Abstract
OBJECTIVE
Myocellular ATP synthesis (fATP) associates with insulin sensitivity in first-degree relatives of subjects with type 2 diabetes. Short-term endurance training can modify their fATP and insulin sensitivity. This study examines the effects of moderate long-term exercise using endurance or resistance training in this cohort.
RESEARCH DESIGN AND METHODS
A randomized, parallel-group trial tested 16 glucose-tolerant nonobese relatives (8 subjects in the endurance training group and 8 subjects in the resistance training group) before and after 26 weeks of endurance or resistance training. Exercise performance was assessed from power output and oxygen uptake (Vo2) during incremental tests and from maximal torque of knee flexors (MaxTflex) and extensors (MaxText) using isokinetic dynamometry. fATP and ectopic lipids were measured with 1H/31P magnetic resonance spectroscopy.
RESULTS
Endurance training increased power output and Vo2 by 44 and 30%, respectively (both P < 0.001), whereas resistance training increased MaxText and MaxTflex by 23 and 40%, respectively (both P < 0.001). Across all groups, insulin sensitivity (382 ± 90 vs. 389 ± 40 mL ⋅ min−1 ⋅ m−2) and ectopic lipid contents were comparable after exercise training. However, 8 of 16 relatives had 26% greater fATP, increasing from 9.5 ± 2.3 to 11.9 ± 2.4 μmol ⋅ mL−1 ⋅ m−1 (P < 0.05). Six of eight responders were carriers of the G/G single nucleotide polymorphism rs540467 of the NDUFB6 gene (P = 0.019), which encodes a subunit of mitochondrial complex I.
CONCLUSIONS
Moderate exercise training for 6 months does not necessarily improve insulin sensitivity but may increase ATP synthase flux. Genetic predisposition can modify the individual response of the ATP synthase flux independently of insulin sensitivity.
First-degree relatives of patients with type 2 diabetes are at greater risk of developing type 2 diabetes, which has been mainly attributed to impaired insulin sensitivity. Insulin-resistant first-degree relatives have reduced insulin-mediated myocellular glucose-6-phosphate (G6P) and glycogen synthesis (1), and also may exhibit lower unidirectional flux through myocellular ATP synthase (fATP) (2), but elevated muscle and liver fat content (2). Lower insulin-stimulated fATP has been observed in overt type 2 diabetes (3,4), suggesting that altered ATP production could be involved in the progression of insulin resistance to diabetes.
In lean, insulin-resistant first-degree relatives, intensive endurance exercise training reduced insulin resistance by increasing insulin-stimulated G6P and glycogen synthesis (1). Such improvement of insulin resistance also has been associated with greater mitochondrial density and/or function in nondiabetic and type 2 diabetic individuals (5). However, the response of insulin sensitivity and mitochondrial function to exercise training may be dissociated depending on age (6) and endocrine function (7). In addition, only one group of first-degree relatives (responders) can improve their insulin sensitivity and fATP after 1 week of endurance exercise training (8). The responder status was linked to the G/G single nucleotide polymorphism rs540467 in the NDUFB6 gene, which encodes a subunit of complex I of the respiratory chain, relates to insulin resistance and type 2 diabetes (9), and is less expressed in the skeletal muscle of type 2 diabetic patients (10). At present, it is unclear whether long-term endurance training can overcome this gene effect in nondiabetic first-degree relatives. Although intensive resistance exercise training also may ameliorate insulin resistance by increasing glucose transport and storage in type 2 diabetes (11), this also has not been reported in first-degree relatives. In addition to the type of exercise, frequency also affects the metabolic outcome of training programs. Lower training frequencies (i.e., twice weekly) may improve insulin resistance using either resistance training in older men with type 2 diabetes (12) or endurance training in obese women (13). Because training regimens may provide higher exercise adherence and practicability for sedentary people, we tested the hypotheses that long-term but moderate exercise training 1) improves fATP and insulin sensitivity and 2) overcomes the impact of the NDUFB6 gene polymorphism observed during short-term exercise and that 3) endurance training is superior to resistance training in increasing fATP in glucose-tolerant, nonobese first-degree relatives.
RESEARCH DESIGN AND METHODS
Twenty-four (50% female) eligible, glucose-tolerant, nonobese subjects with at least one parent with type 2 diabetes, confirmed by hyperglycemia or glucose-lowering treatment, were recruited from a group of first-degree relatives who had participated in a previous 1-week study (8). By design, the 26-week exercise training immediately followed the 1-week exercise training. Four of these first-degree relatives declined to participate in the training study, and another four subjects (two from the endurance training group and two from the resistance training group) did not finish the study because of missing motivation (n = 2), depression (n = 1), and viral infection (n = 1) (Supplementary Fig. 1). Consequently, 16 (50% female) subjects with one (n = 12) or two (n = 4) parents with type 2 diabetes completed the study.
Subjects underwent medical history and physical examinations, including measurement of body weight, height, waist-to-hip ratio (WHR), and 12-lead electrocardiogram; blood and urine analyses; and oral glucose tolerance testing. Exclusion and inclusion criteria have been described (8). Women were neither taking hormonal contraceptives nor were they studied in the luteal phase of their cycle. Inclusion required written informed consent by the volunteer to the study, which was approved by the local institutional ethical board and performed according to the Declaration of Helsinki. Then, participants were randomly assigned to either endurance or resistance training interventions.
Dietary assessment
Dietary intake was assessed at baseline and during weeks 13 and 27 with a modified interviewer-administered, 107-item, open-ended food frequency questionnaire adjusted for local dietary habits (8). Nutrient and fluid intake before the studies were assessed from 24-h recalls. All volunteers were advised to follow a weight-maintaining diet as recommended by the American Diabetes Association (14).
Oral glucose tolerance test
Fasting insulin sensitivity was assessed with the quantitative insulin sensitivity check index, obtained as the inverse of the sum of the logarithms of fasting plasma insulin and glucose (15). A 75-g oral glucose tolerance test (OGTT) was performed for 150 min (8) to assess the dynamic insulin sensitivity with the oral glucose insulin sensitivity index (OGIS). OGIS, obtained from a model-derived equation (16) (http://www.isib.cnr.it/bioing/ogis/home.html), describes glucose clearance and has been validated against the clamp and other insulin sensitivity indices (16,17). OGTT-derived parameters already have been used in our previous exercise study (8,16). β-Cell function was assessed with the insulinogenic index and with the adaptation index, the product of OGIS and insulin secretion from C-peptide (15). The latter describes how the β-cell adapts its glucose-stimulated response to changes of insulin resistance. Hepatic insulin extraction was calculated as previously reported (18).
Magnetic resonance spectroscopy
Participants were studied using a 3-Tesla magnetic resonance spectrometer (Medspec S 300-DBX; Bruker Biospin, Ettlingen, Germany). The procedures of 1H and 31P magnetic resonance spectroscopy (MRS) have been described previously (8). Data for one participant of the resistance training group could not be obtained at the end of the study as a result of a technical failure of the spectrometer.
Habitual physical activity, strength, and exercise testing
Physical activity was assessed with an interviewer-administered questionnaire on a scale from one to five (low to high degree of activity) (8).
Strength testing (isokinetic torque measures).
Maximal isokinetic muscle strength (peak torque) of extension and flexion at the knee was measured via isokinetic dynamometry (Lido Active Multijoint II; Loredan Biomedical, Sacramento, CA) in both legs. In brief, after warm-up and familiarization, the volunteers were instructed to maximally push and pull through the full available range of motion (20° to 90°). Every test included two reciprocal bouts with a 10-s rest period after each bout and a 2-min resting period between the preset test velocities (30°/s and 60°/s). The highest absolute peak torques obtained at each velocity from both limbs were recorded for extension and flexion movements.
Exercise testing (incremental tests).
This test was performed using an electronically braked cycle ergometer (Excalibur Sport; Lode, Groningen, the Netherlands) at 70 revolutions per min in an upright position to the limit of tolerance. Respiratory gas exchange was assessed with an open-air spirometry system (MasterScreen CPX; Jäger/Viasys, Würzburg, Germany) as described (8). From incremental tests power output, oxygen uptake (Vo2) was determined at maximum load and at the aerobic threshold (respiratory compensation point; RCP) (19).
Experimental protocols
All participants were advised to refrain from any physical exercise and to ingest carbohydrate-enriched meals during the 3 days before the start of all tests. In the evenings prior to the tests, the participants consumed identically composed carbohydrate-enriched dinners at identical times and then fasted for 12 h. On day 1, participants did not consume any calories except for the glucose provided during the OGTT until the end of the MRS. They underwent baseline blood sampling, OGTTs, and MRS. On day 2, they performed isokinetic strength tests, and 2 h later they performed the incremental exercise tests. On days 3 and 5, they exercised on a cycling ergometer. On day 7, measurements of day 1 were repeated in identical fashion as described (8). Thereafter, participants were randomly assigned to individually controlled endurance or resistance training, which started 1 week later, with every session supervised and documented by a coach. Blood checks and OGTTs were repeated in week 13. At the end of the study, after 26 weeks of training and 48 h after the last exercise, all measurements from day 1 were repeated in identical fashion (Supplementary Fig. 2). The experimental protocol was designed to yield significant increases in exercise capacities for both endurance and resistance training (Supplementary Fig. 3), as previously reported in humans with obesity or type 2 diabetes (12,13).
Endurance training group.
Volunteers performed two sessions weekly, with increasing duration from 15 min up to 60 min per session. After the 1st week, the intensity of 80% was raised to 90% of the workload determined at the RCP.
Resistance training group.
Participants lifted the greatest possible load for a predetermined number of 12–15 correct repetitions (12–15 RM test) of eight different exercises involving all major lower- and upper-body and trunk muscles on exercise machines (Technogym; Gambettola, Forli, Italy) to predict 1-repetition maximum strength (1RM) with reassessment of 12–15 RM tests every 4 weeks. During weeks 1–4, the intensity was increased from 30 to 50% 1RM. During weeks 5–18, the number of repetitions was raised to 15 for each of the eight exercises. From week 15 on, participants performed two sets of exercises. Starting with week 19, the intensity was increased to 70% 1RM (two sets of 15 repetitions until week 22, increasing to three sets until the end of the training intervention).
Laboratory analyses
Plasma glucose (Glucose analyzer II; Beckman Coulter, http://www.beckmancoulter.com), insulin, and C-peptide and all other parameters were analyzed as described (8). Serum total adiponectin concentrations were measured using the Quantikine Total Human Adiponectin ELISA Kit (R&D Systems, Wiesbaden, Germany) in a subgroup of study participants (five subjects from the resistance training group and six subjects from the endurance training group) for whom sufficient serum was available.
Genotyping
DNA was extracted from blood with a QIAamp DNA Blood Mini Kit (cat. no. 51106; Qiagen, Hilding, Germany); rs540467 of NDUFB6 was genotyped using an allelic discrimination assay performed with an ABI 7900 system (Applied Biosystems, Foster City, CA) and an assay on demand (C_2334430; Applied Biosystems) (9).
Statistics
Statistical analyses were performed using Statistica software (version 6.1; StaSoft, Tulsa, OK). Data were expressed as means ± SD. Unpaired two-tailed Student t tests were used for between-group comparisons, paired t tests were used for comparing pretraining (baseline) with posttraining data within each training group. The association between rs540467 and exercise responses of fATP was analyzed with the χ2 test (NCSS Statistical Software, Kaysville, UT). P values ≤0.05 were considered to indicate significant differences between groups.
RESULTS
Baseline analyses
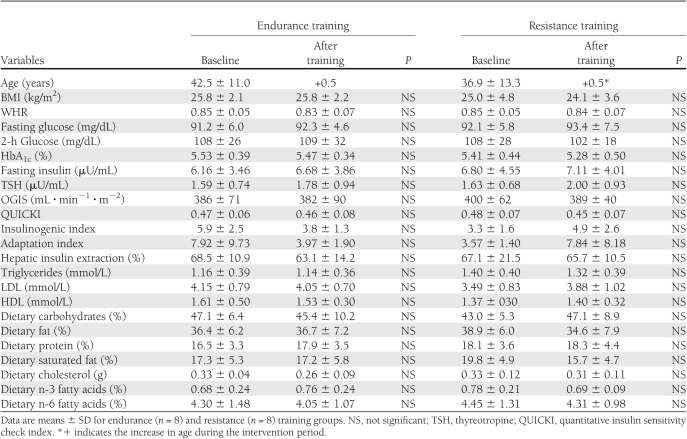
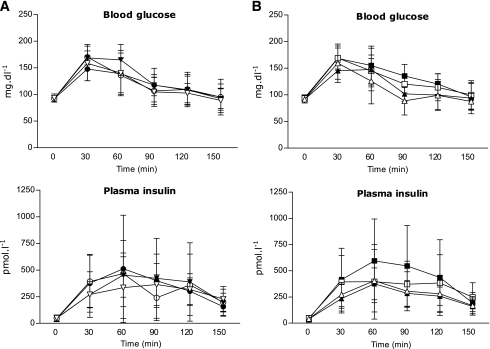
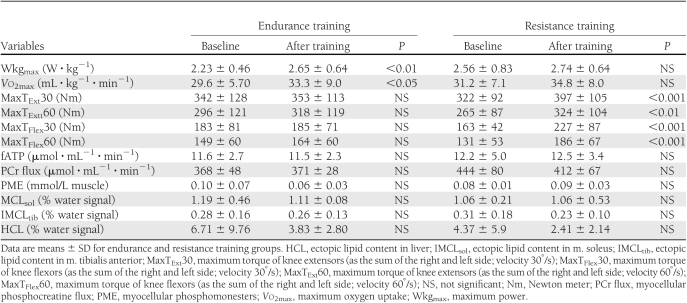
Both the endurance and resistance training groups did not differ in anthropometric, metabolic, and lifestyle parameters (Table 1). During the OGTT, glucose and insulin concentrations also were similar (Fig. 1). None of the participants fulfilled the current criteria for metabolic syndrome. No significant differences comparing men and women were found. Serum adiponectin was not different between endurance and resistance training (12.74 ± 7.99 vs. 13.40 ± 9.29 μg/mL). Performance during strength and exercise testing and fATP, intramyocellular lipids (IMCL), and hepatocellular lipids (HCL) also were not different between endurance and resistance training groups (Table 2).
Table 1.
Anthropometric and laboratory data, parameters of insulin sensitivity and insulin secretion, and dietary intake before (baseline) and after 26 weeks of training
Figure 1.
Blood glucose and plasma insulin concentrations during the OGTT in first-degree relatives of patients with type 2 diabetes. Means ± SD are shown for endurance (circles; n = 8) and resistance (triangles; n = 8) training groups (A) as well as for responders (squares; n = 8) and nonresponders (triangles; n = 7) (B), as defined by the difference of flux through fATP between baseline data (closed symbols) and data after 26 weeks (open symbols) of exercise training.
Table 2.
Exercise performance, myocellular energy metabolism, and ectopic fat contents before (baseline) and after 26 weeks of training
After-training analyses
The volunteers completed 91 ± 3% of the endurance and 90 ± 3% of the resistance training sessions and responded to the respective training interventions (Table 2 and Supplementary Fig. 3). In endurance training, only watt per kg body weight at the respiratory compensation point (WkgRCP) and Vo2RCP rose (P < 0.001) by 44 and 30%, respectively. In resistance training, only MaxText and MaxTflex increased (P < 0.001) by 23 and 40%, respectively. Fasting plasma glucose, lipids, insulin sensitivity, and secretion did not change after endurance and resistance training (Table 1). During the OGTT, glucose and insulin concentrations also were similar (Fig. 1). Adiponectin also remained unchanged in endurance and resistance training (13.10 ± 9.22 vs. 11.24 ± 7.51 μg/mL). fATP and IMCL remained unchanged in both groups (Table 2). Mean HCL tended to be, but was not, significantly lower after endurance and resistance training. Thyroid function neither changed in both groups nor correlated with insulin sensitivity or fATP across groups and within subgroups.
Post hoc subgroup analyses
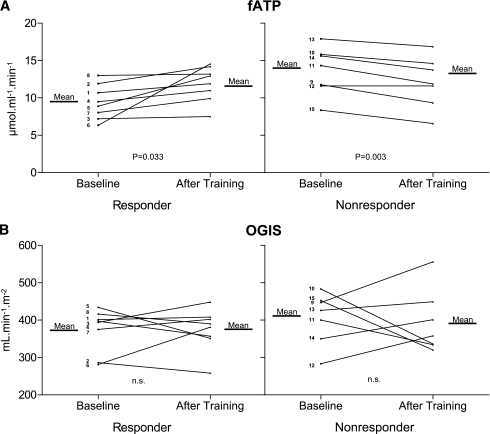
As in our previous short-term exercise training study (8), participants also were analyzed according to the fATP difference between baseline and 26 weeks into responders (four from the endurance training group and four from the resistance training group), who had to show a positive increment in fATP after exercise training, and nonresponders (four from the endurance training group and three from the resistance training group) (Fig. 2). In responders, fATP increased by 26% (P < 0.05), whereas fATP even tended to decrease in nonresponders after 26 weeks. Baseline characteristics between these subgroups were identical except for the slightly older age of the responders (Supplementary Table 1). Exercise training improved Wkg and MaxTFlex60 in both subgroups but increased Vo2 only in responders (Supplementary Table 2). Responders slightly decreased their BMI by 2.7% without changes in WHR. During the OGTT, glucose and insulin concentrations were similar before and after exercise training (Fig. 1).
Figure 2.
Skeletal muscle flux through fATP (A) and insulin sensitivity (OGIS) (B) in first-degree relatives of patients with type 2 diabetes. The graphs show the means and the individual data of responders (subjects 1–8) or nonresponders (subjects 9–15) with regard to increased fATP after 26 weeks of training. The P values are given for the comparison of data at baseline versus 26 weeks of training. NS, not significant. Subject number and training group: subject 1: endurance training; subject 2: endurance training; subject 3: endurance training; subject 4: endurance training; subject 5: resistance training; subject 6: resistance training; subject 7: resistance training; subject 8: resistance training; subject 9: endurance training; subject 10: endurance training; subject 11: endurance training; subject 12: endurance training; subject 13: resistance training; subject 14: resistance training; and subject 15: resistance training.
The presence of the A allele of the NDUFB6 gene polymorphism, rs540467, was associated with resistance to stimulation of fATP. After the training period, 86% of the G/G carriers (six responders and one nonresponder) but only 25% of the A allele carriers increased their fATP (P = 0.019 for a dominant model). In G/G carriers of rs540467, fATP rose by 24% (9.5 ± 2.3 vs. 11.9 ± 2.4 μmol ⋅ mL−1 ⋅ min−1; P = 0.051), whereas fATP was not different in A allele carriers (13.6 ± 3.2 vs. 12.1 ± 3.4 μmol ⋅ mL−1 ⋅ min−1; P = 0.18). Of all responders, 75% (six of eight) were G/G carriers, and the responder status related to the presence of the G allele independent of the mode of exercise training so that endurance and resistance training were combined for this analysis.
CONCLUSIONS
This moderate exercise training program increased the respective exercise capacities with a high degree of compliance in both training groups. Despite no overall improvement of insulin sensitivity and β-cell function across all first-degree relatives, one subgroup (responders) exhibited greater muscular fATP after 26 weeks, which related to the presence of the G allele in the NDUFB6 gene.
This study unmasks a dissociation between the effects of long-term exercise training on fATP and insulin sensitivity and extends our previous observation that the G allele, but not the A allele, in the NDUFB6 gene associates with increases in both fATP and insulin sensitivity after short-term high-intensity exercise training (8). This single nucleotide polymorphism in the NDUFB6 gene nominally associates with risk markers of type 2 diabetes (9), a disorder with impaired insulin-stimulated fATP in skeletal muscle (3,4). Moreover, the function of NDUFB6 is subject to epigenetic regulation by age in human skeletal muscle and by high-fat diet in rat adipose tissue, both of which also impair mitochondrial function (9). These and the current studies suggest that the response to chronic exercise training is independently modulated by mitochondrial function and insulin sensitivity. Inherited factors at least partly control this response and may thereby contribute to the success of physical activity in relatives of patients with type 2 diabetes.
A dissociation between the stimulation of oxidative capacity, mitochondrial function, and insulin sensitivity has been reported recently. Aerobic training for 10 weeks increased oxidative capacity and muscle citrate synthase activity independently of changes in insulin sensitivity in obese humans with or without type 2 diabetes (20). This group further reported similar intrinsic mitochondrial respiration at lower insulin sensitivity in men with type 2 diabetes despite improved insulin resistance after 10 weeks of aerobic training (21). On the other hand, 9 days of intensive exercise training increased fATP and citrate synthase but not whole-body insulin sensitivity in relatives of mothers with type 2 diabetes (22). Likewise, increased mitochondrial capacity also is present in severely insulin-resistant Asian Indians (23), suggesting inherited variability of myocellular mitochondrial function, which is not necessarily coupled to insulin sensitivity.
In the current study, the responders were slightly older and had moderately greater WHR after the training period compared with the nonresponders, but they were not different in any other measured variable. Responders exhibited only nonsignificant trends toward lower resting fTAP and higher HCL, independent of insulin sensitivity and likely corresponding to their older age. Because increasing age and body fatness can be expected to rather reduce the responses of fATP, the finding of increased fATP after exercise training is unlikely to be from nongenetic causes.
Despite successful improvement of the specifically trained exercise capacities after 26 weeks, we could not support our hypothesis that endurance training is superior to resistance training in improving insulin sensitivity and fATP. In line with previous data, circulating adiponectin also did not change during exercise training (24). Of note, Vo2max increased significantly only in responders, suggesting that the nonresponders’ inability to raise Vo2max despite improved maximum power output reflects their inability to increase fATP upon exercise training.
The training intensity for endurance training (80–90% of WkgRCP) was chosen to allow for sufficient stimulation of both glucose and lipid oxidation, which should subsequently decrease myocellular lipid metabolites and IMCL, thereby improving insulin signaling and glucose transport/phosphorylation (i.e., insulin sensitivity) (25). Shorter-duration exercise (~115 min per week) was less effective in improving insulin sensitivity (26). Low-intensity exercise (~40% of heart rate reserve) modestly raised Vo2max but failed to increase insulin sensitivity (27) in line with the current study. Although larger training effects have been demonstrated mainly in obese and type 2 diabetic subjects (28), both dependent (29) and independent of weight loss or fat distribution (30), less is known about their nonobese relatives. Perseghin et al. (1) reported substantially increased insulin-stimulated myocellular G6P and glycogen synthesis but no effect on glucose oxidation after 6 weeks of endurance training in first-degree relatives. The relatives of the current study showed no response in insulin sensitivity but evidence for greater oxidative metabolism in a subgroup, which could be attributed to their lower degree of insulin resistance. In overweight daughters of patients with type 2 diabetes, 7 weeks of endurance training increased the OGTT-derived insulin sensitivity index more than in female subjects without a family history of type 2 diabetes (31). However, OGTTs were performed only 15–24 h after the last exercise bout, indicating acute rather than sustained exercise effects. Finally, subclinical hypothyroidism also may attenuate exercise training–induced improvements of insulin sensitivity (7), but all participants of this study had normal thyroid function.
This study evaluated the effects of a long-term, supervised individual–regulated training, at twice weekly frequency, which is more realistic to be implemented in everyday life. Of note, twice-weekly training frequency was shown to improve insulin resistance using either resistance training in older men with type 2 diabetes (12) or endurance training in obese nondiabetic women (13). On the other hand (1), recent studies found no change in insulin-mediated glucose uptake but increased muscular citrate synthase activity after intensive exercise training for 9 days (22) or 12 weeks (32). The participants of the latter study were older, more obese, and had lower insulin secretion capacity compared with the relatives in our study. In younger relatives, insulin sensitivity also dissociated from Vo2max responses after 10 weeks of exercise training (33). After intensive endurance training for 6 weeks, young first-degree relatives showed a similar incremental improvement of whole-body insulin sensitivity, along with greater muscular insulin-stimulated glucose transport/phosphorylation, but failed to normalize insulin-stimulated muscular glycogen synthesis compared with matched control subjects (1). Even patients with overt type 2 diabetes can normalize their muscular mitochondrial capacity upon 12 weeks of exercise training and still have lower insulin-stimulated nonoxidative glucose disposal than nondiabetic subjects (34). Finally, the HERITAGE study provided evidence that even 20 weeks of exercise training only gradually raised insulin sensitivity, with a broad variability and a surprisingly low rate of ~42% of responders regarding insulin sensitivity (28). Taken together, these data suggest the existence of an exercise resistance in first-degree relatives, which might relate to impaired insulin signaling and specific abnormalities in nonoxidative glucose metabolism (glycogen synthesis) in their skeletal muscle. Nevertheless, other factors, such as differences in local perfusion, muscle fiber composition, and myocellular concentrations of triglycerides and lipid metabolites, also may be involved.
Despite the strengths of this study, such as the continuously supervised, diet-controlled intervention, noninvasive phenotyping, and meticulous exclusion of acute exercise action, possible limitations have to be addressed. The frequent-sampling OGTT was used instead of the hyperinsulinemic-euglycemic clamp, which is considered the gold standard for measuring insulin sensitivity. Nonetheless, OGIS has been repeatedly shown to be equivalent to clamp-derived parameters (16,17) and exhibited similar behavior as fasting insulin levels in this and previous studies (8) and of others (31). Because no muscle biopsies were taken, we cannot differentiate whether changes in fATP were a result of different mitochondrial function or content. Furthermore, fATP measures demand-driven ATP synthesis at rest, not necessarily reflecting maximal mitochondrial capacity as assessed with postexercise phosphocreatine recovery (35).
In conclusion, twice-weekly exercise training for 26 weeks improves exercise performance but may not suffice to increase insulin sensitivity. Nevertheless, muscular ATP synthase flux increases depending on genetic predisposition but independently of the mode of exercise and insulin sensitivity. These findings underline the relevance of gene-environment interactions for the efficacy of lifestyle interventions.
Supplementary Material
Acknowledgments
This study was supported by grants from the European Foundation for the Study of Diabetes (Novo Nordisk and GlaxoSmithKline grants), the Austrian National Bank (OENB11459), and Hochschuljubiläumsstiftung, Vienna (to M.R.); from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (Deutsches Zentrum für Diabetesforschung, DZD e.V.); from the Swedish Research Council and the Wallenberg Foundation (to L.G.); from the Austrian Diabetes Association (to G.K.-B.); and from Regione Veneto (Biotech DGR 2702/10-09-04; to G.P.).
G.K.-B. has served on advisory boards for Takeda. G.P. is a consultant for Novo Nordisk. L.G. has been a consultant and has served on advisory boards for GlaxoSmithKline, Novartis, Merck, sanofi-aventis, Tethys Bioscience, and Xoma and has received lecture fees from Lilly and Novartis. M.R. is or has been a consultant and has served on advisory boards for Hofmann-La Roche, Novo Nordisk, sanofi-aventis, and Takeda and has received lecture fees from AstraZeneca, Bayer, B. Braun-Melsungen, Novo Nordisk, sanofi-aventis, and Takeda. No other potential conflicts of interest relevant to this article were reported.
G.K.-B. researched data and contributed to writing the manuscript. M.K. researched data and reviewed and edited the manuscript. M.C., M.F., C.L., R.P., H.T., J.S., A.I.S., S.G., C.H., M.W., E.M., and G.P. researched data. G.P. carried out the mathematical modeling analysis. G.S. researched data, reviewed and edited the manuscript, and contributed to the discussion. L.G. researched data. M.R. wrote the manuscript, reviewed and edited the manuscript, and is the guarantor of this article.
The authors thank Margareta Svensson, Department of Clinical Sciences, Lund University, Malmö, Sweden, for extracting DNA and Ulrike Poschen, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research, Düsseldorf, Germany, for measuring adiponectin.
Footnotes
Clinical trial reg. no. NCT01145092, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1426/-/DC1.
References
- 1.Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 1996;335:1357–1362 10.1056/NEJM199610313351804 [DOI] [PubMed] [Google Scholar]
- 2.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664–671 10.1056/NEJMoa031314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asmann YW, Stump CS, Short KR, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 2006;55:3309–3319 10.2337/db05-1230 [DOI] [PubMed] [Google Scholar]
- 4.Szendroedi J, Schmid AI, Chmelik M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 2007;4:e154. 10.1371/journal.pmed.0040154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toledo FG, Menshikova EV, Ritov VB, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 2007;56:2142–2147 10.2337/db07-0141 [DOI] [PubMed] [Google Scholar]
- 6.Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003;52:1888–1896 10.2337/diabetes.52.8.1888 [DOI] [PubMed] [Google Scholar]
- 7.Amati F, Dubé JJ, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Improvements in insulin sensitivity are blunted by subclinical hypothyroidism. Med Sci Sports Exerc 2009;41:265–269 [DOI] [PubMed] [Google Scholar]
- 8.Kacerovsky-Bielesz G, Chmelik M, Ling C, et al. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes 2009;58:1333–1341 10.2337/db08-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling C, Poulsen P, Simonsson S, et al. Genetic and epigenetic factors are associated with expression of respiratory chain component NDUFB6 in human skeletal muscle. J Clin Invest 2007;117:3427–3435 10.1172/JCI30938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 10.1038/ng1180 [DOI] [PubMed] [Google Scholar]
- 11.Eves ND, Plotnikoff RC. Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes Care 2006;29:1933–1941 10.2337/dc05-1981 [DOI] [PubMed] [Google Scholar]
- 12.Ibañez J, Izquierdo M, Argüelles I, et al. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 2005;28:662–667 10.2337/diacare.28.3.662 [DOI] [PubMed] [Google Scholar]
- 13.Nowak A, Pilaczynska-Szczesniak L, Sliwicka E, Deskur-Smielecka E, Karolkiewicz J, Piechowiak A. Insulin resistance and glucose tolerance in obese women: the effects of a recreational training program. J Sports Med Phys Fitness 2008;48:252–258 [PubMed] [Google Scholar]
- 14.American Diabetes Association Standards of medical care in diabetes: 2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 10.2337/dc10-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab 2003;17:305–322 10.1016/S1521-690X(03)00042-3 [DOI] [PubMed] [Google Scholar]
- 16.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001;24:539–548 10.2337/diacare.24.3.539 [DOI] [PubMed] [Google Scholar]
- 17.Mari A, Pacini G, Brazzale AR, Ahrén B. Comparative evaluation of simple insulin sensitivity methods based on the oral glucose tolerance test. Diabetologia 2005;48:748–751 10.1007/s00125-005-1683-9 [DOI] [PubMed] [Google Scholar]
- 18.Stadler M, Anderwald C, Karer T, et al. Increased plasma amylin in type 1 diabetic patients after kidney and pancreas transplantation: a sign of impaired β-cell function? Diabetes Care 2006;29:1031–1038 10.2337/dc05-1247 [DOI] [PubMed] [Google Scholar]
- 19.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986;60:2020–2027 [DOI] [PubMed] [Google Scholar]
- 20.Mogensen M, Vind BF, Højlund K, Beck-Nielsen H, Sahlin K. Maximal lipid oxidation in patients with type 2 diabetes is normal and shows an adequate increase in response to aerobic training. Diabetes Obes Metab 2009;11:874–883 10.1111/j.1463-1326.2009.01063.x [DOI] [PubMed] [Google Scholar]
- 21.Hey-Mogensen M, Højlund K, Vind BF, et al. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia 2010;53:1976–1985 10.1007/s00125-010-1813-x [DOI] [PubMed] [Google Scholar]
- 22.Irving BA, Short KR, Nair KS, Stump CS. Nine days of intensive exercise training improves mitochondrial function but not insulin action in adult offspring of mothers with type 2 diabetes. J Clin Endocrinol Metab 2011;96:E1137–E1141 10.1210/jc.2010-2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair KS, Bigelow ML, Asmann YW, et al. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 2008;57:1166–1175 10.2337/db07-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring) 2008;16:241–256 10.1038/oby.2007.53 [DOI] [PubMed] [Google Scholar]
- 25.Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol 2009;20:50–56 10.1097/MOL.0b013e328321b3a8 [DOI] [PubMed] [Google Scholar]
- 26.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 2004;96:101–106 10.1152/japplphysiol.00707.2003 [DOI] [PubMed] [Google Scholar]
- 27.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Effects of endurance training on glucose tolerance and plasma lipid levels in older men and women. JAMA 1984;252:645–649 10.1001/jama.252.5.645 [DOI] [PubMed] [Google Scholar]
- 28.Boulé NG, Weisnagel SJ, Lakka TA, et al. ; HERITAGE Family Study Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care 2005;28:108–114 10.2337/diacare.28.1.108 [DOI] [PubMed] [Google Scholar]
- 29.Racette SB, Evans EM, Weiss EP, Hagberg JM, Holloszy JO. Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50-95 year olds. Diabetes Care 2006;29:673–678 10.2337/diacare.29.03.06.dc05-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, et al. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metabolism 2004;53:1233–1242 10.1016/j.metabol.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 31.Barwell ND, Malkova D, Moran CN, et al. Exercise training has greater effects on insulin sensitivity in daughters of patients with type 2 diabetes than in women with no family history of diabetes. Diabetologia 2008;51:1912–1919 10.1007/s00125-008-1097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dela F, Stallknecht B. Effect of physical training on insulin secretion and action in skeletal muscle and adipose tissue of first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab 2010;299:E80–E91 10.1152/ajpendo.00765.2009 [DOI] [PubMed] [Google Scholar]
- 33.Østergård T, Andersen JL, Nyholm B, et al. Impact of exercise training on insulin sensitivity, physical fitness, and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab 2006;290:E998–E1005 10.1152/ajpendo.00012.2005 [DOI] [PubMed] [Google Scholar]
- 34.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 2010;59:572–579 10.2337/db09-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia 2010;53:1714–1721 10.1007/s00125-010-1764-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.