Abstract
OBJECTIVE
To identify the pattern of relationships between the 17-item Diabetes Distress Scale (DDS17) and diabetes variables to establish scale cut points for high distress among patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Recruited were 506 study 1 and 392 study 2 adults with type 2 diabetes from community medical groups. Multiple regression equations associated the DDS17, a 17-item scale that yields a mean-item score, with HbA1c, diabetes self-efficacy, diet, and physical activity. Associations also were undertaken for the two-item DDS (DDS2) screener. Analyses included control variables, linear, and quadratic (curvilinear) DDS terms.
RESULTS
Significant quadratic effects occurred between the DDS17 and each diabetes variable, with increases in distress associated with poorer outcomes: study 1 HbA1c (P < 0.02), self-efficacy (P < 0.001), diet (P < 0.001), physical activity (P < 0.04); study 2 HbA1c (P < 0.03), self-efficacy (P < 0.004), diet (P < 0.04), physical activity (P = NS). Substantive curvilinear associations with all four variables in both studies began at unexpectedly low levels of DDS17: the slope increased linearly between scores 1 and 2, was more muted between 2 and 3, and reached a maximum between 3 and 4. This suggested three patient subgroups: little or no distress, <2.0; moderate distress, 2.0–2.9; high distress, ≥3.0. Parallel findings occurred for the DDS2.
CONCLUSIONS
In two samples of type 2 diabetic patients we found a consistent pattern of curvilinear relationships between the DDS and HbA1c, diabetes self-efficacy, diet, and physical activity. The shape of these relationships suggests cut points for three patient groups: little or no, moderate, and high distress.
Diabetes distress (DD) refers to the unique, often hidden emotional burdens and worries that are part of the spectrum of patient experience when managing a severe, demanding chronic disease like diabetes (1). High levels of DD are common (prevalence, 18–35%; 18-month incidence, 38–48%) and persistent over time, and they are distinct from clinical depression in their linkages with glycemic control and disease management (2–4). High levels of DD have been significantly associated with poor glycemic control, poor self-care, low diabetes self-efficacy, and poor quality-of-life, even after controlling for clinical depression (5).
A critical concern is the establishment of a reliable and valid cut point or criterion to define high DD so that further assessment and/or intervention might be initiated. Use of a standard cut point also enhances consistency of findings across studies and provides a uniform criterion for identifying patients at risk. In this study, we examined the relationship between the 17-item Diabetes Distress Scale (DDS17) (6), a widely used factor-analyzed, theory-driven, self-report survey, and several diabetes biologic and management measures in two samples of patients with type 2 diabetes. We also included a comparable analysis of the 2-item DDS (DDS2) screener, based on the 17-item scale (7). Our goal was to identify the degree and pattern of relationships between DDS scores and diabetes behavioral and biologic variables to define cut points for high DD for use in clinical and research settings.
Although there is no universally applicable method for determining a scale cut point, empiric approaches fall into two categories (8,9). A data-oriented approach dichotomizes a scale at a prespecified point along the scale distribution and compares those above the cut point with those who reach criterion using a gold standard measure. For example, scores from the Patient Health Questionnaire 9 (10), a depression survey, were compared with scores on a gold standard, structured psychiatric interview like the Comprehensive International Diagnostic Interview (11). A second approach to establishing a scale cut point is most often used when no gold standard measure is available or practical. It observes the covarying relationships between the score distribution of the scale of interest with the same respondents’ scores on other relevant variables or outcomes. Using the DDS, for example, we might observe the linear and quadratic (curvilinear) relationships between DDS scores and scores on diabetes self-efficacy, disease-management, and/or HbA1c. This analysis would tell us not only how substantive the relationships were but also where along the scale distributions these relationships occurred, what form they took, and where a cut point might best be placed.
Because we found no logical, face-valid justification for selecting a specific DDS score for a cut point and because there currently is no gold standard measure of high DD, we adopted the latter approach: the optimal categorization of DD in relation to key diabetes-specific biologic (HbA1c) and behavioral (e.g., diabetes self-efficacy, diet, physical activity) variables, after adjusting for demographics and disease status. Linear and nonlinear terms were included to enhance comprehensiveness. Because the shape and degree of relationship between score distributions can vary by patient sample, we separately analyzed data from two independent community samples of patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Subjects
Baseline samples from two studies of depression and distress among adult type 2 diabetic patients were included. Study 1, our primary sample, used baseline data from the Distress and Depression in Diabetes Study (3D Study), a noninterventional, three-wave, 18-month study of the prevalence and persistence of DD and depressive symptoms among 506 adult type 2 diabetic patients (2). Data were collected between 2003 and 2006. Study 2 used baseline, preintervention data from the Reducing Distress and Enhancing Effective Management (REDEEM) Study, a randomized controlled trial designed to reduce DD and enhance management among 392 type 2 diabetic adults (12). These data were collected between 2008 and 2010. Patients in both studies were recruited using the diabetes registries of several Bay Area community-based medical groups and diabetes education centers.
Inclusion criteria for both studies were patients with type 2 diabetes for 12 months or more, age 21 years or older, read and speak English fluently, no severe diabetes complications, and no active psychosis, substance use, or dementia. Additional, more restrictive criteria for REDEEM Study patients included displaying a mean item score of 1.5 or higher on the DDS2 to indicate elevated DD, displaying a score of 15 or higher on the Patient Health Questionnaire 8 to exclude patients with clinical depression, and displaying a deficit in at least one of three areas of diabetes self-management (diet, physical activity, medication use). A modification of the Summary of Diabetes Self-care Activities (SDSCA) (13) was used to define a deficit as not following their diet or physical activity plan 3 or more days during the last week or not taking prescribed diabetes medications 2 or more days during the last week.
Procedure
For both studies, letters were sent to each patient from their health care facility, cosigned by facility and project representatives, informing them of the project and that they would receive a phone call from the project office if one of two opt-out procedures was not initiated: patient returned an enclosed postcard or called an 800 phone number. A screening phone call followed and, for eligible patients, an appointment was made to explain the project in detail, collect informed consent, and begin the baseline assessment. Baseline assessment in both studies included a 1.5-h home visit for completion of questionnaires, physical measurements, and interviews, as well as a visit to a community laboratory for collection of blood and urine specimens. All data included in the present report were from the baseline assessment only. Both studies were approved by the institutional review boards at the University of California, San Francisco, and at each participating facility.
Measures
Control variables for both studies included patient age, sex (female = 1, male = 0), education, ethnicity (white = 1, nonwhite = 0), years with type 2 diabetes, use of insulin (yes or no), and BMI.
The DDS is a 17-item measure that uses a Likert scale to score each item from 1 (no problem) to 6 (a serious problem) during the last month (α = 0.93) (6). Mean-item scores are then calculated (DDS17). Previous analyses identified that the DDS2 had good sensitivity and specificity with the DDS17 (7) and was considered a reliable screening composite for use in clinical practice.
The DDS17 and DDS2 were associated with four diabetes-related variables. HbA1c was collected at a community facility and processed at the same community laboratory in both studies. Also included were measures of diabetes self-efficacy, diet, and physical activity, although different measures were used in each of the two studies.
3D Study.
Diabetes Self-Efficacy is a 10-item scale (α = 0.88), adapted from Coyne and Smith (14), that assesses the patient’s perceived confidence about taking care of diabetes (15). Items are rated on a 4-point scale from “not at all sure” to “very sure.” Diet and physical activity were assessed by the Summary of Diabetes Self-care Activities (13). Respondents indicated the number of days during the past week that they completed their diet and physical activity regimens.
REDEEM Study.
Diabetes Self-Efficacy was assessed by a 15-item scale developed by Lorig et al. (16) (α = 0.88). Items are rated on a 10-point Likert scale. Diet was assessed by five items from the “Starting the Conversation” survey (17) that asks respondents to indicate the number of times per week during the past 4 months that they ate certain types of unhealthy foods (e.g., fast foods, sodas, sweet tea). Physical activity was assessed by the Community Healthy Activities Model Program for Seniors (18). Nineteen items regarding frequency of specific physical activities (e.g., walk fast or briskly, swim, ride a bicycle) assessed caloric expenditures per week in moderate-intensity physical activity activities, capped at a value of 6,000 calories (19).
Data analysis
Comparisons were undertaken between 3D and REDEEM on key patient demographics using χ2 and t tests. Separate step-wise multiple regression equations were conducted for the DDS17 and DDS2 for each of the four dependent variables for each sample: step 1, demographics and disease status; step 2, DDS score (linear term); step 3, DDS score (quadratic term). Quadratic effects were interpreted only if the related t test, with one degree of freedom at step 3, was significant. The goal in these analyses was to determine if the shape of the relationships yielded a common pattern that would define a meaningful cut point for the DDS17 and DDS2. A minimum change of 0.5 SD units in dependent variables was used to help establish cut points (20). The results of each significant effect were placed on scatterplots, with fitted linear or quadratic lines, to examine the shape and start-point of the relationship between each DDS score and each of the dependent variables. Mean-item DDS values up to 4.0 were plotted because these encompassed more than 90% of the sample and DDS values >4 were based on small numbers with greater error. Note that the direction of the curves varies as a function of the direction of the regression coefficient and the direction of the scale.
RESULTS
3D Study
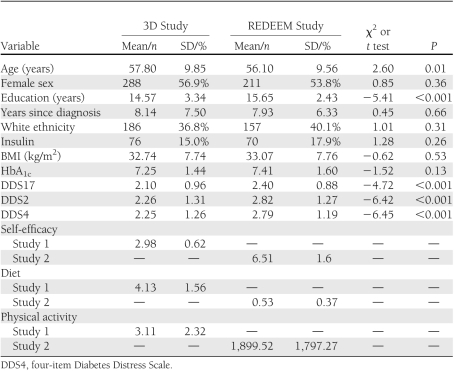
Details of the sample have been presented previously (4). Screening identified 640 eligible patients and 506 participated (79%), with no demographic or diabetes status differences between those who did and did not participate. The final sample was 56.9% women, average age was 57.79 (SD, 9.84) years, and average years since diagnosis was 8.14 (7.49) (Table 1). About 37% self-identified as white, 87.7% completed high school, and 15% of patients were taking insulin.
Table 1.
Sample descriptions of 3D and REDEEM studies
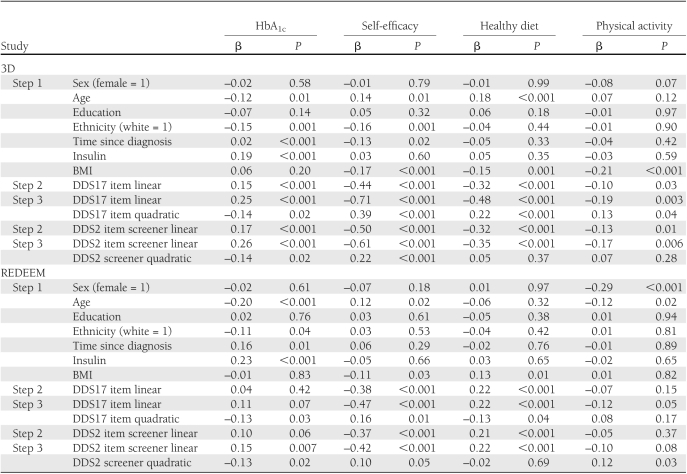
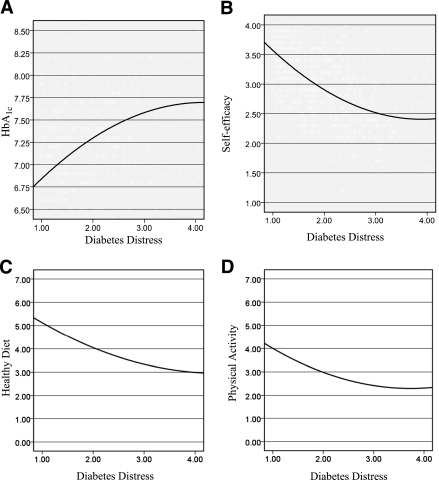
After step1 control variables were entered into the equations, multiple regression analyses indicated significant linear and quadratic effects between the DDS17 and each of the four dependent variables (Table 2), with increases in DD associated with poorer glycemic control (P < 0.02), self-efficacy (P < 0.001), diet (P < 0.001), and physical activity (P = 0.04). Figure 1 indicates that substantive associations between DDS17 and diabetes variables were evident at unexpectedly low levels of DDS17. Relationships between the DDS17 and the dependent variables began linearly between DDS17 scores of 1 and 2, continued but were somewhat muted between DDS17 scores of 2 and 3, and reached a maximum between DDS17 scores of 3 and 4. The significant increases in DDS17 mean-item scores across the four diabetes variables increased by ∼0.5 SD units for each dependent variable for each of three DDS17 score groups: below a DDS mean-item score of 2 (n = 276, 54.6% of patients), between a score of 2 and 3 (n = 139, 27.4%), and above 3 (n = 91, 18.0%).
Table 2.
Standardized coefficients for DDS17 and DDS2 linear and curvilinear effects
Figure 1.
Associations between 3D Study DDS17 scores and the key diabetes variables of HBA1c (A), self-efficacy (B), healthy diet (C), and physical activity (D) using fitted quadratic lines.
Findings from the DDS2 analyses approximated those from the DDS17: after control variables were entered into the equations, significant linear and quadratic effects were found for HbA1c (P = 0.02) and self-efficacy (P < 0.001), but only significant linear effects were found for diet (P < 0.001) and physical activity (P = 0.005; Table 1). Furthermore, where they occurred, the shape of the curvilinear relationships for the DDS2 with the diabetes variables was virtually identical to those found for the full DDS17 scale (data not shown): substantive linear relationships began between a mean item score of 1 and 2, continued but were muted between scores of 2 and 3, and reached a maximum at a mean item score of ∼4. Likewise, about a half-SD unit of change in each dependent variable occurred up to a mean-item score of 2, between 2 and 3, and over 3.
REDEEM Study
Of 588 subjects who were screened as eligible, 392 (67%) completed the baseline assessment (Table 1). There were no significant differences between participating and nonparticipating eligible patients on demographics or disease status. Average age was 56.12 (SD, 9.5) years, 56.5% were women, and diabetes duration was 7.9 (6.33) years. Almost all patients completed high school (97.4%), ∼40% identified as white, and 18% were taking insulin. Although REDEEM patients had to meet more restrictive inclusion criteria than 3D patients, between-group differences were minimal: REDEEM Study patients were slightly younger, had higher education and initial diabetes distress, but displayed no HbA1c differences (Table 1).
Results from the multiple regression analyses for REDEEM paralleled 3D findings. After the control variables were entered into the equations, significant quadratic effects occurred for the DDS17 on HbA1c (P = 0.03), self-efficacy (P < 0.004), and diet (P = 0.04), but not on physical activity (P = NS): high DDS17 was associated with poor glycemic control, low self-efficacy, and poor diet. As in the 3D Study, notable linear associations between DDS17 and the diabetes variables occurred between DDS17 scores of 1 and 2 (n = 135, 34.5% of patients), continued between DDS17 scores of 2 and 3 (n = 162, 41.3%), and a maximum was observed between a mean-item score of 3 and 4 (n = 95, 24.2%), with approximate changes in half-SD units of the dependent variables for each of the 3 DDS score intervals. Where they occurred, the degree and shape of the quadratic effects were virtually identical to those in the 3D Study (Fig. 1).
For the DDS2, significant curvilinear effects occurred for HbA1c (P = 0.02), for self-efficacy (P = 0.05), and for physical activity (P = 0.03; Table 2). Where curvilinear effects were found, substantive linear relationships occurred between a mean-item score of 1 and 2, with more muted linear increases between scores of 2 and 3, and a maximum was reached between mean-item scores of 3 and 4. Approximate half-SD increases in the dependent variables occurred for each DDS2 score interval and the degree and shape of the curves were identical to those in the 3D Study (Fig. 1). Only significant linear effects occurred for the DDS2 on diet (β= 0.21, P < 0.001), with high distress associated with poor diet.
CONCLUSIONS
Significant curvilinear relationships occur between the DDS17 and measures of glycemic control, diabetes-specific self-efficacy, diet, and physical activity in two community-based samples of type 2 diabetic adults, with the exception of physical activity in REDEEM. The degree and shape of these relationships are almost identical for two patient samples of type 2 diabetic adults: a highly diverse community sample (3D Study) and a more restricted community sample that displayed high initial levels of distress, no clinical depression, and poor self-management (REDEEM Study).
The similar degree and shape of relationships across studies, across dependent variables, and across the DDS17 and DDS2 highlight two important findings with respect to establishing a clinically meaningful cut point for high distress. First, the significant relationship of diabetes-specific distress with each of the four dependent variables begins at unexpectedly low levels of distress—far less DD is required to demonstrate a relationship with diabetes-specific biologic and behavioral indicators than was previously considered. A previous report suggested that a mean item score of 3.0 provided a valid cut point for high DD (7). Our new findings confirm and expand these findings: even relatively low levels of DD are associated with diabetes-related indicators.
Second, to help determine where an appropriate DDS cut point should be, we focus on the notable consistency in the shape of the significant curvilinear relationships between the DDS17 across the dependent variables in both 3D and REDEEM: the curve rises linearly from a DDS17 mean item score of 1 to 2, continues linearly but in a more muted fashion between scores of 2 and 3, and reaches a maximum between scores 3 and 4. We note also that, in general, there is an ∼0.5 SD increase in each of the four dependent variables corresponding to increases in DDS17 mean item scores from 1 to 2, 2 to 3, and above 3.
Findings for the DDS2 approximate those for the DDS17, even with the many fewer items and far less information contained in DDS2. The similar pattern of significant linear and curvilinear effects across the four diabetes variables, the similar degree of association, and the similar shape of the respective curves of association all suggest that the DDS2 behaves relatively similarly to the DDS17 with respect to the four diabetes variables we studied.
These findings suggest that dichotomizing the distribution of DDS17 scores to denote a “high distress” group and a “low distress” group does not accurately reflect the shape of the relationships between the DDS17 and the four diabetes indicators. It therefore may be more helpful to define three DDS categories: “little or no DD” (DDS <2.0), “moderate DD” (DDS = 2.0–2.9), and “high DD” (DDS ≥3.0). Creating defined categories of moderate and high DD provides a better reflection of the shape of these relationships across diabetes indicators than a single cut point and allows greater flexibility for use in clinical and research settings.
3D Study results, based on a diverse community sample, indicate that 45.4% of patients with type 2 diabetes display at least moderate DD. (Percentages for REDEEM are considerably higher—65.5%—because this study required high DD as an inclusion criterion.) These findings suggest that significant levels of DD occur among almost half of patients with type 2 diabetes and that the possible reciprocal influence of DD on diabetes indicators indicates increased risk for poor treatment outcomes. Because ∼70% of these patients are not clinically depressed (2), interventions for DD may best be focused on ongoing conversations about DD and diabetes management in the clinical setting. Highly rated DDS17 items can be used to identify areas of specific patient concern, and patients are often relieved when clinicians initiate a discussion that labels feelings overtly, links them with diabetes-related difficulties, and normalizes them in ways that provide both reassurance and perspective (1). Even the verbalization of emotional experiences surrounding diabetes can be therapeutic and can lead to action planning for behavioral change.
Several limitations are worthy of note. First, we included only four diabetes variables for study. The DDS17 may display different levels and patterns of association with other diabetes-related indicators. Second, we did not explore how patient demographic and diabetes-related factors qualify these relationships; instead, we controlled for many of them in equations. Although our goal was to report findings with the greatest generalizability, differences in results may occur for subgroups of patients. Last, our findings are cross-sectional and associational, such that causation between DD and diabetes indicators cannot be inferred. A previous study suggested that these associations are bidirectional (2), with one affecting the other sequentially over time.
We used a data-oriented approach to establish empirically DDS cut points for significant diabetes distress among adult patients with type 2 diabetes. In two community samples we show a consistent pattern of curvilinear relationships between the DDS and HbA1c, diabetes self-efficacy, diet, and physical activity. The degree and shape of these relationships suggest cut points on the DDS17 and the DDS2 for three patient subgroups: those with little or no distress, moderate distress, and high distress.
Acknowledgments
This research was supported by grants DK062732 and DK061937 from the National Institute of Diabetes and Digestive and Kidney Diseases.
No potential conflicts of interest relevant to this article were reported.
L.F. wrote the manuscript. D.M.H. researched data, completed the data analysis, contributed to discussion, and edited the manuscript. W.H.P. and J.M. researched data, contributed to discussion, and edited the manuscript.
Parts of this study were presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The following medical groups and diabetes education centers collaborated in this research: Alta Bates Diabetes Education Center, Berkeley, California; Brown and Toland Medical Group, San Francisco, California; California Pacific Diabetes Education Center, San Francisco, California; Hill Physicians Medical Group, San Ramon, California; and University of California, San Francisco Hospital and Clinics, San Francisco, California.
References
- 1.Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: have we been missing something important? Diabetes Care 2011;34:236–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabet Med 2008;25:1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007;30:542–548 [DOI] [PubMed] [Google Scholar]
- 5.Fisher L, Mullan J, Skaff M, Glasgow R, Arean P, Hessler D. Predicting disease distress among primary care patients with type 2 diabetes: a longitudinal study. Diabet Med 2009;26:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polonsky WH, Fisher L, Earles J, et al. Assesing psychosocial stress in diabetes: development of the diabetes distress scale.. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 7.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med 2008;6:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien SM. Cutpoint selection for categorizing a continuous predictor. Biometrics 2004;60:504–509 [DOI] [PubMed] [Google Scholar]
- 9.Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Finding Optimal Cutpoint for Continuous Covariates With Binary ad Time-to-Event Outcomes. Rochester, MN, Mayo Clinic, Technical Report Series, 2006 [Google Scholar]
- 10.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–1744 [DOI] [PubMed] [Google Scholar]
- 11.Wittchen HU. Reliability and validity studies of the WHO—Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res 1994;28:57–84 [DOI] [PubMed] [Google Scholar]
- 12.Hessler DM, Fisher L, Naranjo D, Mullan JT, Masharani U. Why do Younger Adults Have Higher HbA1c Than Older Adults? The Role of Diabetes Distress and Medication Adherence (abstract). San Diego, CA, American Diasbetes Association, 2011 [Google Scholar]
- 13.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 14.Coyne JC, Fiske V. Couples coping with chronic and catastrophic illness. In Family Health Psychology. Akamatsu TJ, Stephens MA, Hobfoll SE, Crowther JH, Eds. Kent, Ohio, Hemisphere Publishing Corp, 1992, p. 94–113 [Google Scholar]
- 15.Fisher L, Chesla CA, Skaff MM, et al. The family and disease management in Hispanic and European-American patients with type 2 diabetes. Diabetes Care 2000;23:267–272 [DOI] [PubMed] [Google Scholar]
- 16.Lorig K, Stewart A, Riter P, Gonzalez V, Laurent D, Lynch J. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA, Sage Publications, 1996 [Google Scholar]
- 17.Ammerman A. Starting the Conversation - Diet. Instrument Developed by the University of North Carolina, in Conjunction with the NC Prevention Partners, and the Heart Disease and Prevention Branch. Raleigh, NC, North Carolina Department of Health and Human Services, 2004 [Google Scholar]
- 18.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001;33:1126–1141 [DOI] [PubMed] [Google Scholar]
- 19.Glasgow RE, Christiansen SM, Kurz D, et al. Engagement in a diabetes self-management website: usage patterns and generalizability of program use. J Med Internet Res 2011;13:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582–592 [DOI] [PubMed] [Google Scholar]