Abstract
OBJECTIVE
The aim of this study was to examine the relationship between frequent and unrecognized hypoglycemia and mortality in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study cohort.
RESEARCH DESIGN AND METHODS
A total of 10,096 ACCORD study participants with follow-up for both hypoglycemia and mortality were included. Hazard ratios (95% CIs) relating the risk of death to the updated annualized number of hypoglycemic episodes and the updated annualized number of intervals with unrecognized hypoglycemia were obtained using Cox proportional hazards regression models, allowing for these hypoglycemia variables as time-dependent covariates and controlling for the baseline covariates.
RESULTS
Participants in the intensive group reported a mean of 1.06 hypoglycemic episodes (self-monitored blood glucose <70 mg/dL or <3.9 mmol/L) in the 7 days preceding their regular 4-month visit, whereas participants in the standard group reported an average of 0.29 episodes. Unrecognized hypoglycemia was reported, on average, at 5.8% of the intensive group 4-month visits and 2.6% of the standard group visits. Hazard ratios for mortality in models including frequency of hypoglycemic episodes were 0.93 (95% CI 0.9–0.97; P < 0.001) for participants in the intensive group and 0.98 (0.91–1.06; P = 0.615) for participants in the standard group. The hazard ratios for mortality in models, including unrecognized hypoglycemia, were not statistically significant for either group.
CONCLUSIONS
Recognized and unrecognized hypoglycemia was more common in the intensive group than in the standard group. In the intensive group of the ACCORD study, a small but statistically significant inverse relationship of uncertain clinical importance was identified between the number of hypoglycemic episodes and the risk of death among participants.
Hypoglycemia has long been believed to cause serious consequences in patients with diabetes who receive insulin. Severe episodes of hypoglycemia requiring the assistance of another have been shown to be associated with an increased risk of mortality in both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) (1) and the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) (2) studies, where the impact of glycemic control on cardiovascular outcomes was examined in participants with type 2 diabetes. However, more recent evidence suggests that recurrent hypoglycemia that occurs over a few days to weeks actually may provide some protection during future episodes of hypoglycemia, at least in patients with type 1 diabetes (3,4). It is well known that recurrent episodes of hypoglycemia blunt the counterregulatory hormonal response during subsequent hypoglycemia (5), which reduces the symptoms associated with the event and may decrease its impact on the cardiovascular system. Whether recurrent hypoglycemia has such an impact on patients with type 2 diabetes is unknown.
The ACCORD study dataset offers a unique opportunity to examine the effect of recurrent hypoglycemia on mortality in individuals with type 2 diabetes. In the ACCORD study, participants were randomly assigned to an intensive group, in which the hemoglobin A1c (HbA1c) target was <6.0%, or to a standard group, in which the HbA1c target was between 7.1 and 7.9%. During the 3.4 years of the intervention, the incidence of severe hypoglycemia was three times greater in the intensive than in the standard group (1). The ACCORD study was stopped earlier than planned because 20% more deaths were noted to occur in the intensive versus the standard group. Previous analysis has demonstrated that this increase in mortality was not related to the increase in severe hypoglycemia that also was noted in the intensive group (6), but the relationship between recurrent and milder hypoglycemic episodes and mortality is unknown.
In this article, we examine the relationship between participants who experienced frequent and unrecognized hypoglycemia as a surrogate for recurrent hypoglycemia over a short period of time and mortality risk in the ACCORD study cohort. To perform this analysis, we quantified hypoglycemia exposure and identified participants with unrecognized hypoglycemia (as a marker for recurrent hypoglycemia). We then determined whether mortality risk was different in those with or without intervals of unrecognized hypoglycemia.
RESEARCH DESIGN AND METHODS
The rationale, design, and entry criteria for the ACCORD trial have been described elsewhere (1). In brief, the ACCORD trial was conducted at 77 sites in the U.S. and Canada. Between January 2001 and October 2005, 10,251 participants with type 2 diabetes and who had a previous cardiovascular event (35%), anatomical evidence of atherosclerosis, left ventricular hypertrophy, albuminuria, at least two additional cardiovascular risk factors, and a current HbA1c level at 7.5–11.0% were enrolled. Key exclusion criteria included frequent or serious hypoglycemic events or other serious illnesses. Participants were randomly assigned to either an intensive strategy, aiming to achieve an HbA1c <6.0%, or a standard strategy, aiming to maintain an HbA1c level between 7.0 and 7.9%. In addition, in a double two-by-two factorial design, all participants were enrolled in either a randomized blood pressure trial comparing an intensive with a standard blood pressure treatment strategy or a randomized lipid trial comparing treatment with fenofibrate versus placebo while maintaining good control of LDL cholesterol, mainly with simvastatin. The primary end point of all components of the ACCORD trial is a composite of cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke. All-cause mortality is a predefined secondary end point. This report was limited to 10,096 ACCORD trial participants who had follow-up for both hypoglycemia and mortality. All follow-up data until the final closeout visit in 2009 were used in these analyses.
Definition and reporting of hypoglycemia
Participants were asked at every visit if they had experienced episodes of low blood glucose. A full description of the review of such events, including the adjustment of therapeutic goals in response to severe hypoglycemia, has been previously reported (7). That report focused on symptomatic, severe hypoglycemic events requiring medical assistance (hospitalization, visit to the emergency department, or treatment by medical personnel including emergency medical technicians either in a clinical setting or at home), which was defined in the ACCORD trial as either a blood glucose <50 mg/dL (2.8 mmol/L) or symptoms that promptly resolved with oral carbohydrate, intravenous glucose, or glucagon. In addition to this characterization of severe hypoglycemic episodes, at each 4-month visit, participants were queried about the following four areas:
Since the last call or visit, how many times per week, on average, has the participant checked his/her blood sugar?
How many hypoglycemic episodes (SMBG [self-monitoring of blood glucose] <70 mg/dL or <3.9 mmol/L) did the participant have in the last 7 days?
Since the last visit or call, how many times per week, on average, did the participants report having minor, but uncomfortable symptoms suggesting hypoglycemia?
Did any of reported hypoglycemic episodes occur without warning symptoms?
This report made use of the information collected in response to the above questions to define variables representing hypoglycemic episodes characterized by low blood glucose but not requiring medical assistance and hypoglycemia unawareness. Question 3 was not introduced until June 2005. As such, follow-up for mortality before 1 June 2005 was censored for analyses involving that variable, excluding those who died before this date and starting follow-up at the time when use of the questionnaire was initiated.
To obtain time-dependent variables representing the hypoglycemic episodes characterized by low blood glucose, but not requiring medical assistance, we used all data for any visit where a blood glucose level had been checked at least once per week (question 1). Because those who live longer naturally will accumulate more episodes, variables were defined that represented the updated annualized number of episodes reported in response to question 2. We also created an additional variable to use in sensitivity analyses, in which we used only participants who reported checking ≥7 or more times per week on at least 90% of visits. We defined hypoglycemia unawareness in terms of 1) a positive response to question 2 and 2) a positive response to question 4 or a response of zero to question 3. For this variable, we also created a variable representing the updated annualized number of 4-month intervals in which the participant reported hypoglycemia unawareness.
Baseline covariates
Baseline characteristics used in these analyses were those identified by Riddle et al. (8) as being predictive of mortality in the ACCORD study, including demographic and anthropometric characteristics (age, race, education level, and BMI), medical history (smoking history, history of cardiovascular disease, history of congestive heart failure, or previous amputation), and laboratory and clinical measures (urine albumin-to-creatinine ratio, HbA1c level, and serum creatinine). This model also contained terms representing whether the clinic was part of an integrated health plan and group assignments within either the blood pressure or lipid trial.
Statistical analysis
All statistical analyses were conducted at the coordinating center with the use of SAS software (version 9.2; SAS Institute, Cary, NC). All analyses were conducted separately within the intensive and standard glycemia groups.
Across the full period of follow-up, we calculated the cumulative number of reports of low blood glucose by the subject in response to question 2 (listed above) and determined the percentage of participants who died for several ranges of cumulative episodes (0, 0–5, 6–10, 11–15, 16–20, and >20). Likewise, we tabulated the total number of assessment intervals during follow-up (each interval was 4 months long) where the participant experienced hypoglycemia unawareness. The percentage of participants who died was calculated for each cumulative number of intervals.
Within glycemia treatment groups, hazard ratios (95% CI) relating the risk of death to the updated annualized number of hypoglycemic episodes and the updated annualized number of intervals of hypoglycemia unawareness were obtained using Cox proportional hazards regression models, allowing for these hypoglycemia variables as time-dependent covariates and controlling for the baseline covariates identified by Riddle et al. (8). The series of models that were fitted included baseline covariates plus each of the following as additional factors entered into the model: 1) updated annualized number of hypoglycemic episodes; 2) updated annualized number of intervals of hypoglycemia unawareness; 3) updated annualized number of hypoglycemic episodes, a time-varying covariate representing hypoglycemia requiring medical assistance, and the interaction between these two variables; and 4) updated annualized number of intervals of hypoglycemia unawareness, a time-varying covariate representing hypoglycemia requiring medical assistance, and the interaction between these two variables.
RESULTS
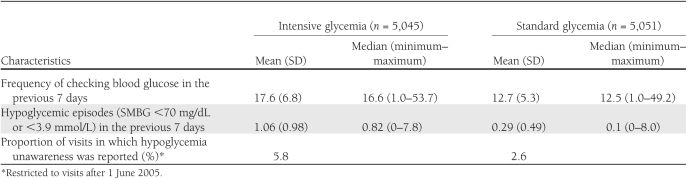
For 10,096 participants with follow-up for hypoglycemia and mortality, there were 780 participants who experienced at least one event of hypoglycemia that required medical assistance (565 participants from the intensive group and 215 from the standard group) (9). Participants from the intensive group reported checking their blood glucose, on average, 17.6 times per week (Table 1), whereas participants from the standard group checked their blood glucose 12.7 times per week. Participants from the intensive group reported a mean of 1.06 hypoglycemic episodes (SMBG <70 mg/dL or <3.9 mmol/L) in the 7 days preceding their regular 4-month visit, whereas participants from the standard group reported an average of 0.29 episodes. Hypoglycemia unawareness was reported, on average, at 5.8% of the intensive group 4-month visits and 2.6% of the standard group visits.
Table 1.
Hypoglycemia characteristics by glycemia group
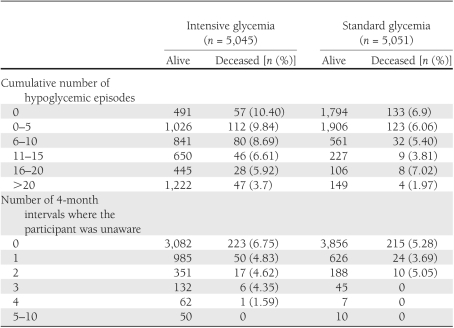
Table 2 reports the number of participants who were alive or deceased relative to the number of reported hypoglycemic episodes at the end of the final follow-up interval. This information is followed by the same tabulation for the number of 4-month intervals where the participant was unaware of their hypoglycemic symptoms. Note that the number of participants used for this second variable is less than that for the first because question 3 was added late in follow-up, and some participants had already dropped out or were deceased by that time. These results suggest that there may be an inverse relationship between the cumulative number of hypoglycemic episodes and death, particularly in the intensive group, but this result could be attributed to the fact that those who live longer are able to accumulate more events. For this reason, the results from the proportional hazards regression models using updated annualized variables may be of greater interest than these tabulations. When we controlled for the baseline covariates that predicted mortality in the ACCORD study cohort (8), we continued to see evidence of an inverse relationship between mortality, particularly in the intensive group, and both the updated annualized number of hypoglycemic events and the updated annualized number of 4-month intervals where participants were unaware of hypoglycemia (see Supplementary Tables 1 and 2 for the model involving only baseline covariates).
Table 2.
Number of participants deceased by cumulative number of hypoglycemic episodes and levels of hypoglycemia unawareness
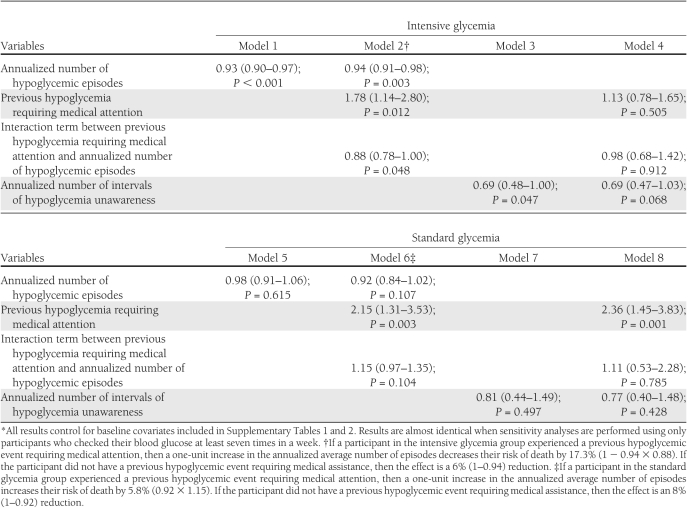
Table 3 reports the hazard ratios relating the risk of death to the updated annualized number of hypoglycemic episodes and the updated annualized number of intervals of hypoglycemia unawareness using Cox proportional hazards regression models. All models control for the baseline covariates contained in the Supplementary Tables. With model 1, participants in the intensive group were found to have a significantly lower risk of death as the annualized number of hypoglycemic episodes increased. Such a relationship was not seen in the standard group (model 5). In model 2, we included a time-dependent variable representing a previous history of severe hypoglycemia requiring medical attention and the interaction between the annualized number of hypoglycemic episodes and this variable. Because the interaction term is statistically significant (P = 0.048), we conclude that a previous event requiring medical assistance modified the relationship between the mortality risk and the annualized number of hypoglycemic episodes in the intensive group. In the intensive group, more frequent hypoglycemia reduced the risk of death more in participants with a history of a hypoglycemic event requiring medical assistance than in those without this previous event. In the standard group, more frequent hypoglycemia increased the risk of death more in participants with a history of a hypoglycemic event requiring medical assistance, whereas it reduced the risk of death in those without this previous event. This apparent differential relationship with mortality is not significantly different between these two groups within participants in the standard group (interaction P = 0.10). A significant relationship was found between the annualized number of intervals of unrecognized hypoglycemia and the risk of death after controlling for baseline covariates in the intensive group, where there is a 31% (hazard ratio 0.69 [95% CI 0.48–1.00]) reduction in the risk of death associated with each additional report of unrecognized hypoglycemia; however, the CI for this estimate is quite wide.
Table 3.
Hazard ratios (95% CI) for mortality in models, including frequency of hypoglycemic episodes and levels of hypoglycemia unawareness*
All analyses were performed for the group as a whole and for a subgroup of those participants who reported checking seven or more times per week on at least 90% of visits (see Supplementary Fig. 1 for a flowchart of the number of participants used in each analysis). This sensitivity analysis did not change the direction of estimates reported in Table 3; however, the interaction term in model 6 was statistically significant under this restriction.
CONCLUSIONS
In this analysis, we found a lower risk of death in those ACCORD study subjects randomly assigned to the intensive treatment group who experienced more episodes of hypoglycemia. Such a relationship was not seen in the standard group. We also found that in participants of the intensive group, the reduction in mortality risk associated with an increased number of hypoglycemic events was more pronounced among those experiencing a previous hypoglycemic event requiring medical assistance than among those without this previous event, but this differential relationship related to a previous event requiring medical assistance was not statistically significant in the standard group. This is in contrast to the positive relationship previously identified between mortality and episodes of severe hypoglycemia requiring medical assistance in both treatment groups (6). These observations suggest that the glycemic target may modify the relationship between mortality and severe hypoglycemia in patients with type 2 diabetes, particularly in patients who experience frequent hypoglycemia.
In this study, hypoglycemia was defined as blood glucose <70 mg/dL or <3.9 mmol/L on a home glucose meter. Assuming the meters used conformed to the International Organization for Standardization guidelines (9), the actual glucose level could have been anywhere between 55 and 85 mg/dL or 3.1 and 4.7 mmol/L. Because participants did not follow a single specific schedule for home glucose testing, we cannot be certain that all episodes in which blood glucose was <70 mg/dL or 3.9 mmol/L were captured on the glucose meters. Participants in the intensive group performed more tests than did participants in the standard group, so it is possible that more normal glucose values were erroneously classified as hypoglycemia in this group. However, an equal number of low blood glucose values would have been erroneously classified as normal. Therefore, it is unlikely that more frequent testing in the intensive group led to an overestimation of the frequency of hypoglycemia. Of interest, the relationship between the number of hypoglycemic episodes experienced in the past 7 days (Table 1) in the intensive versus standard group is similar to the relationship previously defined for the annual incidence of severe hypoglycemia between these groups (6), providing evidence that any overestimation of hypoglycemia occurred equally in both groups.
What factors could be responsible for the observation that frequent hypoglycemia reduces the risk of mortality in participants randomly assigned to a more intensive glucose target? One possibility is that participants in the intensive group were seen more frequently than participants in the standard group and, consequently, may have had more opportunity to learn how to treat and prevent future episodes of hypoglycemia. Such education may have attenuated the severity of future episodes of hypoglycemia and any associated adverse outcomes. Frequency of hypoglycemia is known to increase with improved glycemic control, so it also is possible that participants in the intensive group who did not experience frequent hypoglycemia had comorbidities like cognitive impairment that both increase mortality risk (10) and impair adherence to the complex treatment regimens required to achieve a HbA1c level <6.0%. It also is possible that the counterregulatory response to hypoglycemia is blunted in participants of the intensive group because of their frequent exposure to hypoglycemia, and, as a result, any impact of this stress on cardiovascular function may be reduced.
The definition of hypoglycemia unawareness depended on participants remembering at a 4-month visit whether symptoms of hypoglycemia were present at a time the blood glucose was <70 mg/dL or <3.9 mmol/L on a home glucose meter at any time since the last visit. Such a recollection is subject to error, and this may explain why only a borderline relationship was found between mortality and the annualized number of intervals of unrecognized hypoglycemia. We did note a substantial reduction in the risk of death associated with each additional interval containing a report of unrecognized hypoglycemia in the intensive group, but the wide CI for this analysis probably reflects the imprecision in the measure.
The presence or absence of a previous episode of hypoglycemia requiring medical assistance in the intensive group had an impact on the reduction in mortality seen with increasing episodes of hypoglycemia. For participants in the intensive group with a previous event requiring medical assistance, every one-unit increase in the number of hypoglycemic episodes was associated with a 17.3% reduction in mortality. For participants in the intensive group without a previous event requiring medical assistance, every one-unit increase in the number of hypoglycemic episodes was associated with only a 6% reduction in mortality. The reason for this difference in mortality reduction is uncertain but could include changes in behavior in those who required the attention of a medical professional that made them more aware of impending hypoglycemia.
A history of severe hypoglycemia requiring medical assistance also had an impact on the relationship seen between mortality and increasing episodes of hypoglycemia in the standard group. For participants in the standard group with a history of severe hypoglycemia, the risk of death increased by 5.8% for every one-unit increase in the annualized number of hypoglycemic episodes. Participants in the standard group without a previous hypoglycemic event requiring medical assistance had an 8% reduction in the risk of death for each one-unit increase in the annualized number of hypoglycemic episodes. The reason for this difference is uncertain, but the data confirm, at least for subjects with a HbA1c target of 7.1–7.9%, the findings of the ADVANCE study, in which severe hypoglycemia was found to be a marker of increased vulnerability to adverse outcomes (2).
In the ADVANCE study, severe hypoglycemia was associated with increased mortality from any cause and specifically from cardiovascular disease (2). Similar to the ACCORD study, severe hypoglycemia was more common in the ADVANCE study participants randomly assigned to the intensive glycemic target, but the annual death rates were lower among participants in the intensive group who experienced severe hypoglycemia than among participants in the standard group who experienced severe hypoglycemia. In the ADVANCE study, minor hypoglycemia was associated with a significant reduction in the rate of death and macrovascular events for the entire study population, although whether the relationship was the same in the standard and the intensive groups was not reported. Minor hypoglycemia occurred more than once in almost one-half of the ADVANCE study participants who experienced this event, and although an association between recurrent modest hypoglycemia and mortality in the ADVANCE study was not reported, it is possible that the benefits of minor hypoglycemia on mortality risk could be greatest in those who experienced recurrent episodes.
Our study has many strengths, including the large sample size, the diverse population, and the standardized methods used to collect information about outcomes. We were limited in our ability to accurately determine the number and duration of hypoglycemic episodes experienced by the ACCORD study participants. We found no difference between the results calculated from the whole group and those calculated from participants who reported checking at least seven times per week on at least 90% of visits. Although this latter finding provides some assurance that our conclusions were not biased by the frequency of checking, it is likely that a more meaningful difference could be found between hypoglycemia and mortality if the dataset included multiple daily glucose records from all participants. In addition, our observations do not eliminate the possibility that specific subgroups of ACCORD study participants differ in how hypoglycemia frequency and unrecognized hypoglycemia affects mortality risk. Perhaps future collaboration between the ACCORD trial, ADVANCE trial, and Veterans Affairs Diabetes Trial (VADT) investigators will provide insights into this possibility.
In conclusion, we found a significant inverse relationship between the number of hypoglycemic episodes and the risk of death among participants in the intensive group of the ACCORD trial. However, because hypoglycemia can lead to confusion, coma, and death, this observation does not mean that clinical practice should change to purposefully include frequent episodes of hypoglycemia in patients with type 2 diabetes at high risk for cardiovascular events. Optimal diabetes care should strive to achieve individualized glycemic targets without episodes of hypoglycemia.
Supplementary Material
Acknowledgments
This work was supported by grants (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010) from the National Heart, Lung, and Blood Institute and by other branches of the National Institutes of Health, including the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by general clinical research centers.
The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, sanofi-aventis, and Schering-Plough.
M.E.M. has served as a consultant for Roche. No other potential conflicts of interest relevant to this article were reported.
E.R.S. collected data, contributed to the data analysis, drafted the manuscript, and is the guarantor of the article. M.E.M. performed data analysis and drafted the manuscript. D.E.B. and D.C.G. reviewed and edited the manuscript. M.F. and K.P. collected data and contributed to the manuscript. P.S. contributed to the data interpretation and revision of the manuscript.
Footnotes
Clinical trial reg. no. NCT00000620, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0996/-/DC1.
References
- 1.Gerstein HC, Miller ME, Byington RP, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoungas S, Patel A, Chalmers J, et al. ; ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 3.Jones TW, Borg WP, Borg MA, et al. Resistance to neuroglycopenia: an adaptive response during intensive insulin treatment of diabetes. J Clin Endocrinol Metab 1997;82:1713–1718 [DOI] [PubMed] [Google Scholar]
- 4.Fanelli CG, Paramore DS, Hershey T, et al. Impact of nocturnal hypoglycemia on hypoglycemic cognitive dysfunction in type 1 diabetes. Diabetes 1998;47:1920–1927 [DOI] [PubMed] [Google Scholar]
- 5.Cryer PE. Banting Lecture: Hypoglycemia: the limiting factor in the management of IDDM. Diabetes 1994;43:1378–1389 [DOI] [PubMed] [Google Scholar]
- 6.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonds DE, Kurashige EM, Bergenstal R, et al. ; ACCORD Study Group Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99:80i–89i [DOI] [PubMed] [Google Scholar]
- 8.Riddle MC, Ambrosius WT, Brillon DJ, et al. ; Action to Control Cardiovascular Risk in Diabetes Investigators Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Organization for Standardization In Vitro Diagnostic Test Systems: Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. Geneva, World Health Org., 2003. (ISO rep. no. 15197) [Google Scholar]
- 10.Sachs GA, Carter R, Holtz LR, et al. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Ann Intern Med 2011;155:300–308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.