of 4 years. After excluding individuals who died or had diabetes or bladder cancer before entry, those with type 1 diabetes, and those not using oral antidiabetes medication or insulin, 54,928 patients with type 2 diabetes were recruited.
Age, diabetes duration, comorbidities, and other covariates were determined as a status/diagnosis before entry (6). Bladder cancer was defined as incident cases from January 2006 through December 2009.
Patients prescribed pioglitazone before entry were defined as ever users; never users were those who had never used pioglitazone. The KPNC dose-responsive parameters (4) were used, namely, 1) time since starting pioglitazone: <18, 18–36, and >36 months; 2) therapy duration: <12, 12–24, and >24 months; and 3) cumulative dose: 1–10,500, 10,501–28,000, and >28,000 mg.
Statistical analyses
Incidences of bladder cancer and 95% CIs were calculated (9). Cox regression was used to calculate hazard ratios (HRs). Three sets of confounders were adjusted for: 1) age and sex; 2) variables significantly predictive of bladder cancer in a previous study (for not overfitting [10]), i.e., age, sex, diabetes duration, urinary tract disease (ICD-9 590–599), nephropathy (580–589), chronic obstructive pulmonary disease (490–496), statin use, and region of residence (6); and 3) full model, i.e., age, sex, diabetes duration, nephropathy, urinary tract disease, hypertension (401–405), chronic obstructive pulmonary disease, cerebrovascular disease (430–438), ischemic heart disease (410–414), peripheral arterial disease (250.7, 785.4, 443.81, and 440–448), eye disease (250.5, 362.0, 369, 366.41, and 365.44), dyslipidemia (272.0–272.4), heart failure (398.91, 402.11, 402.91, 404.11, 404.13, 404.91, 404.93, and 428), rosiglitazone, sulfonylurea, meglitinide, metformin, acarbose, insulin, statin, fibrate, ACE inhibitor/angiotensin receptor blocker, calcium channel blocker, region of residence, occupation, and other cancer before baseline (140–208, excluding 188). A P value <0.05 was considered statistically significant.
RESULTS
Among 2,545 ever users and 52,383 never users, there were 10 (0.39%) and 155 (0.30%) incident cases, respectively. Because no users with a duration of therapy >24 months or a cumulative dose >28,000 mg developed bladder cancer, HRs were estimated for duration of therapy <12 and ≥12 months versus never users and for a cumulative dose of 1–10,500 and ≥10,500 mg versus never users. Table 1 shows case numbers and incidences of bladder cancer and HRs for different pioglitazone categories. No HRs were significant.
Table 1.
Incidences and HRs for bladder cancer associated with pioglitazone use
CONCLUSIONS
An insignificant 30% increase in overall risk was observed in the full model (Table 1), which is a result similar to those of the KPNC study (4); however, the HR was attenuated in the model adjusting only for previously identified risk factors (6), which suggests overfitting in the full model.
Although not significant, an increase in risk was observed for time since starting pioglitazone <36 months, duration of therapy <12 months, and cumulative dose <10,500 mg (Table 1). Interestingly, 80% of incident bladder cancers occurred among patients who had <1 year of pioglitazone at baseline and no cancers occurred among those with >2 years at baseline. Unclear is whether this increased risk during the first year at baseline was the result of an early pioglitazone effect on bladder cancer or its late use in patients at high risk for bladder cancer. The lower risk with prolonged use (i.e., time since starting pioglitazone >36 months or duration of therapy >12 months) and cumulative dose >10,500 mg (Table 1) might be a chance effect, as the number of events was very small.
Pioglitazone is a third-line oral antidiabetes medication in Taiwan indicated for treatment of patients with longer diabetes duration or more comorbidities/complications. All of these factors could predispose patients to bladder cancer (6). Because metformin may prevent but insulin might promote some cancers (11,12), their interactions with pioglitazone require investigation.
These results differ from those in whites (4), possibly because of differences in genetic background, diet, socioeconomic status, or cultural background or because the present analysis was underpowered.
We did not analyze time-dependent pioglitazone use because it might have caused bias (13). Furthermore, the 2007 report of an association between rosiglitazone and acute myocardial infarction (14) might have markedly changed prescription practices of physicians, and patients might not have taken thiazolidinediones, including pioglitazone, even when they were prescribed. However, the present findings were unchanged when patients who started pioglitazone after the entry date were excluded from the analyses or when their follow-up was censored at pioglitazone initiation (data not shown).
This study has several strengths. It is population based, with a large, nationally representative sample. Cancer is considered a severe morbidity by the NHI, and most copayments are waived. Therefore, the detection rate is not likely to differ by socioeconomic class. The use of medical records reduced self-report bias.
Study limitations include the lack of actual measurements for confounders such as biochemical data, obesity, smoking, lifestyle, diet, occupational exposure, and genetic parameters. In addition, the underpowered analyses require confirmation.
In summary, there was an insignificant 30% overall increase in bladder cancer risk among pioglitazone users. However, all bladder cancer occurred within 2 years of the start of therapy and no patients with a cumulative dose >28,000 mg developed bladder cancer, which suggests an early effect of pioglitazone on bladder cancer or late pioglitazone use in patients with a high risk of bladder cancer.
Acknowledgments
This study was based on data from the NHI Research Database provided by the Bureau of NHI and the Department of Health, managed by National Health Research Institutes (reg. number 99274).
No potential conflicts of interest relevant to this article were reported.
C.-H.T. collected and analyzed the data, wrote the manuscript, and is the guarantor of the article.
Footnotes
The interpretation and conclusions contained herein do not represent those of the Bureau of NHI, the Department of Health, or the National Health Research Institutes.
References
- 1.Dormandy JA, Charbonnel B, Eckland DJ, et al. ; PROactive investigators Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 2.Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009;32:187–202 [DOI] [PubMed] [Google Scholar]
- 3.Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care 2011;34:1369–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011;34:916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng CH, Chong CK, Tseng CP, Chan TT. Age-related risk of mortality from bladder cancer in diabetic patients: a 12-year follow-up of a national cohort in Taiwan. Ann Med 2009;41:371–379 [DOI] [PubMed] [Google Scholar]
- 6.Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011;54:2009–2015 [DOI] [PubMed] [Google Scholar]
- 7.Tseng CH. Diabetes and risk of prostate cancer: a study using the National Health Insurance. Diabetes Care 2011;34:616–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng CH. Diabetes and non-Hodgkin’s lymphoma: analyses of prevalence and annual incidence in 2005 using the National Health Insurance database in Taiwan. Ann Oncol. 15 July 2011 [Epub ahead of print] [DOI] [PubMed]
- 9.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol 1990;131:373–375 [DOI] [PubMed] [Google Scholar]
- 10.Núñez E, Steyerberg EW, Núñez J. Regression modeling strategies. Rev Esp Cardiol 2011;64:501–507 [in Spanish] [DOI] [PubMed] [Google Scholar]
- 11.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 12.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461 [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Chan JC. Comment: analyses using time-dependent pioglitazone usage in Cox models may lead to wrong conclusions about its association with cancer. Diabetes Care 2011;34:e136–; author reply 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471 [DOI] [PubMed] [Google Scholar]