Abstract
OBJECTIVE
To investigate whether SIRT1, a nutrient-sensing histone deacetylase, influences fetal programming during malnutrition.
RESEARCH DESIGN AND METHODS
In 793 individuals of the Dutch Famine Birth Cohort, we analyzed the interaction between three SIRT1 single nucleotide polymorphisms (SNPs) and prenatal exposure to famine on type 2 diabetes risk.
RESULTS
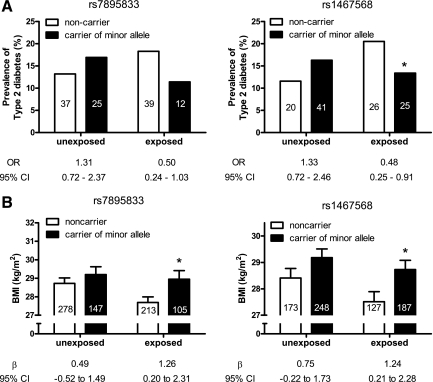
In the total population (exposed and unexposed), SIRT1 variants were not associated with type 2 diabetes. A significant interaction was found between two SIRT1 SNPs and exposure to famine in utero on type 2 diabetes risk (P = 0.03 for rs7895833; P = 0.01 for rs1467568). Minor alleles of these SNPs were associated with a lower prevalence of type 2 diabetes only in individuals who had been exposed to famine prenatally (odds ratio for rs7895833 0.50 [95% CI 0.24–1.03], P = 0.06; for rs1467568 0.48 [0.25–0.91], P = 0.02).
CONCLUSIONS
SIRT1 may be an important genetic factor involved in fetal programming during malnutrition, influencing type 2 diabetes risk later in life.
Fetal malnutrition may predispose to type 2 diabetes by altering gene expression profiles through epigenetic mechanisms (1–3). SIRT1, a nutrient-sensing histone deacetylase, is, in addition to deacetylation of histones, involved in glucose and insulin metabolism by regulating the expression of various transcription factors. Dietary factors influence the NAD+-to-NADH ratio regulating SIRT1 activity (4). We hypothesized that genetic variants in SIRT1 may interact with fetal malnutrition, influencing type 2 diabetes risk later in life. This was addressed in the Dutch Famine Birth Cohort.
RESEARCH DESIGN AND METHODS
The Dutch Famine Birth Cohort is composed of individuals born as term singletons in Amsterdam around the famine in the Netherlands during World War II, as described in detail earlier (5). A total of 2,414 singletons were born between 1 November 1943 and 28 February 1947. A total of 810 of 1,423 invited subjects agreed to participate in the cohort study in 2002. The study was approved by the local medical ethics committee. All participants gave written informed consent. Prenatal exposure to famine was defined as a daily food ration for the pregnant mother of <1,000 calories during any 13-week period of gestation.
Study parameters
The primary outcome measure of the current study was type 2 diabetes, which was defined as using antidiabetes medications or fasting glucose levels >7.0 mmol/L or 120-min glucose levels >11.0 mmol/L in an oral glucose tolerance test (OGTT). Secondary outcome measures were glucose metabolism parameters (120-min glucose and insulin levels, area under the curve [AUC] for glucose and insulin) and BMI.
Genotyping
DNA was available from 793 subjects for Taqman allelic discrimination assays. Three tagging single nucleotide polymorphisms (SNPs) were selected, covering most of the common variants of the SIRT1 gene in whites (rs7895833, rs1467568, and rs497849, as described earlier) (6). Minor allele frequencies of the SNPs were 19, 36, and 22%, respectively. The SNPs were in Hardy-Weinberg equilibrium (χ2 <3.1; 1 df; P > 0.07).
Statistical methods
Associations of genetic variants and diabetes were investigated with binary logistic regression and associations with BMI, AUC, and 120-min levels of glucose and insulin with linear regression. Interactions were tested by creating interaction terms for each genetic variant with the exposure group. The minor allele was coded 1 and 0 for noncarriers. Unexposed subjects born before or conceived after the Dutch famine were coded 0 and 1 for exposed subjects. P < 0.05 was considered significant.
RESULTS
A total of 337 subjects were exposed to famine in utero, and 456 subjects were born before or after the famine. In adulthood, BMI and type 2 diabetes prevalence were not different between subjects exposed and nonexposed to famine. A total of 117 of 791 individuals developed diabetes in adulthood (15.8% in the exposed and 14.1% in the unexposed group). Plasma glucose levels at 120 min of the OGTT were 0.37 mmol/L (P = 0.03) higher for those exposed to famine in utero.
In the total population (exposed and unexposed), carriers of the minor allele of rs7895833 (GG and AG) and rs1467568 (AA and GA) had a higher BMI than noncarriers (β for rs7895833: 0.82 kg/m2 [95% CI 0.09–1.56], P = 0.028; β for rs1467568: 0.96 kg/m2 [0.25–1.67], P = 0.008). There was no association between the SIRT1 variants and diabetes and AUCs of glucose and insulin levels during OGTT.
Next, we investigated the interactions of the SIRT1 SNPs with prenatal exposure to famine on type 2 diabetes risk and BMI at age 58 years. An interaction was found between prenatal exposure to famine and rs7895833 (P = 0.03) and rs1467568 (P = 0.01) but not with rs497849 on diabetes risk. In stratified analyses in subjects not prenatally exposed to famine, the SNPs and type 2 diabetes were not associated (Fig. 1A). In the exposed subjects, there was a borderline significant association between rs7895833 and diabetes and a significant association for rs1467568, with diabetes risk decreasing for minor allele carriers (Fig. 1A). We did not observe an interaction between any of the SIRT1 variants and exposure to famine on BMI, glucose, and insulin values. However, minor allele carriers of rs7895833 tended to have lower 120-min glucose and insulin levels as well as lower AUC glucose/insulin values in the exposed group, whereas such a trend was not evident in the unexposed subjects (data not shown).
Figure 1.
The relationship of the rs7895833 and rs1467568 SIRT1 SNPs with the prevalence of diabetes (A) and BMI (B) according to prenatal exposure to famine. Odds ratios (ORs; A) and β-coefficients (β; B) are shown for carriers of the minor alleles. The number of subjects per group (i.e., diabetic patients for A) are shown inside each bar. *P < 0.05 vs. noncarriers.
In those prenatally exposed to famine, BMI was significantly higher in carriers of minor alleles of rs7895833 and rs1467568 (Fig. 1B). In subjects, who had not been exposed to famine prenatally, no significant association was found, although the direction of the effect was the same (Fig. 1B).
CONCLUSIONS
Our results show that an interaction between two SNPs in SIRT1 (rs7895833 and rs1467568) and in utero exposure to malnutrition significantly influenced type 2 diabetes risk in adulthood. Minor allele carriers of these two genetic SIRT1 variants who had been exposed to famine in utero had a 50% lower risk of developing diabetes than noncarriers but, surprisingly, had a higher BMI.
SIRT1 is susceptible to intracellular fluctuations in the NAD+-to-NADH ratio and may influence type 2 diabetes risk by its known epigenetic effects and β-cell apoptosis (4,7,8). Recently, Sandovici et al. (9) showed that suboptimal nutrition in rats during early development led to epigenetic silencing and reduced the expression of the transcription factor Hnf4a, which is required for pancreatic β-cell differentiation and glucose homeostasis. Valtat et al. (10) also showed that food restriction during gestation impaired glucose tolerance and decreased β-cell mass later in life. In line, we also found that exposure to famine in utero reduced glucose tolerance (5).
We previously reported an interaction between variants of the PPAR-γ2 gene and the IGF2BP2 gene and exposure to famine in utero on the prevalence of impaired glucose tolerance and type 2 diabetes (11,12). Whether SIRT1 interacts with these genes on pathways influencing diabetes risk should be elucidated by additional studies.
Unexpectedly, associations between two SIRT1 SNPs and BMI in subjects who had been exposed to famine were in the opposite direction as expected (13–15). Nevertheless, the SIRT1 variants interacted with exposure to famine on diabetes risk independent of BMI.
Interactions have been reported between SIRT1 variants and niacin (6) and vitamin E (16) intake. It is possible that associations between SIRT1 variants and diabetes risk can only be found when interactions with environmental factors are taken into account, such as fetal nutrition in our study.
Although our results are based on relatively small numbers and therefore have to be interpreted with caution, they support that SIRT1 plays a role in the fetal programming of type 2 diabetes through fetal malnutrition.
Acknowledgments
The Dutch Famine Birth Cohort study was funded by grants from the Dutch Heart Foundation (NHS2007B087 and NHS2003B165), the European Science Foundation (EUROCORES/STRESS), and The Netherlands Organization for Scientific Research (NWO).
No potential conflicts of interest relevant to this article were reported.
I.P.G.B. and M.C.Z. researched data, contributed to the discussion, and wrote the manuscript. S.R.R. and T.J.R. researched data, contributed to the discussion, and reviewed and edited the manuscript. J.G.L. and A.H.J.D. contributed to the discussion and reviewed and edited the manuscript. E.J.G.S. is the guarantor of the article, contributed to the discussion, and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the 21st European Meeting on Hypertension and Cardiovascular Prevention, Milan, Italy, 17–20 June 2011, and at the High Blood Pressure Research 2011 Scientific Sessions, Orlando, Florida, 21–24 September 2011.
References
- 1.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993;341:938–941 [DOI] [PubMed] [Google Scholar]
- 2.Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab 2008;22:1–16 [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care 2009;12:431–437 [DOI] [PubMed] [Google Scholar]
- 5.Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998;351:173–177 [DOI] [PubMed] [Google Scholar]
- 6.Zillikens MC, van Meurs JB, Sijbrands EJ, et al. SIRT1 genetic variation and mortality in type 2 diabetes: interaction with smoking and dietary niacin. Free Radic Biol Med 2009;46:836–841 [DOI] [PubMed] [Google Scholar]
- 7.Tang MM, Zhu QE, Fan WZ, et al. Intra-arterial targeted islet-specific expression of Sirt1 protects β cells from streptozotocin-induced apoptosis in mice. Mol Ther 2011;19:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafontaine-Lacasse M, Doré G, Picard F. Hexosamines stimulate apoptosis by altering SIRT1 action and levels in rodent pancreatic β-cells. J Endocrinol 2011;208:41–49 [DOI] [PubMed] [Google Scholar]
- 9.Sandovici I, Smith NH, Nitert MD, et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci U S A 2011;108:5449–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valtat B, Dupuis C, Zenaty D, et al. Genetic evidence of the programming of beta cell mass and function by glucocorticoids in mice. Diabetologia 2011;54:350–359 [DOI] [PubMed] [Google Scholar]
- 11.de Rooij SR, Painter RC, Phillips DI, et al. The effects of the Pro12Ala polymorphism of the peroxisome proliferator–activated receptor-γ2 gene on glucose/insulin metabolism interact with prenatal exposure to famine. Diabetes Care 2006;29:1052–1057 [DOI] [PubMed] [Google Scholar]
- 12.van Hoek M, Langendonk JG, de Rooij SR, Sijbrands EJ, Roseboom TJ. Genetic variant in the IGF2BP2 gene may interact with fetal malnutrition to affect glucose metabolism. Diabetes 2009;58:1440–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zillikens MC, van Meurs JB, Rivadeneira F, et al. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes 2009;58:2828–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Berg SW, Dollé ME, Imholz S, et al. Genetic variations in regulatory pathways of fatty acid and glucose metabolism are associated with obesity phenotypes: a population-based cohort study. Int J Obes (Lond) 2009;33:1143–1152 [DOI] [PubMed] [Google Scholar]
- 15.Peeters AV, Beckers S, Verrijken A, et al. Association of SIRT1 gene variation with visceral obesity. Hum Genet 2008;124:431–436 [DOI] [PubMed] [Google Scholar]
- 16.Zillikens MC, van Meurs JB, Rivadeneira F, et al. Interactions between dietary vitamin E intake and SIRT1 genetic variation influence body mass index. Am J Clin Nutr 2010;91:1387–1393 [DOI] [PubMed] [Google Scholar]