Abstract
OBJECTIVE
To assess the effect of dietary flavonoids on cardiovascular disease (CVD) risk in postmenopausal women with type 2 diabetes on established statin and hypoglycemic therapy.
RESEARCH DESIGN AND METHODS
Despite being medicated, patients with type 2 diabetes have elevated CVD risk, particularly postmenopausal women. Although dietary flavonoids have been shown to reduce CVD risk factors in healthy participants, no long-term trials have examined the additional benefits of flavonoids to CVD risk in medicated postmenopausal women with type 2 diabetes. We conducted a parallel-design, placebo-controlled trial with type 2 diabetic patients randomized to consume 27 g/day (split dose) flavonoid-enriched chocolate (containing 850 mg flavan-3-ols [90 mg epicatechin] and 100 mg isoflavones [aglycone equivalents)]/day) or matched placebo for 1 year.
RESULTS
Ninety-three patients completed the trial, and adherence was high (flavonoid 91.3%; placebo 91.6%). Compared with the placebo group, the combined flavonoid intervention resulted in a significant reduction in estimated peripheral insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR] −0.3 ± 0.2; P = 0.004) and improvement in insulin sensitivity (quantitative insulin sensitivity index [QUICKI] 0.003 ± 0.00; P = 0.04) as a result of a significant decrease in insulin levels (−0.8 ± 0.5 mU/L; P = 0.02). Significant reductions in total cholesterol:HDL-cholesterol (HDL-C) ratio (−0.2 ± 0.1; P = 0.01) and LDL-cholesterol (LDL-C) (−0.1 ± 0.1 mmol/L; P = 0.04) were also observed. Estimated 10-year total coronary heart disease risk (derived from UK Prospective Diabetes Study algorithm) was attenuated after flavonoid intervention (flavonoid +0.1 ± 0.3 vs. placebo 1.1 ± 0.3; P = 0.02). No effect on blood pressure, HbA1c, or glucose was observed.
CONCLUSIONS
One-year intervention with flavan-3-ols and isoflavones improved biomarkers of CVD risk, highlighting the additional benefit of flavonoids to standard drug therapy in managing CVD risk in postmenopausal type 2 diabetic patients.
The global prevalence of diabetes is increasing, with recent predictions suggesting that complications of diabetes accounts for 7% of all-cause mortality and 12% of healthcare costs worldwide (1). Pharmacological treatment is critical in delaying the underlying progression of diabetes (2), yet it remains inadequate in preventing the increased risk of cardiovascular disease (CVD) in patients with type 2 diabetes, especially women (3). Gender disparities in the treatment and control of CVD risk factors have been reported (3,4), and observational studies suggest poorer metabolic control in women with type 2 diabetes (4), suggesting potential gender differences in the mechanisms underlying disease progression. These data reinforce the need for effective preventative and disease management strategies to reduce CVD risk associated with type 2 diabetes, particularly in women.
Dietary flavonoids are bioactive constituents, with observational data supporting an association between high intakes of some flavonoid subclasses and a reduced risk of CVD (5). In particular, there is growing evidence to support the cardioprotective effects of two specific subclasses, the flavan-3-ols (present in cocoa and tea) and isoflavones (present almost exclusively in soy). Observational studies support the association between high cocoa intake and reduced CVD risk and mortality (6), and improvements in flow-mediated dilatation, hyperemic blood flow, and blood pressure (BP) have followed short-term interventions with cocoa flavan-3-ols (7,8). A growing body of in vitro evidence also supports the potentially beneficial effects of flavan-3-ols on CVD risk, via effects on nitric oxide and glucose transporters (9,10). In Asian populations, where soy is consumed as a staple, increased habitual intake of soy isoflavones has also been associated with a reduced risk of CVD and type 2 diabetes (11), and recent meta-analyses suggest beneficial effects of increased isoflavone intake on CVD risk biomarkers and glycemic control (12–14).
In patients with type 2 diabetes, no long-term trials have been conducted to examine the effects of either isoflavones or flavan-3-ols on CVD risk biomarkers, although the available data from short-term studies (maximum duration 12 weeks in both flavonoid subclasses) are suggestive of favorable effects (15–17). To our knowledge, no studies have examined the long-term effects of a combined intervention of these bioactive flavonoids on CVD risk in medicated patients with type 2 diabetes, and given our knowledge of their specific mechanisms of action, there is the potential of synergistic effects. Therefore, we conducted a randomized, double-blind, placebo–controlled, parallel trial to examine the vascular protective effects of a 1-year combined intervention of flavan-3-ols and isoflavones in postmenopausal women with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Subjects
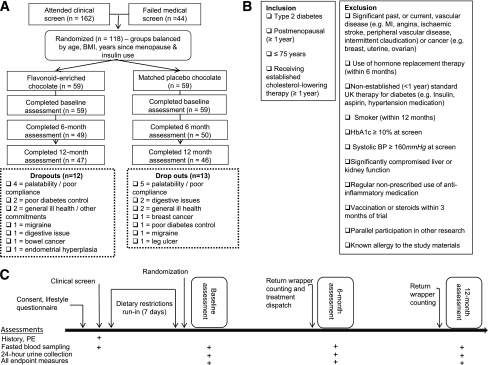
One hundred and eighteen postmenopausal women (no menstruation for ≥12 months), aged 51–74 years and receiving standard U.K. care for type 2 diabetes (including statin therapy, established for ≥12 months and glycemic target of HbA1c of 7.0%) were recruited through general practitioners, specialist diabetes clinics, and local media advertisements (Fig. 1A). Exclusion criteria included use of hormone replacement therapy (within 6 months), known allergy to the study foods, history of vascular disease or cancer, and poor diabetes control or raised BP at screening (as outlined in Fig. 1B). All study investigations were conducted according to the principles expressed in the Declaration of Helsinki and the study was approved by the Norfolk Research Ethics Committee. All subjects provided written informed consent.
Figure 1.
Enrollment, randomization, and trial design. A: Enrollment and randomization. B: Inclusion/exclusion criteria. C: Assessment overview. MI, myocardial infarction; PE, physical exam.
Study protocol
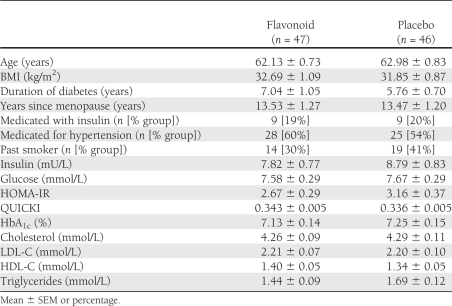
Subjects were randomized to placebo (n = 59) or flavonoid-enriched (n = 59) chocolate daily for 1 year. Treatment allocation was stratified according to age, BMI, years since menopause, insulin use, and patient characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the 93 postmenopausal patients with type 2 diabetes who completed the 1-year flavonoid intervention
The daily intake of 27 g flavonoid-enriched chocolate (conceptualized by the research team and formulated and produced by Barry Callebaut [Lebbeke-Wieze, Belgium] specifically for the trial) provided 90 mg epicatechin (850 mg total flavan-3-ols) and 100 mg isoflavones (aglycone equivalents), the optimal dose for biological efficacy on the basis of published findings from previous short-term trials (18,19). Flavonoid extracts were purchased (flavan-3-ols and Acticoa cocoa [Barry Callebaut]; isoflavones and SoyLife40 [Frutarom, Veenendaal, the Netherlands]), and intervention and placebo chocolates were produced by Barry Callebaut, which were matched for macronutrient content, appearance, and taste. Batch-to-batch variability and effect of storage on flavonoid content were independently determined using high-performance liquid chromatography (HPLC) (20).
Chocolate (13.5 g) was consumed twice daily (lunchtime and evening) to maintain circulating levels of metabolites, given our knowledge of the half-life of the compounds (21). Subjects were advised to exchange the study chocolate with foods of similar nutritional content (total daily intake provided 152 kcal, 10 g fat, and 13 g carbohydrate). Adherence to intervention was determined by counting returned wrappers and objectively assessed through quantification of 24-h urinary total epicatechin (sum of epicatechin, 3′-methyl epicatechin, 4′-methyl epicatechin, epicatechin-3-sulfate, and epicatechin-methyl-sulfate) and isoflavone (daidzein, genistein, and equol) excretion levels at 6 and 12 months determined by HPLC with mass spectrometric (MS) detection after enzyme hydrolysis using established methods. Intra-assay coefficients of variation (CVs) for total epicatechin, daidzein, genistein, and equol levels were 7.7, 6.3, 8.0, and 8.1%, respectively, and interassay CVs were 9.1, 11.0, 14.1, and 8.6%, respectively.
One week preceding and during the 1-year trial, habitual diet and exercise routines were maintained, with the exception of restricting the intake of specific flavonoid-rich foods (a list of which was provided). Four-day diet diaries were used to assess habitual dietary intake at baseline, 6 months, and 12 months (Supplementary Table 1), and adherence to study protocol was monitored by research staff between study assessments. Additionally, for 24 h before study assessments, subjects were asked to refrain from caffeine, alcohol, and strenuous exercise. Each volunteer consumed a low-flavonoid meal the evening before each assessment followed by an overnight fast (>10 h).
At baseline and 12 months, vascular measures were made at the research facility, including 2-h ambulatory BP monitoring (Spacelabs Healthcare, Issaquah, WA), with recordings every 10 min. Biological samples (24-h urine and fasting blood) were also collected (Fig. 1C), with levels of fasting glucose and lipoproteins (total cholesterol, HDL-cholesterol [HDL-C], and triglycerides) measured photometrically using a clinical chemistry autoanalyzer (ARCHITECT c Systems autoanalyzer; Abbott Laboratories, Abbott Park, IL). Fasting HbA1c was assessed by HPLC (A. Menarini Diagnostics, Florence, Italy). LDL-cholesterol (LDL-C) was determined using the Friedewald equation (22). Furthermore, plasma/urine aliquots were stored at −80°C for subsequent analyses. Anthropometric measures (body weight and height) were taken in duplicate, according to standardized protocols. From banked plasma, insulin concentrations were assessed using routine methods (insulin ELISA; Mercodia, Uppsala, Sweden). The intra-assay coefficients of variation were 2.90% (HbA1c), 2.82% (glucose), 1.24% (total cholesterol), 3.01% (HDL-C), 1.43% (triglycerides), and 3.84% (insulin). Insulin resistance and insulin sensitivity were calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) equation, HOMA-IR = glucose × insulin/22.5 (23), and the quantitative insulin sensitivity index (QUICKI) (24) equation, QUICKI = 1/(log [fasting insulin] + log [fasting glucose]), respectively. HOMA-IR, QUICKI, glucose, and insulin data are reported for n = 45 (in both groups), triglycerides levels for n = 45 flavonoid and n = 44 placebo subjects, and LDL-C levels for n = 45 flavonoid and n = 42 placebo subjects.
Ten-year estimated risks for coronary heart disease (CHD), fatal CHD, stroke, and fatal stroke were calculated using the UK Prospective Diabetes Study (UKPDS) risk engine (version 2.0, www.dtu.ox.ac.uk/ukpds_trial/index.php; Diabetes Trials Unit, University of Oxford, Oxford, U.K.). The UKPDS risk engine includes age, diabetes duration, sex, presence of atrial fibrillation, ethnicity, smoking status, HbA1c (%), systolic BP (mmHg), total cholesterol (mmol/L), and HDL-C (mmol/L). The last observation carried forward was used to interpolate incomplete data (n = 3 observations for HbA1c, cholesterol, and HDL-C).
Statistical analysis
Results are expressed as mean ± SEM. At baseline (0 mol/L) and 12 months (12 mol/L), differences in patient characteristics were assessed using Student independent-sample t test (continuous data) and Pearson χ2 test (categorical data). Data are presented for those completing the 1-year intervention per protocol, and the primary test of effect was univariate ANCOVA analysis (intervention group as fixed factor [flavonoid/placebo] versus change in variable [pre (0 mol/L)/post (12 mol/L)], with baseline value of variable as covariate). Further sensitivity analysis was conducted to exclude for low compliers (returning ≤70% wrappers), those with annual change in BMI of greater than or equal to ±5 kg/m2, and one volunteer with atrial fibrillation (n = 5 flavonoid; n = 4 placebo). Statistical analyses were performed using SPSS, release 18.0.0 (IBM Corp., New York, NY), and statistical significance was defined as P < 0.05.
RESULTS
Ninety three patients completed the trial, and subject characteristics and key measures of diabetes control were similar between groups at baseline (Table 1); age ranged from 51 to 74 years, and BMI was 21.5–57.9 kg/m2. There were 25 withdrawals during the trial (21% of those randomized), with almost equal numbers in both groups (Fig. 1A). There were no differences in baseline characteristics of the 118 subjects randomized to treatment (n = 118 in total; n = 59 in flavonoid and placebo group) (data not shown). In the 93 participants who completed the study, the mean 1-year adherence to intervention treatment was high, with 91.3 and 91.6% of chocolate bars consumed for the flavonoid and placebo groups, respectively (determined by wrapper returns). Adherence in the flavonoid group was confirmed by the sustained increases in total epicatechin and isoflavone levels in 24-h urine at 6 and 12 months (total epicatechin 148.5 ± 12.3 and 154.1 ± 11.1 μmol/day; specifically 3-methyl-epicatechin 6.4 ± 0.6 and 7.0 ± 0.6 μmol/day, 4-methyl-epicatechin 1.7 ± 0.2 and 1.5 ± 0.2 μmol/day, epicatechin-3-sulfate 52.7 ± 4.8 and 58.6 ± 5.1 μmol/day, epicatechin-methyl-sulfate 75.5 ± 7.1 and 73.1 ± 5.6 μmol/day, daidzein 77.5 ± 4.9 and 75.2 ± 5.3 μmol/day, respectively, in hydrolyzed samples at 6 and 12 months). Thirty six percent of the flavonoid group were equol producers, determined according to standard methods (25).
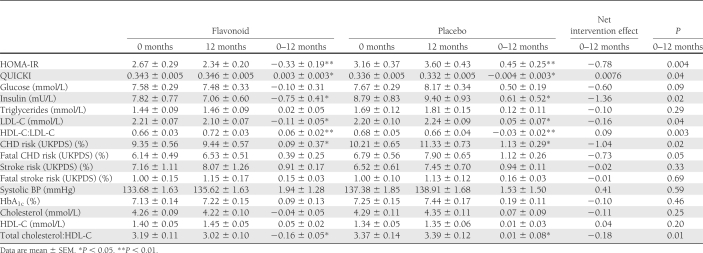
The intervention attenuated insulin resistance (−0.3 ± 0.2 HOMA-IR flavonoid group, 0.4 ± 0.2 placebo group; P = 0.004) and improved insulin sensitivity, effects that were driven by a significant decrease in insulin levels (Table 2). We also observed significant changes in lipoprotein status: total-C:HDL-C ratio (flavonoid −0.2 ± 0.1, placebo 0.0 ± 0.6; P = 0.01), LDL-C levels (flavonoid −0.1 ± 0.1 mmol/L, placebo 0.1 ± 0.1 mmol/L; P = 0.04), and HDL-C:LDL-C ratio (flavonoid 0.06 ± 0.0, placebo −0.03 ± 0.0; P = 0.003) (Table 2). The intervention also had a significant impact on estimated percent CHD risk, with risk attenuated in the intervention group relative to placebo for CHD (0.1 ± 0.3 vs. 1.1 ± 0.3%, respectively; P = 0.02) and a trend toward a reduction in fatal CHD (P = 0.05) (Table 2). The intervention had no effect on BP or glycemic control (HbA1c and glucose levels) (Table 2). At 12 months, mean BMI was similar in both groups.
Table 2.
Effect of 1-year combined intervention with flavan-3-ols and isoflavones on biomarkers of CVD risk in medicated postmenopausal patients with type 2 diabetes
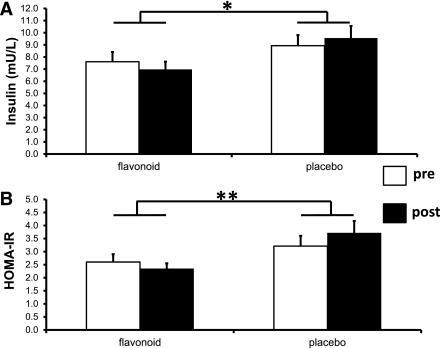
In sensitivity analyses, a similar magnitude of effect of the flavonoid intervention on HOMA-IR and insulin (Fig. 2) and estimated 10-year risk of CHD (flavonoid 0.0 ± 0.4%, placebo 1.2 ± 0.4%; P = 0.02) was observed. Moreover, stronger statistical associations were observed for the effects on estimated 10-year risk of fatal CHD (flavonoid 0.3 ± 0.3%, placebo 1.1 ± 0.3%; P = 0.04) and lipoprotein status: total C:HDL-C ratio (flavonoid −0.2 ± 0.1, placebo 0.0 ± 0.1; P = 0.004) and LDL-C (flavonoid −0.1 ± 0.1 mmol/L, placebo 0.1 ± 0.1 mmol/L; P = 0.03).
Figure 2.
The effect of the 1-year flavonoid intervention on insulin resistance in compliant patients. A: Plasma insulin. B: HOMA-IR (mean ± SEM). *P < 0.05, ** P = 0.01; n = 42 flavonoid, n = 42 placebo.
CONCLUSIONS
In this 1-year, randomized, placebo-controlled intervention trial, a combined intake of flavan-3-ols and isoflavones significantly improved lipoprotein status and markers of insulin sensitivity and attenuated the estimated 10-year risk of CHD in postmenopausal women receiving standard therapy for type 2 diabetes. To our knowledge, this is the only long-term flavonoid trial that has been conducted in medicated postmenopausal patients with type 2 diabetes, and these data highlight the additional benefit of dietary flavonoids to standard drug therapy in managing CVD risk in these patients.
All patients were receiving statin therapy (≥40 mg simvastatin or ≥10 mg atorvastatin) as part of standard medical care, but flavonoid intervention resulted in further improvements in lipoprotein status, specifically significant reductions in LDL-C and total-C:HDL-C ratio. The observed reduction of 0.16 mmol/L LDL-C is consistent with data from the meta-analysis of short term (maximum 12 weeks) cocoa (−0.15 mmol/L) (26) and soy isoflavone (−0.13 mmol/L) (14) intervention trials in healthy participants and with the magnitude of change observed in the few short-term trials on soy isoflavones (16,17) or chocolate (15) in type 2 diabetic patients. Our findings do not suggest any additive effect of our combined intervention on lipoprotein status in this medicated group.
The observed effect is of potential clinical significance. A 1.0 mmol/L reduction in LDL-C has been associated with a 21% decrease in vascular events in patients with type 2 diabetes (27), suggesting that long-term flavonoid intervention may be associated with a 3.4% reduction in vascular events. These effects are also supported by in vitro work, suggesting that flavan-3-ols increase apolipoprotein A1 and decrease apolipoprotein B production as a result of upregulation of sterol regulatory element binding proteins and an increase in LDL receptor activity (28). Likewise, isoflavones have been shown to decrease apolipoprotein B secretion in HepG2 cells through multiple mechanisms and may augment the efficacy of statins (29).
We also observed a significant reduction in insulin resistance (HOMA-IR) and improvements in insulin sensitivity (QUICKI) that were driven by a significant decrease in insulin concentrations. These findings are clinically important, as insulin resistance is not only a key determinant of the metabolic syndrome but is also associated with increased arterial stiffening (30) and risk of cardiovascular events even in individuals with no diabetes (31). Our recent meta-analysis of short-term cocoa/chocolate trials (≤4 months duration) also showed that intervention improved insulin resistance (HOMA-IR −0.67 [95% CI −0.98, −0.36]) due to significant reductions in serum insulin, although only 3 of the 42 studies were in patients with type 2 diabetes (A. Cassidy, personal communication). However another recent meta-analysis observed no effect of soy isoflavones on measures of glycemic control (32); although only 3 of the 24 trials were in type 2 diabetic patients, there was wide variability in source and dose of soy isoflavones fed, and few studies reported changes in HOMA-IR. In another review, only soy isoflavones and genistein exerted beneficial effects on HOMA-IR (14). There are supporting underlying mechanisms for these effects; in vitro, flavan-3-ols, isoflavones, and their metabolites enhanced insulin-stimulated glucose uptake in adipocytes through increased expression of GLUT4, and improved insulin secretory function of pancreatic β-cells (10,33).
Our combined intervention attenuated the UKPDS-estimated, 10-year CHD risk as a result of beneficial effects on lipoprotein status. The UKPDS algorithm incorporates additional risk factors for diabetes (i.e., HbA1c and duration of diabetes) beyond traditional risk factors, and, although validation studies have identified a tendency to overestimate CHD risk (34), it is still considered a useful tool (35) to compliment other CHD risk assessments. Over half of our study population was on antihypertensive medication, and this may have influenced our lack of effect on BP. In the few previous short-term studies with type 2 diabetic patients, flavan-3-ols alone did not improve BP (15,36), although in one study, isoflavones reduced BP in patients with type 2 diabetes who were not on medication for hypertension (37). This is in contrast to flavonoid trials in participants without type 2 diabetes, where systolic BP and diastolic BP were reduced by short-term flavan-3-ol (by 4.5 and 2.5 mmHg, respectively) (38) and isoflavone intake (by 2.5 and 1.5 mmHg, respectively) (39).
Our study has a number of limitations. The dropout rate during the intervention was high (21%), with the most frequent reason for participant withdrawal (in both groups) attributable to palatability (Fig. 1A); however, these numbers are comparable to other nutrition trials in type 2 diabetic patients (40), and as observed in other studies, a small number of patients had changes in BP medication during the 1-year trial period (n = 10). Our findings also relate specifically to a combined flavonoid intervention given to postmenopausal women receiving standard diabetes therapy (including lipid-lowering medication), and further studies are now required to determine the relative influence of each flavonoid subclass (flavan-3-ols and isoflavones) on biomarkers of CVD risk in this population and to examine whether similar effects are observed in male patients or in other patient groups. Finally, although the flavonoid intake in our trial could potentially be attained through high intake of dietary flavonoid, an emerging range of flavonoid-rich functional foods may offer additional opportunities to consume flavonoids at optimal levels. Despite these limitations, our trial is the longest intervention to date to assess the effects of flavonoids on CVD risk factors in a population of medicated, postmenopausal patients with type 2 diabetes and benefits from objective assessment of compliance.
This pragmatic 1-year trial provides evidence to suggest that the intake of flavonoids results in sustained improvements in lipid profile and insulin sensitivity and an attenuation of estimated CHD risk, highlighting the additional benefit of flavonoids to standard drug therapy in managing CVD risk in patients with type 2 diabetes. Our study is the only combined flavonoid trial and the longest flavan-3-ol intervention to date, and our findings have potential clinical relevance. Long-term studies are now required to determine whether these effects are restricted to populations of medicated postmenopausal women with established type 2 diabetes and to determine whether chronic intake of flavan-3-ols or isoflavones is as effective when consumed independently.
Supplementary Material
Acknowledgments
This study was funded by Diabetes UK (06/0003397, principal investigator A.C.), with raw materials purchased from Frutarom Netherlands (isoflavones) and Barry Callebaut Belgium (Acticoa cocoa) and trial chocolates manufactured by Barry Callebaut.
P.A.K. and A.C. have received research funding from Unilever Research to conduct an anthocyanin trial and in vitro experimental work (BBSRC-CASE PhD studentship). P.A.K. has been an independent member of the Coressence Science Board since 2006 and is in receipt of research funding from Danisco A/S. A.C. has received unrestricted funding from the chocolate industry to conduct a systematic review on chocolate and oxidative stress. No other potential conflicts of interest relevant to this article were reported.
P.J.C. provided input for the study conception and design, coordinated the trial, conducted laboratory and statistical analyses, interpreted data, and drafted the manuscript. M.S., J.P., and K.D. provided input for the study conception and design and read and approved the final draft of the manuscript. P.A.K. provided input for the study conception and design, analyzed levels of flavonoids in foods and biological samples, and read and approved the final draft of the manuscript. A.C. conceived and designed the study, interpreted data, drafted the manuscript, and is the guarantor of this article.
The authors thank Barry Callebaut Belgium and Frutarom Netherlands for their assistance in developing the trial foods and the Local Diabetes Research Network for invaluable nursing support.
Footnotes
Clinical trial reg. no. NCT00677599, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1443/-/DC1.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, Fourth Edition Brussels, Belgium, International Diabetes Federation, 2009 [Google Scholar]
- 2.Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J 2006;152:27–38 [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N, Gao P, Seshasai SR, et al. ; Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kautzky-Willer A, Kamyar MR, Gerhat D, et al. Sex-specific differences in metabolic control, cardiovascular risk, and interventions in patients with type 2 diabetes mellitus. Gend Med 2010;7:571–583 [DOI] [PubMed] [Google Scholar]
- 5.Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 2007;85:895–909 [DOI] [PubMed] [Google Scholar]
- 6.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med 2006;166:411–417 [DOI] [PubMed] [Google Scholar]
- 7.Hooper L, Kroon PA, Rimm EB, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88:38–50 [DOI] [PubMed] [Google Scholar]
- 8.Wang-Polagruto JF, Villablanca AC, Polagruto JA, et al. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J Cardiovasc Pharmacol 2006;47(Suppl. 2):S177–S186; discussion S206–S209 [DOI] [PubMed]
- 9.Schewe T, Steffen Y, Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys 2008;476:102–106 [DOI] [PubMed] [Google Scholar]
- 10.Ueda M, Nishiumi S, Nagayasu H, Fukuda I, Yoshida K, Ashida H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem Biophys Res Commun 2008;377:286–290 [DOI] [PubMed] [Google Scholar]
- 11.Odegaard AO, Koh WP, Butler LM, et al. Dietary patterns and incident type 2 diabetes in Chinese men and women: the Singapore Chinese Health Study. Diabetes Care 2011;34:880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr 2007;85:1148–1156 [DOI] [PubMed] [Google Scholar]
- 13.Li SH, Liu XX, Bai YY, et al. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. Am J Clin Nutr 2010;91:480–486 [DOI] [PubMed] [Google Scholar]
- 14.Ricci E, Cipriani S, Chiaffarino F, Malvezzi M, Parazzini F. Effects of soy isoflavones and genistein on glucose metabolism in perimenopausal and postmenopausal non-Asian women: a meta-analysis of randomized controlled trials. Menopause 2010;17:1080–1086 [DOI] [PubMed] [Google Scholar]
- 15.Balzer J, Rassaf T, Heiss C, et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol 2008;51:2141–2149 [DOI] [PubMed] [Google Scholar]
- 16.Pipe EA, Gobert CP, Capes SE, Darlington GA, Lampe JW, Duncan AM. Soy protein reduces serum LDL cholesterol and the LDL cholesterol:HDL cholesterol and apolipoprotein B:apolipoprotein A-I ratios in adults with type 2 diabetes. J Nutr 2009;139:1700–1706 [DOI] [PubMed] [Google Scholar]
- 17.Jayagopal V, Albertazzi P, Kilpatrick ES, et al. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care 2002;25:1709–1714 [DOI] [PubMed] [Google Scholar]
- 18.Schroeter H, Heiss C, Balzer J, et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 2006;103:1024–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol 2003;23:1066–1071 [DOI] [PubMed] [Google Scholar]
- 20.Cooper KA, Campos-Giménez E, Jiménez Alvarez D, Nagy K, Donovan JL, Williamson G. Rapid reversed phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationships of their concentrations in chocolate. J Agric Food Chem 2007;55:2841–2847 [DOI] [PubMed] [Google Scholar]
- 21.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 2005;81(Suppl.):243S–255S [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 24.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 25.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr 2006;136:2188–2193 [DOI] [PubMed] [Google Scholar]
- 26.Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2010;92:218–225 [DOI] [PubMed] [Google Scholar]
- 27.Kearney PM, Blackwell L, Collins R, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125 [DOI] [PubMed] [Google Scholar]
- 28.Yasuda A, Natsume M, Osakabe N, Kawahata K, Koga J. Cacao polyphenols influence the regulation of apolipoprotein in HepG2 and Caco2 cells. J Agric Food Chem 2011;59:1470–1476 [DOI] [PubMed] [Google Scholar]
- 29.Duncan RE, El-Sohemy A, Archer MC. Regulation of HMG-CoA reductase in MCF-7 cells by genistein, EPA, and DHA, alone and in combination with mevastatin. Cancer Lett 2005;224:221–228 [DOI] [PubMed] [Google Scholar]
- 30.Webb DR, Khunti K, Silverman R, et al. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010;53:1190–1198 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Sakurai M, Miura K, et al. Homeostasis model assessment of insulin resistance and the risk of cardiovascular events in middle-aged non-diabetic Japanese men. Diabetologia 2010;53:1894–1902 [DOI] [PubMed] [Google Scholar]
- 32.Liu ZM, Chen YM, Ho SC. Effects of soy intake on glycemic control: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2011;93:1092–1101 [DOI] [PubMed] [Google Scholar]
- 33.Fu Z, Liu D. Long-term exposure to genistein improves insulin secretory function of pancreatic beta-cells. Eur J Pharmacol 2009;616:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Dieren S, Peelen LM, Nöthlings U, et al. External validation of the UK Prospective Diabetes Study (UKPDS) risk engine in patients with type 2 diabetes. Diabetologia 2011;54:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Heijden AA, Ortegon MM, Niessen LW, Nijpels G, Dekker JM. Prediction of coronary heart disease risk in a general, pre-diabetic, and diabetic population during 10 years of follow-up: accuracy of the Framingham, SCORE, and UKPDS risk functions: the Hoorn Study. Diabetes Care 2009;32:2094–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellor DD, Sathyapalan T, Kilpatrick ES, Beckett S, Atkin SL. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med 2010;27:1318–1321 [DOI] [PubMed] [Google Scholar]
- 37.Howes JB, Tran D, Brillante D, Howes LG. Effects of dietary supplementation with isoflavones from red clover on ambulatory blood pressure and endothelial function in postmenopausal type 2 diabetes. Diabetes Obes Metab 2003;5:325–332 [DOI] [PubMed] [Google Scholar]
- 38.Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens 2010;23:97–103 [DOI] [PubMed] [Google Scholar]
- 39.Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG, Hui RT. Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 8 February 2011 [Epub ahead of print] [DOI] [PubMed]
- 40.Jenkins DJ, Kendall CW, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA 2008;300:2742–2753 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.