Abstract
OBJECTIVE
Metformin is the first-line oral medication recommended for glycemic control in patients with type 2 diabetes. We reviewed the literature to quantify the effect of metformin treatment on glycated hemoglobin (HbA1c) levels in all types of diabetes and examine the impact of differing doses on glycemic control.
RESEARCH DESIGN AND METHODS
MEDLINE, EMBASE, and the Cochrane Library were searched from 1950 to June 2010 for trials of at least 12 weeks’ duration in which diabetic patients were treated with either metformin monotherapy or as an add-on therapy. Data on change in HbA1c were pooled in a meta-analysis. Data from dose-comparison trials were separately pooled.
RESULTS
A total of 35 trials were identified for the main analysis and 7 for the dose-comparison analysis. Metformin monotherapy lowered HbA1c by 1.12% (95% CI 0.92–1.32; I2 = 80%) versus placebo, metformin added to oral therapy lowered HbA1c by 0.95% (0.77–1.13; I2 = 77%) versus placebo added to oral therapy, and metformin added to insulin therapy lowered HbA1c by 0.60% (0.30–0.91; I2 = 79.8%) versus insulin only. There was a significantly greater reduction in HbA1c using higher doses of metformin compared with lower doses of metformin with no significant increase in side effects.
CONCLUSIONS
Evidence supports the effectiveness of metformin therapy in a clinically important lowering of HbA1c used as monotherapy and in combination with other therapeutic agents. There is potential for using higher doses of metformin to maximize glycemic control in diabetic patients without increasing gastrointestinal effects.
Metformin is the most commonly prescribed antihyperglycemic medication for diabetes in the U.S. (1) and the U.K. (2) and is the recommended first choice for oral therapy (2–4). The role of metformin in glucose lowering has been associated with a reduction in cardiovascular outcomes (5,6). However, its effectiveness in glycemic control is not well documented, although estimates based on trials suggest that it reduces glycated hemoglobin (HbA1c) by 1–2% (11–22 mmol/mol) (3,7). A recent systematic review (8) suggested that this is an overestimate of effect, but the meta-analysis included only seven trials of metformin, and it did not separately examine metformin use as a monotherapy or in combination with other antihyperglycemic medications. We therefore conducted a systematic review and meta-analysis of randomized controlled trials of metformin with the aim of 1) quantifying its reduction in HbA1c, 2) exploring the different treatment effects when administered as a monotherapy or as an add-on therapy, and 3) examining head-to-head trials of low versus high metformin doses to understand the effect metformin dose has on HbA1c reduction.
RESEARCH DESIGN AND METHODS
Search strategy and study selection
MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials were searched from 1950 to June 2010. Abstracts of identified articles were reviewed by two reviewers, and full texts were examined by two reviewers (J.A.H. and R.A.). For inclusion, trials were required to fulfill the following criteria: 1) be a randomized controlled design; 2) report data on participants with diabetes; 3) have a patient follow-up of at least 12 weeks; 4) have a treatment group of metformin monotherapy, or metformin as an add-on therapy; 5) have a placebo or background treatment comparator group; 6) randomize patients to a fixed dose of metformin; 7) blind patients to oral medications; 8) use the same metformin dose for each patient in the trial; and 9) use the same fixed dose of any other oral glucose-lowering medication used in combination with metformin in both the metformin and comparator arms.
Data extraction and quality assessment
Data were abstracted using standardized forms to include trial characteristics (design and duration), interventions, trial quality, patient characteristics, and outcome measures. The quality of trials was assessed using items for selection bias, treatments, outcome measurement statistical methods, and outcome assessment. Outcome measures were the change in HbA1c levels from baseline to the end of the trial, total adverse events, and gastric adverse events (diarrhea and abdominal cramps). In 12 trials where change in HbA1c was not reported, it was calculated from baseline and end point data, and SD of the change was estimated from baseline and end point SDs (9). In five trials, SD of HbA1c was not given, and it was imputed by averaging the SDs from trials in which it was reported (9). Trials in which data were estimated or imputed were excluded from the meta-analysis in a sensitivity analysis. When a study had two metformin arms of different doses, we included both in the analysis and present results ordered by dose, splitting the comparator group into two to avoid covariance problems (9). When a study had more than two metformin arms, we selected the lowest and highest dose arms for inclusion in the analysis. Sensitivity analyses of trials of 24 weeks or longer were conducted to verify that the treatment effect observed was sustained in the longer trials.
A second review comparing head-to-head trials of two metformin doses was conducted. The same inclusion criteria were applied with the exception that the comparator arm included a different dose of metformin to the intervention arm. In each multiarm trial, we pooled arms with metformin doses of 1,000 and 1,500 mg into a single “low-dose” arm and arms with metformin dose >1,500 mg into a single “high-dose” arm for comparisons.
All of the trials covered in this review reported HbA1c units as a percentage of total hemoglobin standardized to the methods of the Diabetes Control and Complications Trial (DCCT). Results are therefore reported in DCCT units as a percentage and have been converted into the new Standard International units using International Federation of Clinical Chemistry and Laboratory Medicine units of millimoles per mole of hemoglobin.
Statistical analysis
Data analysis was performed in Stata 11.1 (Stata Corporation, College Station, TX) using a random-effects model based on the DerSimonian and Laird method to pool the data, reporting the mean difference in change in HbA1c levels between the metformin and comparator arms or high-dose versus low-dose metformin. Adverse-events data were analyzed using the Mantel-Haenszel method, with a random-effects model reporting risk ratio under the approximating assumption that adverse events occur independently. Heterogeneity was explored using subgroup analyses and metaregression to look at the effect of type of diabetes, trial size, BMI, age, metformin dose, baseline HbA1c levels, length of follow-up, year of publication, country of trial, and change in HbA1c in the comparator group.
RESULTS
Study characteristics
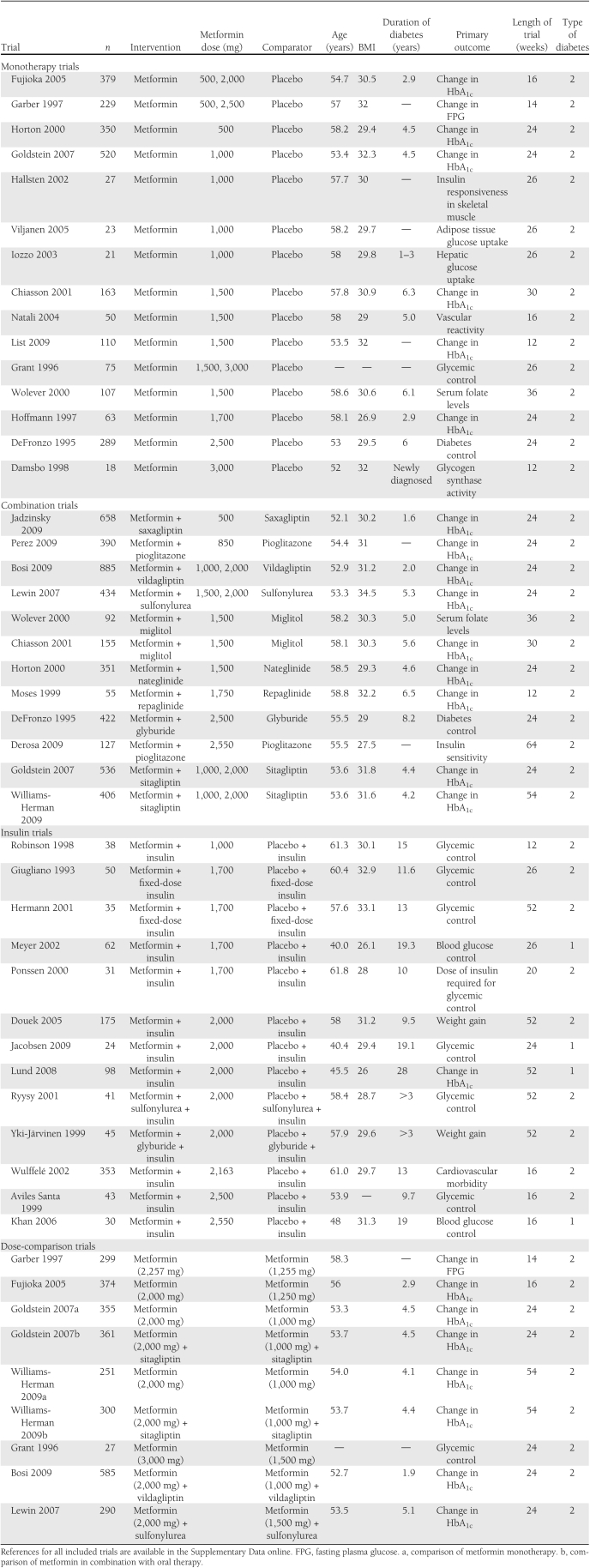
Searches identified 2,680 trials (Supplementary Fig. A1). These were screened on title and abstract to give 293 articles requiring examination of the full text. A total of 35 trials, representing 7,960 participants, met inclusion criteria (a full list of references of included trials is given in the Supplementary Data online). Of these, 15 were receiving metformin monotherapy compared with placebo, no treatment, or diet (2,424 participants); 12 were receiving metformin treatment in combination with another oral antihyperglycemic medication compared with the other medication (4,511 participants); and 13 were receiving metformin in combination with insulin treatment compared with patients on insulin treatment only (1,025 participants). Five trials with multiple arms (10–14) were included in the meta-analysis of both monotherapy and metformin combination. Seven trials were included in the dose-comparison analysis (2,842 participants). Details of the trial characteristics are shown in Table 1.
Table 1.
Included studies
Quality of trials
All of the included trials were double blinded, with the exception of one trial from the insulin subgroup (15) in which the comparator group received an extra insulin injection and, thus, was only partially blinded. Of the 35 included trials, only 8 stated the method of randomization.
Metformin effectiveness
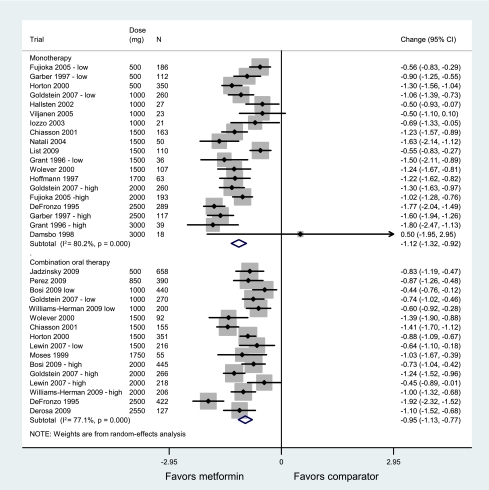
All monotherapy and combination oral therapy trials were conducted on patients with type 2 diabetes. In the metformin monotherapy trials, HbA1c was reduced by 1.12% (95% CI 0.92–1.32; P < 0.00001, I2 = 80.2%), corresponding to a reduction of 12 mmol/mol more with metformin than placebo (Fig. 1). When we restricted the analysis to trials of ≥24 weeks, the HbA1c in 10 trials was 1.19% lower (0.98–1.41; I2 = 71.2%) in the metformin groups versus placebo. In the trials of metformin as add-on to oral therapy, HbA1c was reduced by 0.95% (0.77–1.13; P < 0.00001, I2 = 77.1%), corresponding to a reduction of 11 mmol/mol more with metformin than in the comparator group. When we restricted the analysis to trials of ≥24 weeks, the HbA1c in 11 trials was 0.94% lower (0.76–1.13; I2 = 78.6%) in the metformin groups versus comparator groups.
Figure 1.
Mean difference in change in HbA1c of metformin treatment versus comparator (boxes) and pooled estimates (diamonds) calculated by the random-effects DerSimonian and Laird method, stratified by metformin monotherapy and metformin added to an oral antidiabetes medication. Horizontal bars and diamond widths denote 95% CIs, and box sizes indicate relative weight in the analysis. (A high-quality color representation of this figure is available in the online issue.)
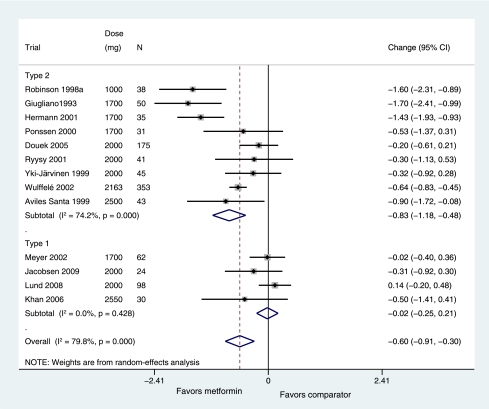
In trials of metformin as add-on to insulin therapy, HbA1c was reduced by 0.60% (95% CI 0.30–0.91; P = 0.0001, I2 = 79.8%), corresponding to a reduction of 6 mmol/mol more in the metformin groups than in the comparator group. A subgroup analysis of these trials performed on type of diabetes (Fig. 2) found that patients with type 2 diabetes taking metformin with their insulin treatment had HbA1c levels 0.83% lower (0.48–1.18; P = 0.000, I2 = 74.2%) in nine trials, corresponding to HbA1c of 9 mmol/mol lower than patients on insulin alone. Patients with type 1 diabetes, however, showed no change in their HbA1c levels when metformin was added to their insulin treatment (change in HbA1c −0.02% [95% CI −0.25 to 0.21]; P = 0.43, I2 = 0%) in four trials. Restricting the analysis to trials of ≥24 weeks included three trials of type 1 diabetes that gave no change in HbA1c (−0.01% [−0.22 to 0.25]; I2 = 0%) and five trials of type 2 diabetes in which HbA1c was 0.79% lower (95% CI 0.15–1.42; I2 = 83.4%) in metformin versus comparator groups.
Figure 2.
Mean difference in change in HbA1c of metformin added on to insulin versus placebo and insulin comparator (boxes) and pooled estimates (diamonds) calculated by the random-effects DerSimonian and Laird method, stratified by type of diabetes. Horizontal bars and diamond widths denote 95% CIs, and box sizes indicate relative weight in the analysis. (A high-quality color representation of this figure is available in the online issue.)
Metaregression, carried out to investigate the effect of other variables and to explore sources of heterogeneity, found that no single factor could explain the heterogeneity. The I2 statistic for the insulin trials was reduced by 24.3% by mean BMI. Year of publication reduced the I2 by 11.8% for metformin combination trials and 18.5% for insulin trials. No other factor reduced heterogeneity by >10.4% in any of the analyses, even though year of publication, metformin dose, mean BMI, mean patient age, and mean duration of diabetes were significantly associated with the mean outcome in some of the analyses.
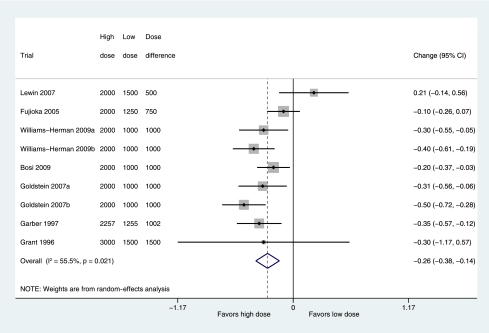
The dose-comparison review identified seven trials with head-to-head comparisons of two different metformin doses for inclusion, two of which could be used for more than one comparison, giving nine comparisons. Meta-analysis of data from these trials (Fig. 3) found significantly greater change in HbA1c in the higher-dose arms with a reduction in HbA1c of 0.26% (95% CI 0.14–0.38; P < 0.0001, I2 = 55.5%) more in these arms.
Figure 3.
Mean difference (boxes) in change in HbA1c of high dose metformin, combining trial arms allocated to at least 2,000 mg metformin, versus low dose metformin, combining trial arms allocated to 1,000–1,500 mg metformin, showing pooled estimates (diamonds) calculated by the random-effects DerSimonian and Laird method. Horizontal bars and diamond widths denote 95% CIs, and box sizes indicate relative weight in the analysis. (A high-quality color representation of this figure is available in the online issue.)
Adverse events
The most commonly reported adverse events were gastrointestinal events, such as diarrhea, nausea, vomiting, flatulence, and abdominal pain, but also included were hypoglycemia, dizziness, headache, urinary tract infection, hypertension, coughing, and palpitations. The meta-analysis of reported adverse events (Supplementary Fig. A2) found an increase in the number of adverse events in metformin-treated groups in comparison with comparator groups in the monotherapy trials (with an approximate risk ratio of 1.13 [95% CI 1.06–1.21]; I2 = 3%, P = 0.0003), and in oral combination trials (1.03 [0.95–1.12]; I2 = 82%, P = 0.45). The number of adverse events in insulin trials was not significantly different between the metformin group and the comparator group (2.37 [0.65–8.67]; I2 = 73%, P = 0.19). There was no significant difference in adverse events between higher and lower dose in the dose-comparison trials (approximate risk ratio = 1.23, P = 0.13). However, the assumption of independence was clearly an approximation (Supplementary Fig. A2).
An analysis of gastric adverse events (diarrhea and abdominal cramps) showed significantly more adverse events in the metformin arms of the monotherapy trials (approximate risk ratio 2.26 [95% CI 1.60–3.20]; I2 = 0%) and combination trials (1.55 [1.29–1.87]; I2 = 0%) and nonsignificantly more adverse events in the metformin arms of insulin trials (2.18 [0.68–7.01]; I2 = 78%). There were also more gastric adverse events in the higher-dose trials compared with the lower-dose trials, although this was not significant (1.18 [0.98–1.42]; I2 = 7%, P = 0.08).
CONCLUSIONS
This systematic review of double-blinded, randomized, controlled trials has separately examined metformin treatment as a stand-alone therapy and as an add-on therapy both to other oral medications and to insulin to quantify its effect on glycemic control. Metformin monotherapy reduced HbA1c by 1.12%, and metformin in combination with other oral antihyperglycemic treatments or insulin reduced HbA1c by 0.95 and 0.83%, respectively, for type 2 diabetes, and these effects were sustained at 24 weeks. Of particular interest is that the addition of metformin treatment to insulin treatment improves glycemic control in type 2 diabetes by a clinically significant level despite protocol-permitted insulin dose adjustments in both arms of these trials. Treatment of type 1 diabetic patients with metformin did not reduce HbA1c. We have also clearly demonstrated that an increase in metformin dose results in a further modest reduction in HbA1c of 0.26% in trials comparing lower doses to higher doses up to a metformin dose of 2,000 mg. It was not possible to establish whether there is further benefit when metformin dose is increased beyond this level because there were too few trials with higher doses, although the trial on which much of the evidence for cardiovascular benefit is based, the UK Prospective Diabetes Study (UKPDS) (16), used a median dose of 2,550 mg metformin. Establishing how the dose-effect relationship may vary at different doses and what the maximum effective dose may be is an area for future work.
A previous systematic review found lower rates of cardiovascular mortality in people randomized to metformin in six trials of >11,000 patients (6). Compared with that review, our analysis has the disadvantage of using the surrogate outcome of glucose lowering, albeit a well-established surrogate on which treatment guidelines are based (4,17,18), but it has the advantage of an estimate based on more trials. This has allowed us to examine subgroups, such as monotherapy, combination oral therapy, and insulin therapy, and to establish a dose-response relationship.
This is the most comprehensive systematic review to date on the effect of metformin treatment on HbA1c levels. In addition, to our knowledge, this is the first meta-analysis of metformin dose-comparison studies. Significant unexplained heterogeneity observed between the trials, however, is a limitation of this review and, consequently, results need to be treated with caution. Our searches identified 5 times more trials than a previous review, which examined the effect of metformin on glycemic control in seven trials (8). This is partly explained by the other reviewer’s more stringent inclusion criteria, which included a minimum of 50 subjects in each arm of the trial and an explicit statement that informed consent was obtained. Inclusion of a larger number of trials has made it possible to separately analyze data from metformin monotherapy, oral combination therapy, and insulin trials. The greatest reduction in HbA1c in our analysis was 1.1%, which is at the low end of the 1–2% estimated by Nathan et al. (3). The results of our review suggest that these estimated reductions are most likely to be achieved with the highest metformin doses. Previous trials that compared different metformin doses from various trials could not establish a dose-effect relationship of metformin (8). By including head-to-head trials of metformin in our systematic review, we clearly demonstrate the benefit of using a higher metformin dose to maximize HbA1c reduction, although there may be an associated increase in gastric side effects. We have not been able to demonstrate a relationship between baseline HbA1c and change in HbA1c with treatment observed in other systematic reviews (8,19). A previous systematic review of 61 trials of oral glucose-lowering therapy found an association between baseline HbA1c and the change in HbA1c on treatment, but metformin was a randomized therapy in only seven of these trials (8). We were not able to find such a relationship in 15 trials of metformin monotherapy, 12 trials of metformin in combination with oral therapy, and 13 trials of metformin in combination with insulin. However, our analysis is based on metaregression, which compares mean values across trials. The best data to address this question would make comparisons between individuals (20). These results, therefore, need to be interpreted with caution.
Metformin’s known benefit in reducing cardiovascular mortality (6), as well as its neutral effects on body weight and low risk of hypoglycemia (16), has led to wide recommendations for routine prescribing. However, until now, it has not been clear how different patients may respond to treatment. By separately examining the effect of metformin treatment in various groups of patients, depending on their previous antidiabetes medication, we are providing a tool to assist decisions on treatment combinations and optimal doses.
This review demonstrates that metformin treatment can be used to reduce HbA1c in all patients with type 2 diabetes regardless of prior antihyperglycemic medication or insulin treatment. Use of higher doses of metformin resulted in modestly higher decreases in HbA1c compared with lower doses. Metformin use in type 1 diabetes may not, however, reduce HbA1c. Despite this, there may be other indications for treating type 1 diabetic patients with metformin because a reduction in insulin dose required in the metformin arm of these trials was observed consistent with metformin’s role as an insulin sensitizer (21).
Supplementary Material
Acknowledgments
This project was funded by the U.K.’s National Health Services Diabetes. A.J.F. has received financial support from the National Institute of Health Research Oxford Biomedical Research Centre.
No potential conflicts of interest relevant to this article were reported.
J.A.H. researched data and wrote the manuscript. A.J.F. contributed to discussion and reviewed and edited the manuscript. R.A. and N.W.R. researched data and reviewed and edited the manuscript. R.J.S. researched data, contributed to discussion, and reviewed and edited the manuscript.
The authors thank Ben Cairns and Francesca Crowe from the Cancer Epidemiology Unit, University of Oxford; Clare Bankhead, Julie McLellan, Peter Rose, Rafael Perera, Annette Plüddemann, and Sian Harrison from the Department of Primary Care Health Sciences, University of Oxford; and Angelyn Bethel from the Diabetes Trials Unit, Oxford Centre for Diabetes Endocrinology and Metabolism, University of Oxford, for their input in reviewing and screening the original articles.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1465/-/DC1.
The views expressed by the authors may not necessarily reflect the views or policies of the National Institute for Health Research.
References
- 1.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Arch Intern Med 2008;168:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health Service. Prescribing for diabetes in England: 2004/5 to 2009/10 [article online], 2010. Available from http://www.ic.nhs.uk/webfiles/publications/Primary%20Care/Prescriptions/prescribingdiabetes/Prescribing%20for%20Diabetes%20in%20England%2020045%20to%20200910.pdf Accessed 5 December 2011
- 3.Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association; European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Clinical Excellence. Type 2 diabetes: national clinical guideline for management in primary and secondary care (update) [article online], 2008. Available from http://www.nice.org.uk/nicemedia/live/11983/40803/40803.pdf Accessed 5 December 2011
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Bolen S, Yeh H-C, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med 2008;168:2070–2080 [DOI] [PMC free article] [PubMed]
- 7.Nathan DM. Finding new treatments for diabetes—how many, how fast... how good? N Engl J Med 2007;356:437–440 [DOI] [PubMed] [Google Scholar]
- 8.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care 2010;33:1859–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Deeks JJ, Altman DG, Eds (2011). Chapter 16: Special topics in statistics. Cochrane Handbook for Systematic Reviews of Interventions [Internet], 2011, Version 5.1.0. The Cochrane Collaboration. Available from http://www.cochrane-handbook.org/ Accessed 5 December 2011
- 10.Chiasson JL, Naditch L; Miglitol Canadian University Investigator Group The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care 2001;24:989–994 [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 1995;333:541–549 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE; Sitagliptin 036 Study Group Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007;30:1979–1987 [DOI] [PubMed] [Google Scholar]
- 13.Wolever TMS, Assiff L, Basu T, et al. Miglitol, an alpha-glucosidase inhibitor, prevents the metformin-induced fall in serum folate and vitamin B12 in subjects with type 2 diabetes. Nutr Res 2000;20:1447–1456 [Google Scholar]
- 14.Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin 2009;25:569–583 [DOI] [PubMed] [Google Scholar]
- 15.Yki-Järvinen H, Ryysy L, Nikkilä K, Tulokas T, Vanamo R, Heikkilä M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1999;130:389–396 [DOI] [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 17.British Medical Association. QOF guidance, fourth revision 2011-2012 [article online], 2011. Available from http://www.bma.org.uk/employmentandcontracts/independent_contractors/quality_outcomes_framework/qofguidance2011.jsp Accessed 5 December 2011
- 18.American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed]
- 19.DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet Med 2010;27:309–317 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, Eds. Cochrane Handbook for Systematic Reviews of Interventions [Internet], 2011. Version 5.1.0. The Cochrane Collaboration. Available from www.cochrane-handbook.org. Accessed 5 December 2011
- 21.Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334:574–579 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.