Abstract
OBJECTIVE
Insulin resistance and type 2 diabetes are associated with an increased risk of neurodegenerative diseases. Brain-derived neurotrophic factor (BDNF) regulates neuronal differentiation and synaptic plasticity, and its decreased levels are supposed to play a role in the pathogenesis of Alzheimer’s disease and other disorders. The aim of the current study was to estimate the effects of hyperinsulinemia and serum free fatty acids (FFA) elevation on circulating BDNF concentration in humans.
RESEARCH DESIGN AND METHODS
We studied 18 healthy male subjects (mean age 25.6 ± 3.0 years; mean BMI 26.6 ± 4.8 kg/m2). Serum and plasma BDNF concentration was measured in the baseline state and in the 120 and 360 min of euglycemic hyperinsulinemic clamp with or without intralipid/heparin infusion. Furthermore, plasma BDNF was measured in 20 male subjects (mean age 22.7 ± 2.3 years; mean BMI 24.9 ± 1.5 kg/m2) 360 min after a high-fat meal.
RESULTS
Insulin sensitivity was reduced by ∼40% after 6 h of intralipid/heparin infusion (P < 0.001). During both clamps, serum and plasma BDNF followed the same pattern. Hyperinsulinemia had no effect on circulating BDNF. Raising FFA had no effect on circulating BDNF in 120 min; however, it resulted in a significant decrease by 43% in serum and by 35% in plasma BDNF after 360 min (P = 0.005 and 0.006, respectively). High-fat meal also resulted in a decrease by 27.8% in plasma BDNF (P = 0.04).
CONCLUSIONS
Our data show that raising FFA decreases circulating BDNF. This might indicate a potential link between FFA-induced insulin resistance and neurodegenerative disorders.
Several studies have proven that phenotypes associated with insulin resistance are at increased risk for developing cognitive decline and neurodegeneration (1). The incidences of Alzheimer’s disease, vascular dementia, and major depression were higher in individuals with type 2 diabetes than in those without (1). Furthermore, cognitive impairment is also increased in patients with prediabetes and metabolic syndrome (1).
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family, which interacts with high affinity with tyrosine receptor kinase B (2). BDNF is abundantly expressed in central and peripheral nervous system and can cross the blood-brain barrier in both directions (3). Recent studies have shown that BDNF is also produced in nonneurogenic tissues, including skeletal muscle and vascular endothelium, and is stored in platelets (4,5). Because serum contains the factors released from the platelets, it could be an important issue whether BDNF concentration is measured in serum or in plasma. In the study of Rasmussen et al. (6), it was reported that the brain contributed to more than 70% of plasma BDNF in healthy humans. The changes in plasma BDNF were considered to reflect its changes in the brain (7). However, serum BDNF concentration has also been reported to closely correlate with the cortical BDNF level (8), suggesting that it can reflect the BDNF level in the brain as well. Dietary restriction increases the number of newly generated neuronal cells, induces BDNF expression in the dentate gyrus of rats (9), and increases serum BDNF in humans (10). Physical activity increases BDNF measured both in serum and in plasma (7,11).
BDNF is a key protein in regulating neuronal survival, differentiation, and synaptic plasticity and seems important for learning and memory function (12). Numerous data indicate that BDNF has specific effects on central pathways involved in appetite regulation and energy expenditure (2). BDNF might also regulate glucose metabolism (13). It reduces food intake and lowers blood glucose in genetically modified (db/db) obese mice (13). The hypoglycemic effect of BDNF cannot be ascribed solely to the hypophagic effect of BDNF, because BDNF administration improves insulin resistance in db/db mice, even when food intake is controlled.
Low plasma BDNF levels were observed not only in neurodegenerative diseases but also in type 2 diabetes and obesity (14). We observed decreased serum BDNF concentration in young nonobese subjects with low insulin sensitivity (15). Insulin resistance might play a role in common pathogenesis of neurodegenerative and metabolic disorders, and decreased BDNF may explain the clustering of these diseases. However, the mechanism connecting insulin resistance with neurodegeneration is still unclear. It is widely accepted that free fatty acids (FFA) induce insulin resistance (16). It is noteworthy that experimental studies indicate that a high-fat diet (HFD) disrupts cognition and contributes to neurodegenerative diseases (17). Therefore, the aim of the current study was to estimate the effects of hyperinsulinemia and serum FFA elevation on circulating BDNF concentration in humans.
RESEARCH DESIGN AND METHODS
Participants
In protocol 1, we examined 18 young (mean age 25.6 ± 3.0 years, mean BMI 26.6 ± 4.8 kg/m2), apparently healthy male subjects with normal glucose tolerance. In protocol 2, we studied 20 male subjects with similar clinical characteristics (mean age 22.7 ± 2.3 years; mean BMI 24.9 ± 1.5 kg/m2). All study participants were recruited from the medical staff and students and by the local advertisements. All subjects were nonsmokers, without serious diseases, and were not taking any drugs. Physical examination and appropriate laboratory tests were performed. Analyses were performed after an overnight fast. Subjects underwent an oral glucose tolerance test, and all had normal glucose tolerance according to the World Health Organization criteria. The study protocol was approved by the Ethics Committee of Medical University of Bialystok. All subjects gave written informed consent before entering the study.
Anthropometry
Anthropometric parameters were measured in all subjects. The BMI was calculated as body weight per height and expressed in kilograms per meter squared. The waist circumference was measured at the smallest circumference between the rib cage and the iliac crest, with the subject in the standing position. The percent of body fat was assessed by bioelectric impedance analysis using the Tanita TBF-511 Body Fat Analyzer (Tanita Corporation, Tokyo, Japan).
Insulin sensitivity (protocol 1)
Insulin sensitivity was measured with the euglycemic hyperinsulinemic clamp technique, according to DeFronzo et al. (18). On the morning of the study, two venous catheters were inserted into antecubital veins, one for the infusion of insulin and glucose and the other in the contralateral hand for blood sampling, with that hand heated to ~60°C. Insulin (Actrapid HM; Novo Nordisk, Copenhagen, Denmark) was given as a primed-continuous intravenous infusion for 360 min at 40 mU × m−2 × min−1, resulting in constant hyperinsulinemia of ∼80 μIU/mL. Arterialized blood glucose was obtained every 5 min, and 20% dextrose (1.11 mol/L) infusion was adjusted to maintain plasma glucose levels at 5.0 mmol/L. The rate of whole-body glucose uptake (M value) was calculated every hour from 2 to 6 h of the clamp as the mean glucose infusion rate during the last 40 min of the respective hour, corrected for the glucose space, and normalized for fat-free mass.
After 1 week, another 6-h clamp, with concurrent intralipid/heparin infusion, was performed. In this experiment, 20% intralipid (Fresenius Kabi, Uppsala, Sweden) was given at 0.013 mL/kg per min, and heparin was given at 0.2 units/kg per min. No difference in steady-state insulin concentration between these protocols was observed.
High-fat meal (protocol 2)
We gave Calogen (Nutricia Poland, Warsaw, Poland), in which energy comes almost in total from fat (450 kcal/100 mL), 250 mL twice within 1 h. A venous catheter was inserted into antecubital vein, and blood was drawn every 30 min for 360 min for the determination of FFA.
Laboratory analyses
Serum and plasma-EDTA BDNF concentration was measured in the baseline state and in the 120 and 360 min of euglycemic hyperinsulinemic clamp with or without intralipid/heparin infusion. Plasma-EDTA BDNF was also measured at baseline and 360 min after a high-fat meal.
To obtain plasma, blood samples were drawn into tubes containing EDTA and centrifuged at 4,000 g for 10 min at 4°C. Plasma samples then were again centrifuged at ∼10,000 g for 10 min at 4°C for complete platelet removal. A second centrifugation was performed after freezing at −80°C and then thawing samples in protocol 1 and immediately in protocol 2.
Plasma glucose was measured immediately by the enzymatic method using a glucose analyzer (YSI 2300 STAT PLUS). Serum total cholesterol, HDL cholesterol, and triglycerides were assessed by enzymatic method (Cormay, Warsaw, Poland). The concentration of LDL cholesterol was calculated from Friedewald’s formula.
Before estimation of concentrations of BDNF, insulin and FFA, serum and plasma samples were kept frozen at −80°C. Serum insulin was measured with the monoclonal immunoradiometric assay (IRMA; DIAsource ImmunoAssays S.A., Nivelles, Belgium) with the sensitivity of 1 μIU/mL and with intra-assay and interassay coefficients of variation below 2.2% and 6.5%, respectively. Serum FFA were assayed using a commercially available kit (Wako Chemicals, Richmond, VA). The level of serum and plasma BDNF was measured using immunoenzymatic method (Quantikine; R&D SYSTEMS, Minneapolis, MN) with the sensitivity of below 20 pg/mL and with intra-assay and interassay coefficients of variation below 6.3 and 11.4%, respectively.
Statistical analysis
The statistics were performed with the STATISTICA 9.0 (Statsoft, Krakow, Poland). The variables, which did not have normal distribution, were log-transformed before analyses. For the purpose of the data presentation these variables were again anti–log-transformed to absolute values in RESULTS. To estimate differences between the clamp without or with intralipid/heparin infusion and before and after a high-fat meal, the paired Student t test was used. To assess the differences in serum and plasma BDNF before and during the clamp, repeated-measures ANOVA with post hoc Scheffe test was performed. Relationships between variables were analyzed with the Pearson product-moment correlation analysis. Statistical significance was accepted at P < 0.05.
RESULTS
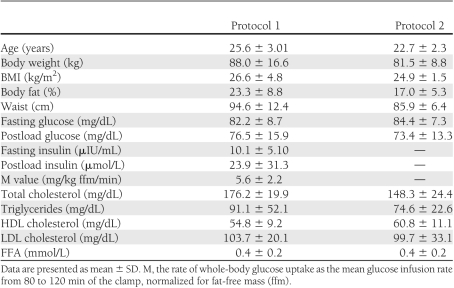
Clinical characteristics of the studied groups are given in Table 1.
Table 1.
Anthropometric, biochemical, and metabolic characteristics of the studied group in protocol 1 (clamp without or with intralipid/heparin infusion; n = 18) and protocol 2 (high-fat meal; n = 20)
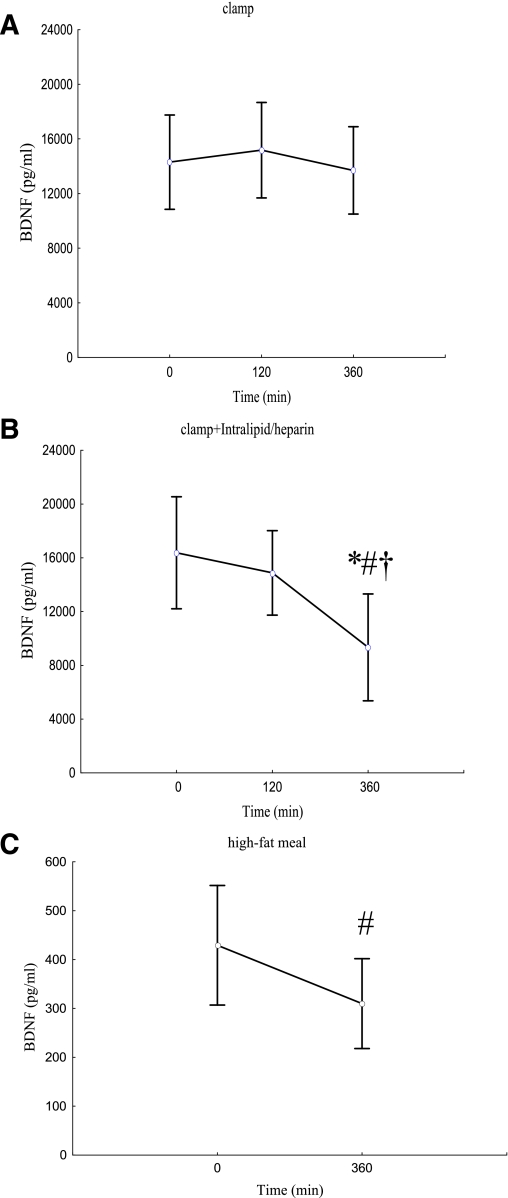
There was more than a fourfold increase in serum FFA from the initial values during intralipid/heparin infusion (P < 0.001). Insulin sensitivity was not different during the 2nd h of both clamps (P = 0.47). We observed a significantly decreased insulin sensitivity from 4 to 6 h of intralipid/heparin infusion, resulting in a reduction by ∼40% after 6 h (all P < 0.001). Hyperinsulinemia had no effect on serum BDNF concentration (P = 0.75) (Fig. 1A). We observed a significant effect of raising circulating FFA on serum BDNF (P = 0.003) (Fig. 1B). BDNF value in 120 min of the clamp with intralipid/heparin infusion was not different from the baseline (P = 0.75); however, serum BDNF value after 360 min was significantly lower than in 0 and 120 min (P = 0.005 and 0.03, respectively), resulting in a decrease by 43% in comparison with the initial value. Furthermore, although the baseline serum BDNF concentrations were not different between the protocols (P = 0.30), BDNF 360-min value of intralipid/heparin clamp was markedly lower in comparison with BDNF 360-min value of the clamp without raising FFA (P = 0.007). Serum BDNF in 360 min of the clamp was related to a decrease in insulin sensitivity during intralipid/heparin infusion (r = −0.50, P = 0.035).
Figure 1.
A–C: Serum BDNF concentrations during the clamp without (A) and with (B) intralipid/heparin infusion and plasma BDNF concentration after high-fat meal (C). Data are presented as mean ± SD. *P < 0.05 from the paired Student t test, for the difference vs. clamp without intralipid/heparin; #P < 0.05 for the difference vs. basal value; †P < 0.05 for the difference vs. value in 120 min. Signs of significance are taken from post hoc Scheffé test, which was performed, when repeated-measures ANOVA gave significant result (A and B) and from the paired Student t test (C). (A high-quality color representation of this figure is available in the online issue.)
Plasma BDNF followed the same pattern as serum values during both clamps. Hyperinsulinemia had no effect on plasma BDNF (0 min, 502.6 ± 322.7 pg/mL; 120 min, 542.7 ± 380.9 pg/mL; 360 min, 474.7 ± 264.3 pg/mL; P = 0.98). Intralipid/heparin infusion resulted in a decrease in plasma BDNF (0 min, 445.6 ± 285.2 pg/mL; 120 min, 487.6 ± 332.1 pg/mL; 360 min, 289.4 ± 173.2 pg/mL; P = 0.001). BDNF value in 6 h was significantly lower in comparison with the baseline and 2-h levels (P = 0.006 and 0.005, respectively); the reduction in comparison with the baseline value was ∼35%.
After a high-fat meal, we observed a significant and sustained elevation of serum FFA maintaining from 180 to 360 min (baseline, 0.4 ± 0.2; 360 min, 0.9 ± 0.3 mmol/L; P < 0.0001). Although this elevation was smaller than observed during intralipid/heparin infusion, we were able to demonstrate a significant decrease in plasma BDNF after a high-fat meal (baseline, 429.4 ± 261.1; 360 min, 310.0 ± 196.2 pg/mL; P = 0.04; 27.8% reduction) (Fig. 1C).
The change in circulating BDNF (ΔBDNF) in response to fat overload was almost identical in lean (BMI <25 kg/m2) and overweight/obese individuals (BMI >25 kg/m2). This was true for both serum and plasma BDNF after intralipid infusion (P = 0.88 and 0.86, respectively) and for plasma BDNF after a high-fat meal (P = 0.84).
In both protocols, no correlation between baseline serum or plasma BDNF and FFA and between serum or plasma BDNF and FFA at the respective time points during all experiments was observed.
Insulin sensitivity was inversely related to body weight, BMI, waist circumference, percent of body fat, fasting insulin, and fasting serum FFA (all P < 0.05). Fasting serum BDNF inversely correlated with total cholesterol (r = −0.53; P = 0.025).
CONCLUSIONS
The main finding of our study is the observation that raising FFA, obtained during intralipid plus heparin infusion or a high-fat meal, resulted in a significant decrease in circulating BDNF.
Low levels of plasma BDNF were observed in conditions associated with insulin resistance, including obesity and type 2 diabetes (14). It has also been reported that plasma BDNF was inversely associated with homeostasis model assessment insulin resistance version 2, which is indirect measure of insulin resistance (14). Furthermore, chronic physical activity, which enhances insulin sensitivity (19), influences secretion of BDNF. Three months of endurance training increased cerebral release of BDNF (20). However, the mechanism by which insulin resistance may decrease BDNF is still unclear. Krabbe et al. (14) showed that the cerebral output of BDNF was inhibited when blood glucose levels were elevated during clamp conditions. In contrast, when plasma insulin was increased while maintaining normal blood glucose, the cerebral output of BDNF was not inhibited. In our study, hyperinsulinemia had also no effect on serum BDNF concentration.
On the other hand, some authors observed inverse correlation between basal serum BDNF and cardiorespiratory fitness level in healthy Korean men. Furthermore, serum BDNF was positively related to the risk factors of metabolic syndrome, such as BMI, total cholesterol, and triglycerides (21). Given the results mentioned above on the increased circulating BDNF after exercise training (7,9,20), this topic requires further investigation.
Our results demonstrate for the first time that 6 h of intralipid/heparin infusion during hyperinsulinemic euglycemic clamp significantly decreased by 43% serum BDNF and by 35% plasma BDNF level. Of note, the acute increase in serum FFA concentrations, obtained during 6-h clamp with intralipid/heparin infusion, reduced insulin sensitivity by ∼40%. Plasma BDNF decreased also by 27.8% in 6 h after a high-fat meal. It has been demonstrated that FFA induced insulin resistance in humans through inhibition of glucose transport activity (16). It was also suggested that intramuscular accumulation of fatty acids, or their metabolites such as ceramides, played an important role in the pathogenesis of human insulin resistance (22). It is noteworthy that ceramides have inhibitory effects on insulin signaling in brain and induce neuronal cell death (23).
Experimental studies indicate that HFD increases plasma FFA and impairs neurogenesis through oxidative stress followed by the accumulation of peroxidized lipids, including malondialdehyde (MDA), and decreases level of BDNF protein in the hippocampus (24). HFD increased brain MDA levels by almost 50% (24). The toxic effects of MDA were evaluated on neuronal progenitor cells (NPCs) proliferation in the dentate gyrus of the hippocampus without affecting neuronal differentiation (24). MDA reduced the growth of NPCs, but BDNF treatment restored NPCs proliferation (24). Other authors demonstrated that HFD affected not only newborn cell proliferation but also differentiation (25). It has also been shown that a diet high in fat or refined sugar can reduce the expression of hippocampal BDNF in rats (26). Furthermore, Wu et al. (27) provide novel evidence that oxidative damage mediates the deleterious effects of the diet high in fat on synaptic function and cognition by reducing expression of BDNF and its downstream effectors synapsin I and cyclic AMP-responsive element–binding protein in hippocampus. Treatment with vitamin E significantly prevented this reduction, suggesting that oxidative damage was involved in the adverse effects of HFD on the transcription of BDNF (27). All of these data indicated that HFD could downregulate BDNF production and neurogenesis in the hippocampus, which is important for learning and memory. In our study, we showed that elevation in serum FFA itself decreased serum BDNF levels, because during clamp, normal blood glucose levels were maintained. We also demonstrated that this decrease was a rapid effect and did not require changes in body weight.
It is possible that decrease in circulating BDNF might be a result of its increased clearance. However, although there are experimental data, which support mechanistically the hypothesis that FFA might decrease BDNF production. To our knowledge, no such data exist on BDNF degradation. Circulating BDNF is cleared mainly by the liver (28). In fact, elevated FFAs are supposed to have a protein-sparing effect (29).
Another possible limitation is an acuity of our studies. This might not reflect long-standing metabolic derangements present in diabetes. BDNF was demonstrated to exert beneficial metabolic effects in diabetic mice during prolonged treatment (13). These experimental conditions do not correspond to the acute changes in circulating BDNF observed in our study. However, association between circulating BDNF and low insulin sensitivity might also occur in nondiabetic population (14,15). Thus, our protocols might resemble a condition to which modern healthy people are exposed regularly, i.e., a high-fat meal. Although it is difficult to draw direct clinical conclusion on the basis of our results, we think that our data give an interesting assumption for further studies on the associations between neurodegenerative disorders, low insulin sensitivity, and an HFD.
In conclusion, raising FFA decreases circulating BDNF. This might indicate a potential link between FFA-induced insulin resistance and neurodegenerative disorders.
Acknowledgments
This work was supported by Grant UDA-POIG.01.03.01-00-128/08 from the Program Innovative Economy 2007-2013 and financed in part by the European Union within the European Regional Development Fund and by the statutory funds of Institute of Animal Reproduction and Food Research, Polish Academy of Sciences, Olsztyn, Poland.
No potential conflicts of interest relevant to this article were reported.
M.K.-K. researched data, performed the statistics, and wrote the manuscript. I.K. researched data and reviewed the manuscript. A.N., A.A., M.Z., N.K., and E.O. researched data. M.G. reviewed the manuscript. M.S. designed the study, researched data, performed the statistics, and reviewed the manuscript and is guarantor of this article, taking full responsibility for the content of the article.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 July 2011, and as a poster at the 47th Scientific Sessions of the European Association for the Study of Diabetes, Lisbon, Portugal, 12–16 September 2011.
References
- 1.S Roriz-Filho J, Sá-Roriz TM, Rosset I, et al. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta 2009;1792:432-443 [DOI] [PubMed]
- 2.Soppet D, Escandon E, Maragos J, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991;65:895–903 [DOI] [PubMed] [Google Scholar]
- 3.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998;37:1553–1561 [DOI] [PubMed] [Google Scholar]
- 4.Hohn A, Leibrock J, Bailey K, Barde YA. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature 1990;344:339–341 [DOI] [PubMed] [Google Scholar]
- 5.Matthews VB, Aström MB, Chan MH, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009;52:1409–1418 [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol 2009;94:1062–1069 [DOI] [PubMed] [Google Scholar]
- 7.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol 2008;59(Suppl. 7):119–132 [PubMed] [Google Scholar]
- 8.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett 2002;328:261–264 [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci 2000;15:99–108 [DOI] [PubMed] [Google Scholar]
- 10.Araya AV, Orellana X, Espinoza J. Evaluation of the effect of caloric restriction on serum BDNF in overweight and obese subjects: preliminary evidences. Endocrine 2008;33:300–304 [DOI] [PubMed] [Google Scholar]
- 11.Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett 2008;431:62–65 [DOI] [PubMed] [Google Scholar]
- 12.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem 2002;9:224–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa T, Tsuchida A, Itakura Y, et al. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes 2000;49:436–444 [DOI] [PubMed] [Google Scholar]
- 14.Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007;50:431–438 [DOI] [PubMed] [Google Scholar]
- 15.Karczewska-Kupczewska M, Strączkowski M, Adamska A, et al. Decreased serum brain-derived neurotrophic factor concentration in young nonobese subjects with low insulin sensitivity. Clin Biochem 2011;44:817–820 [DOI] [PubMed] [Google Scholar]
- 16.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 1999;103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solfrizzi V, D’Introno A, Colacicco AM, et al. Dietary fatty acids intake: possible role in cognitive decline and dementia. Exp Gerontol 2005;40:257–270 [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 19.King DS, Dalsky GP, Clutter WE, et al. Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J Appl Physiol 1988;64:1942–1946 [DOI] [PubMed] [Google Scholar]
- 20.Seifert T, Brassard P, Wissenberg M, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol 2010;298:R372-R377 [DOI] [PubMed]
- 21.Jung SH, Kim J, Davis JM, Blair SN, Cho HC. Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy Korean men. Eur J Appl Physiol 2011;111:303–311 [DOI] [PubMed] [Google Scholar]
- 22.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arboleda G, Huang TJ, Waters C, Verkhratsky A, Fernyhough P, Gibson RM. Insulin-like growth factor-1-dependent maintenance of neuronal metabolism through the phosphatidylinositol 3-kinase-Akt pathway is inhibited by C2-ceramide in CAD cells. Eur J Neurosci 2007;25:3030–3038 [DOI] [PubMed] [Google Scholar]
- 24.Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett 2010;482:235–239 [DOI] [PubMed] [Google Scholar]
- 25.Hwang IK, Kim IY, Kim DW, et al. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res 2008;1241:1–6 [DOI] [PubMed] [Google Scholar]
- 26.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002;112:803–814 [DOI] [PubMed] [Google Scholar]
- 27.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci 2004;19:1699–1707 [DOI] [PubMed] [Google Scholar]
- 28.Pardridge WM, Kang YS, Buciak JL. Transport of human recombinant brain-derived neurotrophic factor (BDNF) through the rat blood-brain barrier in vivo using vector-mediated peptide drug delivery. Pharm Res 1994;11:738–746 [DOI] [PubMed] [Google Scholar]
- 29.Gormsen LC, Gjedsted J, Gjedde S, et al. Dose-response effects of free fatty acids on amino acid metabolism and ureagenesis. Acta Physiol (Oxf) 2008;192:369–379 [DOI] [PubMed] [Google Scholar]