Abstract
OBJECTIVE
To delineate the associations of total adiponectin, high-molecular-weight (HMW) adiponectin, and the HMW-to-total adiponectin ratio with diabetes in older adults.
RESEARCH DESIGN AND METHODS
Total and HMW adiponectin were measured in a population-based study of older adults. The relations of total adiponectin, HMW adiponectin, and their ratio with incident diabetes (n = 309) were assessed in 3,802 individuals.
RESULTS
Total and HMW adiponectin were highly correlated (r = 0.94). Analysis using cubic splines revealed that the associations between total and HMW adiponectin and new-onset diabetes were not linear. Specifically, after adjustment for confounders, there were similar inverse relationships for total (hazard ratio per SD 0.49 [95% CI 0.39–0.63]) and HMW adiponectin (0.42 [0.32–0.56]) with diabetes up to values of 20 and 10 mg/L, respectively, above which the associations plateaued. These associations persisted after adjustment for potential mediators (blood pressure, lipids, C-reactive protein, and homeostasis model assessment of insulin resistance [HOMA-IR]). There was, however, evidence of interaction by HOMA-IR in the lower range of adiponectin, with stronger inverse associations among insulin-sensitive than insulin-resistant participants. HMW-to-total adiponectin ratio showed a linear adjusted association with outcome, but this was abolished by inclusion of mediating variables.
CONCLUSIONS
In this older cohort, increasing concentrations of total and HMW adiponectin were associated with comparably lower risks of diabetes, but these associations leveled off with further increases above concentrations of 20 and 10 mg/L, respectively. The more pronounced risk decreases at the lower range among participants without insulin resistance support a role for adiponectin that is independent of baseline hyperinsulinemia, but this will require further investigation.
The widening epidemic of type 2 diabetes poses a major challenge to the public health (1). Although the increasing burden of diabetes in the U.S. has been borne by adults in all age categories, the greatest share has fallen upon the older segment, with nearly one-third of adults aged ≥65 years affected (1,2). As in younger age groups, the prevalence of dysglycemia in elders has risen in tandem with excess adiposity, yet the proportion of older adults with diabetes who are overweight or obese is more modest (3). This has led to the concept that diabetes in older adults may have different pathophysiologic features, with a greater role for impaired pancreatic insulin secretion relative to the obesity-associated insulin resistance that predominates in diabetes occurring earlier in life (3).
Still, the prevailing link between adiposity and diabetes across the age spectrum has focused attention on the role of various bioactive peptides secreted by adipocytes as potential mediators of obesity-associated dysglycemia (4). Of these adipokines, adiponectin circulates in concentrations three orders of magnitude greater than any other, although plasma levels of adiponectin exhibit a paradoxic decline with increasing adiposity (5). In animal models, elimination of adiponectin production leads to insulin resistance, whereas repletion of adiponectin restores insulin sensitivity (6). Acting through cognate receptors, adiponectin stimulates AMP-activated protein kinase and peroxisome-proliferator activated receptor-α (PPAR-α) to promote fatty acid catabolism and enhance insulin sensitivity in the liver and skeletal muscle (6). The strongest insulin-sensitizing actions appear to reside with the high-molecular-weight (HMW) fraction of adiponectin, which increases preferentially in response to PPAR-γ agonists and may mediate the insulin-sensitizing effects of these agents (7). Yet adiponectin also influences the function of pancreatic β-cells, and its antiapoptotic actions on such cells could contribute to its favorable glycometabolic properties (8).
Consistent with preclinical investigations, a meta-analysis of prospective epidemiologic studies demonstrated an inverse association between total circulating adiponectin levels and incident diabetes (9). This systematic review reported that the association tended to be stronger for younger than older adults, based principally on two published studies (10,11) focusing on older people, although effect-measure modification by age was not significant. Fewer prospective studies (12–14) have evaluated the association for HMW adiponectin, documenting similar protective associations as for total adiponectin, but no study to date has addressed this question longitudinally in an older population. We sought to examine in detail the relative associations of HMW and total adiponectin, as well as their ratio (7), with diabetes incidence in a prospective cohort of older adults, in order to determine the extent to which findings from middle-aged cohorts apply to older individuals.
RESEARCH DESIGN AND METHODS
Study population
The Cardiovascular Health Study (CHS) is a population-based investigation of cardiovascular disease (CVD) and its determinants in older adults. As reported previously (15), participants consisted of community-dwelling individuals aged 65 years and older identified from Medicare eligibility lists. Enrollment occurred at four U.S. field centers in California, Maryland, North Carolina, and Pennsylvania. An original cohort (n = 5,201) was recruited in 1989–1990, followed in 1992–1993 by a supplemental cohort of African Americans (n = 687). Standardized health evaluations of participants were performed at site clinics using previously described protocols (15,16).
The 1992–1993 examination included 5,553 returning or newly added individuals, of whom 4,715 had samples available for adiponectin measurement. For the present analyses, we excluded 708 participants with prevalent diabetes, and 205 with missing data for determination of baseline or incident diabetes status, leaving 3,802 eligible individuals.
Ascertainment and definition of diabetes
Glucose was measured in blood samples (17) collected in 1989–1990, 1992–1993, 1994–1995, 1996–1997, 1998–1999, and 2005–2006. All visits except for 1994–1995 stipulated a prior overnight fast, and all measurements were in serum except for 1998–1999, which used EDTA-plasma. Time since last intake of food or drink was obtained by questionnaire at all visits. An inventory of medication use was compiled at baseline and annually thereafter (18). Prevalent and incident diabetes was defined by 1) glucose ≥126 mg/dL when participants had reported fasting ≥8 h before venipuncture; 2) glucose ≥200 mg/dL when last oral intake was <8 h from venipuncture; or 3) use of hypoglycemic medication. Prediabetes was defined by fasting blood glucose of 100–125 mg/dL or nonfasting blood glucose of 140–199 mg/dL.
Risk factor definitions
Hypertension was defined by systolic and diastolic blood pressure cutoffs of 140 and 90 mmHg or by self-report and antihypertensive therapy. Anthropometric measurements were performed in standardized fashion by trained personnel, as reported elsewhere (19). Leisure-time physical activity was calculated as a weighted sum of kilocalories expended in specific physical tasks (20). Prevalent CVD included coronary heart disease (CHD), heart failure, atrial fibrillation, stroke, transient ischemic attack, and peripheral arterial disease, ascertained at the 1989–1990 and 1992–1993 examinations by combining the CHS questionnaire, medical-record review, and physician confirmation (16).
Additional laboratory measurements on fasting baseline samples (17) included creatinine, lipids, insulin, and high-sensitivity C-reactive protein (hsCRP) (21). The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5 (22). The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation (23).
Measurement of glucose and harmonization procedures
All CHS glucose assays were performed at the University of Vermont Central Blood Analysis Laboratory. Glucose was analyzed using enzymatic methods with analytical coefficients of variation (CVs) under 2%. Samples from major exam years (1989–1990, 1992–1993, and 1996–1997) along with samples from 2005–2006, were analyzed shortly after collection using the Kodak Ektachem 700 (Eastman Kodak, Rochester, NY) (17) or the Johnson & Johnson Vitros 950 IRC (Johnson & Johnson Clinical Diagnostics, Rochester, NY). Samples from other years were analyzed in 2010 using the Roche Integra 400 (Roche Diagnostics, Indianapolis, IN).
To minimize measurement error and misclassification of participants that can result from differences in glucose measurement over time, we harmonized measurements performed before 2010 with those obtained contemporaneously. This was accomplished by selecting a subset of 48 participants who had specimens available from all previous examinations and reassaying their samples in 2010 with the Roche instrument. Additionally, to identify any differences in glucose measurements attributable to plasma, glucose was measured in 48 samples of serum and plasma from stored specimens in 1996–1997, when both specimen types were available. We then compared the new glucose measurements with the original ones among the subset of participants with paired measurements by evaluating correlation coefficients, regression lines, Bland-Altman plots, and mean differences. In all years, the correlation between the original and new assays was high (0.91–0.99), and there was no statistical evidence of a multiplicative effect (regression slopes did not differ from 1). There were differences in the mean values, however, and adjustments were undertaken based on these mean differences to align all glucose measurements to those from 1989–1990. Harmonized glucose measurements were used for all analyses, including ascertainment of prediabetes and diabetes.
Measurement of adiponectin
Measurements of adiponectin were performed on EDTA-plasma samples stored at −70°C since collection. Total and HMW adiponectin were measured using an enzyme-linked immunosorbent assay (Millipore, Billerica, MA); interassay analytical CVs were 6.9% and 11.1%, respectively.
Statistical analysis
Correlations between adiponectin and baseline covariates were assessed by computing Pearson coefficients after natural log transformation of highly skewed variables. Differences in adiponectin concentrations by levels of categorical variables were evaluated with the Student t test or ANOVA. The functional forms of the associations of total adiponectin, HMW adiponectin, and HMW-to-total adiponectin ratio with incident diabetes were examined using general additive model plots, with the measure of interest fit using a penalized cubic spline. Nonlinearity of associations was tested with the gain statistic, which compares the difference in normalized deviance between the cubic spline model and a model fit with a linear term for the predictor of interest (24). Continuous associations of total adiponectin and HMW adiponectin with incident diabetes were modeled using linear splines with knots chosen at 20 and 10 mg/L, respectively, based on visual inspection of general additive model plots and, for total adiponectin, also the association with death observed previously in a follow-up study involving this cohort (25).
Associations of quartiles of each adiponectin measure with outcome were also evaluated. Although mean adiponectin levels were significantly higher in women than in men, there was substantial overlap in values. Because there was no evidence of interaction by sex (P = 0.20) in the relation of total or HMW adiponectin with outcome, we examined overall quartiles rather than sex-specific quartiles. Cox proportional hazards models were used to evaluate associations with time to incident diabetes. The proportional hazards assumption was tested by the Schoenfeld goodness-of-fit procedures, which did not reveal meaningful violations.
Models were adjusted for age, sex, and race, as well as for potential confounders, wherein covariates that were found to materially influence the risk estimate (>10% change) were retained. Subsequent models considered the effect of putative mediators, namely, systolic blood pressure, HDL cholesterol, triglycerides, hsCRP, HOMA-IR, and fasting glucose. To evaluate for interactions with sex, age, race, BMI, and HOMA-IR, appropriate cross-product terms were included. This was performed by excluding adiponectin outliers at the upper tail (extreme 2.5% of values) and by considering covariates both continuously and dichotomized by their median (age and BMI) or 75th percentile (HOMA-IR, to define insulin resistance). Owing to the detection of nonlinear relationships for both total and HMW adiponectin, cross-product terms were included for adiponectin values below and above the observed inflection points. Significance was assessed separately for each cross-product term (Wald test), as well as overall for the multivariable model with both cross-product terms versus neither (likelihood ratio test).
We did not examine total and HMW adiponectin jointly in multivariable models because the two measures were very highly correlated (r = 0.94 or 0.89 when log-transformed), which would render their mutually adjusted regression coefficients uninterpretable. Last, we conducted sensitivity analyses focusing on events occurring >5 years of follow-up or based on antidiabetes medication use only, or that excluded participants with unintentional weight loss >10 lb in the previous year or with prevalent CHD, heart failure, and atrial fibrillation. All analyses were performed with STATA 11.0 software (StataCorp LP, College Station, TX).
RESULTS
Participants with adiponectin measurements included more women and African Americans, and were younger and in better health, than those without such measurements, consistent with specimen depletion for members of the original cohort with early CVD events. The mean age of the study sample was 74.8 ± 5.2 years, of which 63.3% were women. Total and HMW adiponectin distributions were positively skewed, with geometric means (95% CIs) of 12.8 mg/L (12.6–13.0) and 6.2 mg/L (6.0–6.3), respectively, whereas HMW-to-total adiponectin ratio was normally distributed (0.51 [0.50–0.52]).
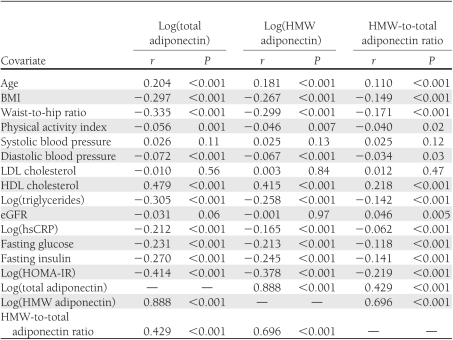
Total and HMW adiponectin were highly correlated, as reported in Table 1. Moderate positive correlations were observed for total adiponectin, HMW adiponectin, and HMW-to-total adiponectin ratio each with age and HDL cholesterol, as were marginal correlations with systolic blood pressure (Table 1). Moderate negative correlations were in turn present for both total adiponectin and HMW adiponectin (and their ratio) with BMI, waist-to-hip ratio, triglycerides, hsCRP, fasting glucose, fasting insulin, and HOMA-IR, with weaker negative correlations observed with diastolic blood pressure.
Table 1.
Correlations between adiponectin and baseline covariates
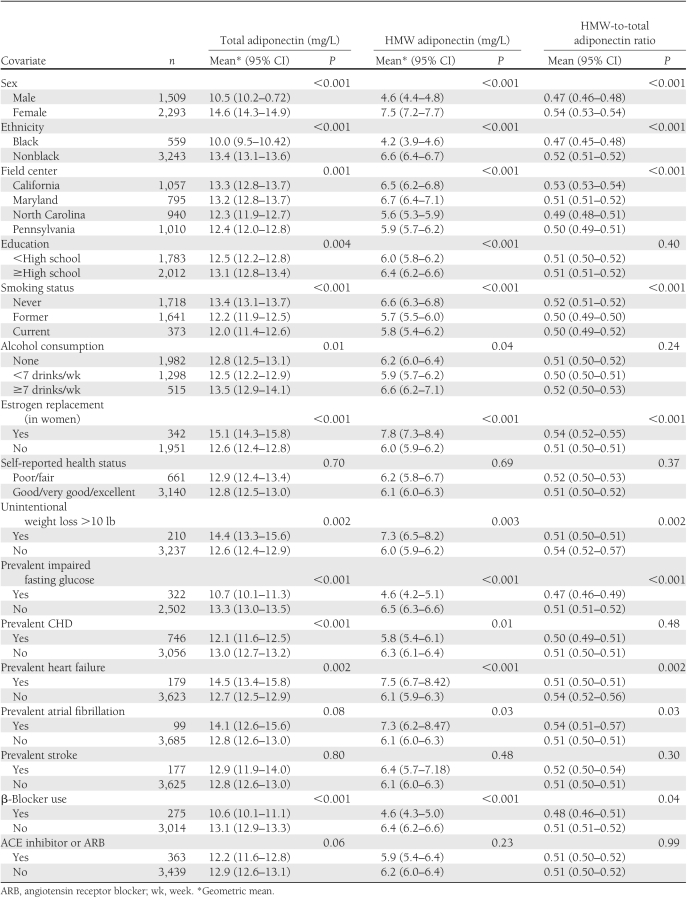
Table 2 presents values of total adiponectin, HMW adiponectin, and HMW-to-total adiponectin ratio according to sociodemographic and clinical subgroups. Higher values of all three adiponectin measures were observed in women, particularly those receiving estrogen replacement therapy, as well as in participants of nonblack ethnicity; in individuals from the California and Maryland field centers; in participants with greater alcohol intake; and in those who never smoked, had normal fasting glucose, or experienced >10-lb unintentional weight loss in the past year (only HMW adiponectin and HMW-to-total adiponectin ratio). In turn, participants with prevalent heart failure or atrial fibrillation had greater levels of all adiponectin measures, while those with CHD or using β-blockers exhibited lower concentrations.
Table 2.
Levels of adiponectin in clinical subgroups at baseline
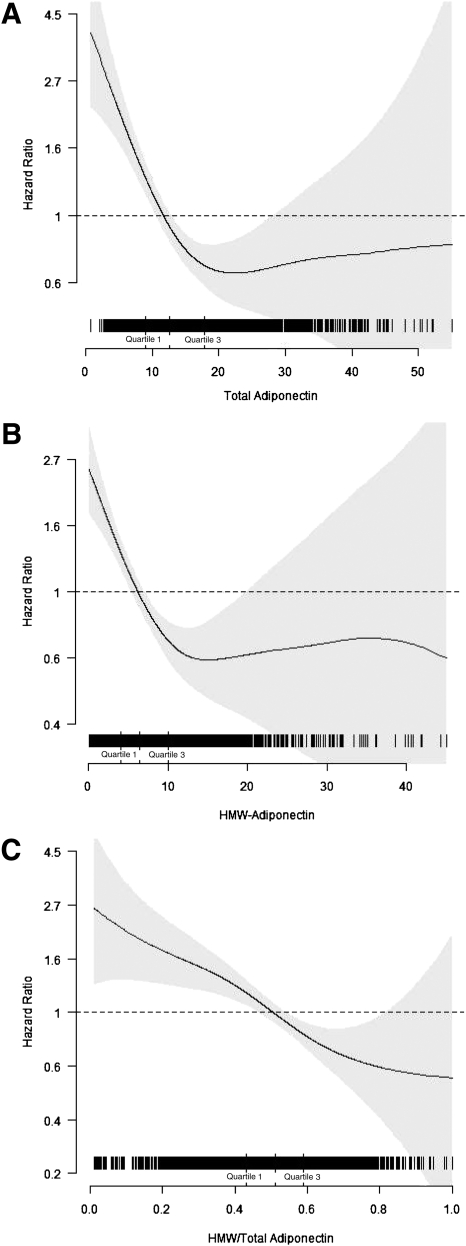
During a median follow-up of 10.6 (maximum, 14.9) years, 309 cases of incident diabetes occurred. Inspection of cubic spline plots, with or without adjustment for potential confounding, revealed that total and HMW adiponectin were inversely associated with incident diabetes up to circulating concentrations of ∼20 mg/L (∼80th percentile) and ∼10 mg/L (∼75th percentile), respectively, above which such associations plateaued (P < 0.001 for nonlinearity for both; Fig. 1). By contrast, spline plots showed that the association of HMW-to-total adiponectin ratio with incident diabetes was linear throughout its distribution (P = 0.60 for nonlinearity).
Figure 1.
Spline regression graphs depict the associations of continuous levels of total adiponectin (A), HMW adiponectin (B), and the HMW-to-total adiponectin ratio (C) with incident diabetes. The 95% CIs are presented in light gray. All models are adjusted for age, sex, race, income, smoking, alcohol, eGFR, prevalent heart failure, prevalent atrial fibrillation, prevalent CHD, β-blocker use, health status, and BMI.
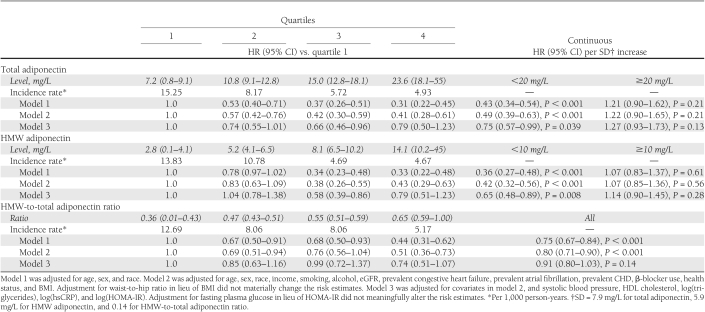
Consistent with the forms of these associations, analyses of quartiles of total and HMW adiponectin revealed graded decreases in risk for quartiles 2 and 3 compared with quartile 1, with quartiles 3 and 4 exhibiting similar effect estimates (Table 3). Significantly reduced risks of diabetes persisted after full adjustment for confounding variables (model 2), with quartiles 3 and 4 showing ∼60% lower risk than the referent quartile. These risk estimates were virtually identical for total and HMW adiponectin. When putative mediators of the association were included in these multivariable models, and notably baseline HOMA-IR (or fasting glucose), the reduced risks observed for the quartile 3 versus quartile 1 comparisons of total and HMW adiponectin remained significant, but those for quartile 4 versus quartile 1 became nonsignificant.
Table 3.
Total adiponectin, HMW adiponectin, and HMW-to-total adiponectin ratio in relation to incident diabetes
The associations of continuous levels of total and HMW adiponectin with incident diabetes, stratified by their corresponding inflection points, are also presented in Table 3. There were significant and comparable inverse associations for total adiponectin and HMW adiponectin with outcome up to concentrations of 20 and 10 mg/L, respectively. These associations were attenuated but remained statistically significant after adjustment for mediators, characterized by risk reductions of 25% and 35% per SD increase for total adiponectin and HMW adiponectin, respectively, with overlapping 95% CIs (Table 3). By contrast, no significant associations with outcome were observed for further increases of total and HMW adiponectin beyond levels of 20 and 10 mg/L, respectively, with or without adjustment for confounders or mediators.
As reported in Table 3, the ratio of HMW to total adiponectin showed a significant inverse association with outcome as well, which, in keeping with lack of departure from linearity in splines analyses, did not exhibit the same apparent leveling off of risk reductions for the upper quartiles. Although significantly lower risks were observed for upper quartiles compared with quartile 1 in models fully adjusted for confounders, these significant associations disappeared after adjustment for mediators. Likewise, the analysis of the HMW-to-total adiponectin ratio as a continuous variable showed a significant 20% lower risk of incident diabetes associated with every SD increase in the ratio after complete adjustment for confounders, but the relation was no longer significant with additional inclusion of mediators.
In the foregoing analyses, there were no significant overall interactions between total and HMW adiponectin with dichotomous age (P = 0.82 and 0.71, respectively), sex (P = 0.15 and 0.059), race (P = 0.51 and 0.45), or BMI (P = 0.51 and 0.41). Findings were similar for continuous age and BMI. Nor was there evidence of significant effect-modification by these covariates above or below the corresponding adiponectin cut points. There were, however, significant interactions with binary HOMA-IR for total and HMW adiponectin below (P = 0.010 and 0.030) but not above (P = 0.17 and 0.37) their respective cut points, such that effect modification was significant overall for total (P = 0.035) but not HMW adiponectin (P = 0.098). For HOMA-IR <3.21 (75th percentile), total and HMW adiponectin were significantly inversely related to incident diabetes (adjusted [model 2] HR per SD 0.44 [95% CI 0.32–0.62], and 0.40 [0.27–0.59], respectively), but these associations were blunted for HOMA-IR ≥3.21 (0.81 [0.57–1.14] and 0.73 [0.48–1.10]). The significant associations within the insulin-sensitive stratum (HOMA-IR <3.21) remained minimally altered with additional adjustment for HOMA-IR (0.50 [0.36–0.70] for total adiponectin; 0.44 [0.29–0.66] for HMW adiponectin). A similar pattern of effect modification was present when HOMA-IR was modeled continuously. There were again significant interactions for total and HMW adiponectin below their cut points (P = 0.013 and 0.030, respectively), but not above (P = 0.12 and 0.30, respectively), with the overall interaction achieving significance for total (P = 0.039) but not HMW adiponectin (P = 0.20).
Last, findings were not materially changed when only incident events after the first 5 years of follow-up were considered, when diabetes diagnosis was based solely on medications, or after exclusion of participants with involuntary weight loss and prevalent CHD, heart failure, and atrial fibrillation.
CONCLUSIONS
To our knowledge, this is the largest prospective study to evaluate the relationship of adiponectin with new-onset diabetes in older people, and to do so concurrently for both total and HMW adiponectin. As such, this investigation yields several novel findings with regard to the adiponectin–diabetes association in older adults. First among them is a departure from linearity in the relationships of total and HMW adiponectin with incident diabetes. As determined by the use of linear splines, there was a strong inverse association between total and HMW adiponectin levels and diabetes up to concentrations of approximately 20 and 10 mg/L, respectively, above which the risk associated with further increases plateaued.
A departure from linearity in the association between adiponectin and diabetes has only been reported to date in a cohort of middle-aged women, wherein there was a stronger decrease in risk for the lower range of the adipokine's distribution than for the higher range (12). Unlike our findings, the inverse association continued to be evident at the higher end of adiponectin concentrations, even if in attenuated form, but total and HMW adiponectin concentrations in that study were lower than those observed in our older cohort, as were their corresponding inflection points (12). Nevertheless, because the numbers of incident diabetes cases >20 mg/L of total adiponectin and >10 mg/L of HMW adiponectin in our study were modest (n = 30 and n = 40, respectively), the relationships in the upper range lack precision for adequate comparison. Moreover, in the absence of an adiponectin measurement standard, firm conclusions about differences in absolute values of adiponectin concentrations and the cut points observed in the two studies are not possible.
Still, the leveling off of risk observed here for the higher range of total and HMW adiponectin concentrations has important implications. Because prior studies have not identified a plateauing of the association at the higher end of values (9), but have modeled it instead as linear throughout the distribution of the adipokine, effect estimates for continuous associations may have been underestimated. In fact, when expressed per log-mg/L increase, the relative risk for total adiponectin levels <20 mg/L observed here (0.41 [95% CI 0.31–0.55]) is substantially lower than that reported for older cohorts (mean age >60 years) in the meta-analysis (0.77 [0.70–0.84]) (9), although the caveat about lack of measurement standardization across cohorts applies.
Furthermore, the shape of the association defined by the present analyses may shed light on the adiponectin “paradox” (26). This refers to the conundrum that despite the insulin-sensitizing and antiatherogenic properties demonstrated for adiponectin in laboratory studies, and the protective relationship with cardiovascular events documented for total adiponectin in healthy younger populations, the association with cardiovascular outcomes and all-cause mortality in older or higher-risk populations has instead been adverse.
The plateauing of the association >20 mg/L observed here is consistent with a recently reported U-shaped relation with all-cause mortality in CHS survivors enrolled in the follow-up CHS All Stars study, whose adiponectin levels were measured in 1996–1997 and again 9 years later (25). In the CHS All Stars Study, an inflection point at approximately the same value of 20 mg/L was likewise manifest, even though samples were collected 13 years after the present ones (25). The current results could provide a plausible framework for understanding the mortality finding, indicating as they do that although higher adiponectin levels have favorable glycometabolic consequences within the lower range of values, once levels exceed the 80th percentile, further increases in adiponectin appear to afford no additional glycometabolic benefits. If levels at the higher range reflect adiponectin increases occurring in response to homeostatic dysregulation or aging-related disease processes (e.g., vascular disease, inflammation) (25), they would tend to be associated with the unfavorable glycometabolic outlook and otherwise adverse prognosis that accompanies such processes. This might explain the offset of further gains with regard to diabetes risk at the high end of adiponectin values, and with it, the heightened risk of all-cause mortality documented previously.
Another notable finding concerns the relative associations of total and HMW adiponectin with incident diabetes, evaluated concurrently for the first time in an older population. Although HMW adiponectin showed slightly stronger effect estimates than total adiponectin, the relative risks were not significantly different. And although the difference was sufficient to confer a linear inverse association for the HMW-to-total adiponectin ratio that held throughout its distribution, the relationship ceased to be significant once putative mediators were taken into account. Taken together, these findings argue against a substantial advantage to measuring HMW adiponectin over, or in addition to, total adiponectin for assessment of glycometabolic risk.
Our analyses did not document significant effect-measure modification by sex (27) or BMI (10), as suggested in earlier studies. They did, however, show evidence of interaction by a proxy measure of insulin resistance, HOMA-IR, wherein the strong inverse associations documented for the lower range of the adiponectin measures held for HOMA-IR values <3.21 but were blunted at higher values. Interestingly, this finding is contrary to those of a previous report, in which inverse associations between total adiponectin and incident diabetes were documented only among insulin-resistant (HOMA-IR ≥75th percentile) participants in two population-based cohorts, but not in their insulin-sensitive counterparts (28). The basis for the different findings is uncertain, although the numbers of diabetes cases for exploring the nature of the relationship were modest in each of the two cohorts, a significant interaction by HOMA-IR was detected only in one, and the study populations were younger than the one studied here (28). Nevertheless, our finding has relevance for an important unresolved question in the field, namely, whether the association of hypoadiponectinemia with incident diabetes in humans results from insufficient insulin-sensitizing (or pancreatic β-cell enhancing) effects otherwise exerted directly by the adipokine or instead reflects the suppressive effects of hyperinsulinemia on adiponectin production by adipocytes (29). That the inverse associations detailed here persisted after adjustment for HOMA-IR, and were stronger in insulin-sensitive participants at baseline, supports a role for adiponectin that is independent of hyperinsulinemia. More definitive assessment of the complex interplay between insulin and adiponectin, however, will require an approach predicated on serial measurements of insulin and adiponectin. Additional work is necessary to elucidate the pathophysiologic pathways involved, and whether development of therapies that specifically raise adiponectin levels could result in glycometabolic and cardiovascular health benefits.
Several limitations merit consideration. Because adiponectin measurements were obtainable only in a healthier subset of CHS participants in 1992–1993, the present findings may not apply to more disease-prone older cohorts. Diabetes ascertainment was based on medication inventory throughout follow-up, but regular determinations of fasting blood glucose were only possible during the initial 6 years, with a final measurement 13 years later in a subgroup. There was no evidence of differential associations, however, when the outcome was limited to treated diabetes. In addition, we lacked 2-h glucose tolerance testing, which may have resulted in misclassification of prevalent and incident diabetes in our cohort. Laboratory testing in this cohort also did not include direct measures of insulin sensitivity or insulin secretion, which would have permitted more accurate, detailed assessment of the relations of interest. Last, as noted, our study had limited power to define the precise shape of the relationship of adiponectin with diabetes in the upper range. Larger studies will be required to better characterize the relationship at the higher end and to explore the underlying basis for its apparent attenuation.
In conclusion, in this large older cohort, total and HMW adiponectin exhibited a nonlinear association with incident diabetes, wherein levels up to 20 and 10 mg/L, respectively, showed strong inverse associations that were independent of potential confounders and even proposed intermediates, but additional increases above these levels conferred no further detectable lowering in incident diabetes risk. The inverse associations within the lower range were more pronounced among insulin-sensitive than insulin-resistant individuals. These data do not demonstrate meaningful superiority of HMW over total adiponectin for assessment of diabetes risk and argue against baseline hyperinsulinemia as the underlying basis for the observed associations.
Acknowledgments
This work was supported by R01-HL-094555 from the National Heart, Lung, and Blood Institute (NHLBI). The CHS was supported by NHLBI contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01-HC-15103, N01-HC-55222, N01-HC-75150, N01-HC-45133, and NHLBI Grant HL-080295, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging. See also http://www.chs-nhlbi.org/pi.htm.
No potential conflicts of interest relevant to this article were reported.
J.R.K. participated in study conception and design, acquired the data, analyzed and interpreted the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. A.M.A. participated in study conception and design, analyzed and interpreted the data, performed the statistical analysis, and critically revised the manuscript for important intellectual content. D.B. performed the statistical analysis and critically revised the manuscript for important intellectual content. J.H.I., L.D., and S.J.Z. participated in study conception and design, acquired the data, analyzed and interpreted the data, and critically revised the manuscript for important intellectual content. J.I.B. critically revised the manuscript for important intellectual content. R.P.T. participated in study conception and design, acquired the data, and critically revised the manuscript for important intellectual content. C.S.M. critically revised the manuscript for important intellectual content. D.S.S. and K.J.M. participated in study conception and design, acquired the data, analyzed and interpreted the data, and critically revised the manuscript for important intellectual content. J.R.K. takes responsibility for the contents as guarantor of this article.
References
- 1.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 10.2337/dc08-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloan FA, Bethel MA, Ruiz D, Jr, Shea AM, Feinglos MN. The growing burden of diabetes mellitus in the US elderly population. Arch Intern Med 2008;168:192–199; discussion 199 10.1001/archinternmed.2007.35 [DOI] [PubMed] [Google Scholar]
- 3.Meneilly GS. Diabetes in the elderly. Med Clin North Am 2006;90:909–923 10.1016/j.mcna.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 4.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev 2007;21:1443–1455 10.1101/gad.1550907 [DOI] [PubMed] [Google Scholar]
- 5.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr 2010;91:258S–261S 10.3945/ajcn.2009.28449C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–1792 10.1172/JCI29126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 2004;279:12152–12162 10.1074/jbc.M311113200 [DOI] [PubMed] [Google Scholar]
- 8.Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic beta-cell function. Metabolism 2011;60:1664–1672 [DOI] [PubMed] [Google Scholar]
- 9.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2009;302:179–188 10.1001/jama.2009.976 [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Lowe GD, Rumley A, Cherry L, Whincup PH, Sattar N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care 2007;30:1200–1205 10.2337/dc06-2416 [DOI] [PubMed] [Google Scholar]
- 11.Kanaya AM, Wassel Fyr C, Vittinghoff E, et al. Adipocytokines and incident diabetes mellitus in older adults: the independent effect of plasminogen activator inhibitor 1. Arch Intern Med 2006;166:350–356 10.1001/archinte.166.3.350 [DOI] [PubMed] [Google Scholar]
- 12.Heidemann C, Sun Q, van Dam RM, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med 2008;149:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakashima R, Kamei N, Yamane K, Nakanishi S, Nakashima A, Kohno N. Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab 2006;91:3873–3877 10.1210/jc.2006-1158 [DOI] [PubMed] [Google Scholar]
- 14.Zhu N, Pankow JS, Ballantyne CM, et al. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J Clin Endocrinol Metab 2010;95:5097–5104 10.1210/jc.2010-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276 10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 16.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 1995;5:270–277 10.1016/1047-2797(94)00092-8 [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–270 [PubMed] [Google Scholar]
- 18.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318 [PubMed] [Google Scholar]
- 19.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA 2010;303:2504–2512 10.1001/jama.2010.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort—the Cardiovascular Health Study All Stars Study. J Am Geriatr Soc 2009;57:432–440 10.1111/j.1532-5415.2008.02152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem 1997;43:52–58 [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 24.Hastie TJ, Hibishani R. Generalized additive models. Boca Raton, Florida, Chapman & Hall/CRC, 1990 [Google Scholar]
- 25.Kizer JR, Arnold AM, Jenny NS, et al. Longitudinal changes in adiponectin and inflammatory markers and relation to survival in the oldest old: the Cardiovascular Health Study All Stars Study. J Gerontol A Biol Sci Med Sci 2011;66A:1100–1107 10.1093/gerona/glr098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattar N, Nelson SM. Adiponectin, diabetes, and coronary heart disease in older persons: unraveling the paradox. J Clin Endocrinol Metab 2008;93:3299–3301 10.1210/jc.2008-1435 [DOI] [PubMed] [Google Scholar]
- 27.Snijder MB, Heine RJ, Seidell JC, et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the Hoorn Study. Diabetes Care 2006;29:2498–2503 10.2337/dc06-0952 [DOI] [PubMed] [Google Scholar]
- 28.Hivert MF, Sullivan LM, Shrader P, et al. Insulin resistance influences the association of adiponectin levels with diabetes incidence in two population-based cohorts: the Cooperative Health Research in the Region of Augsburg (KORA) S4/F4 study and the Framingham Offspring Study. Diabetologia 2011;54:1019–1024 10.1007/s00125-011-2067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kizer JR, Biggs ML, Ix JH, et al. Measures of adiposity and future risk of ischemic stroke and coronary heart disease in older men and women. Am J Epidemiol 2011;173:10–25 10.1093/aje/kwq311 [DOI] [PMC free article] [PubMed] [Google Scholar]