Abstract
OBJECTIVE
The pathogenesis of brain disorders in type 1 diabetes (T1D) is multifactorial and involves the adverse effects of chronic hyperglycemia and of recurrent hypoglycemia. Kidney-pancreas (KP), but not kidney alone (KD), transplantation is associated with sustained normoglycemia, improvement in quality of life, and reduction of morbidity/mortality in diabetic patients with end-stage renal disease (ESRD).
RESEARCH DESIGN AND METHODS
The aim of our study was to evaluate with magnetic resonance imaging and nuclear magnetic resonance spectroscopy (1H MRS) the cerebral morphology and metabolism of 15 ESRD plus T1D patients, 23 patients with ESRD plus T1D after KD (n = 9) and KP (n = 14) transplantation, and 8 age-matched control subjects.
RESULTS
Magnetic resonance imaging showed a higher prevalence of cerebrovascular disease in ESRD plus T1D patients (53% [95% CI 36–69]) compared with healthy subjects (25% [3–6], P = 0.04). Brain 1H MRS showed lower levels of N-acetyl aspartate (NAA)-to-choline ratio in ESRD plus T1D, KD, and KP patients compared with control subjects (control subjects vs. all, P < 0.05) and of NAA-to-creatine ratio in ESRD plus T1D compared with KP and control subjects (ESRD plus T1D vs. control and KP subjects, P ≤ 0.01). The evaluation of the most common scores of psychological and neuropsychological function showed a generally better intellectual profile in control and KP subjects compared with ESRD plus T1D and KD patients.
CONCLUSIONS
Diabetes and ESRD are associated with a precocious form of brain impairment, chronic cerebrovascular disease, and cognitive decline. In KP-transplanted patients, most of these features appeared to be near normalized after a 5-year follow-up period of sustained normoglycemia.
In patients with type 1 diabetes (T1D), a hyperglycemia-related generalized multiorgan failure (e.g., eye, kidney, central/peripheral nervous system, and cardiovascular system) is evident (1,2). A reduction in the volume of the cerebral cortex and a significant loss in neocortical neurons are also present in mice with streptozotocin-induced hyperglycemia (3). Diffuse central nervous system changes have been documented among patients with T1D: neuronal and axonal generalized lesions were reported at multiple sites, such as brain, optical nerve, and posterior and anterior horns of spinal cord (4). Neuropsychological tests performed in diabetic patients have demonstrated that the longer the exposure to hyperglycemia, the more significant the impairment of superior brain functions (1,2). Patients with so-called primary diabetic encephalopathy experience a reduction in mnemonic, abstract reasoning, problem-solving, and hand-eye coordination abilities and an increased risk of Alzheimer disease (5–7). Furthermore, among patients with T1D a neurophysiologic modification with a diffuse slowing in electroencephalographic tracks, somatosensory/motor evoked potentials, and brainstem auditory evoked potentials has also been reported (8). The degree of these changes is associated with metabolic control; thus, tight glycemic control is the only available option to slow or prevent an early cognitive decline and possibly dementia (3,8–10). Recurrent hypoglycemic episodes are likely to play a role in decline of cognitive functions as well (3,6,11–14). Thus far, various imaging techniques have been used to gain insight into the diabetic brain; among them, an important role is played by proton magnetic resonance spectroscopy (1H MRS). 1H MRS is a noninvasive technique capable of detecting metabolic changes in normal-appearing magnetic resonance imaging (MRI) examinations by allowing the measurement of total creatine (associated with energy metabolism and considered an internal standard), choline-containing compounds (Cho) (having an important role in the turnover of cellular membrane), and N-acetyl aspartate (NAA) (a marker of neuronal density and function), whose relative decrease has been attributed to neuroaxonal loss in number and function (15,16). Kidney-pancreas (KP) but not kidney-alone (KD) transplantation has been shown to be capable of halting the progression and in many cases reversing diabetes complications (17,18). Conversely, no studies are available thus far on the effects of KP transplantation on the central nervous system. The aim of our study was to assess the effect of KD and KP transplantation on morphological (e.g., brain volume), metabolic (e.g., neuronal/assonal modifications as evaluated with 1H MRS parameters), and functional (e.g., psychological and neuropsychological abnormalities) central nervous system features generally characterizing T1D patients’ central nervous system. We thus carried out brain MRI, localized single-voxel spectroscopy, and nonlocalized whole-brain NAA (WBNAA) spectroscopy in patients with end-stage renal disease (ESRD) and T1D and in KD- and KP-transplanted patients after ∼5 years of follow-up. Our hypothesis was that in the long term, the persistence of normoglycemia will be able to halt the progression and potentially to normalize some of the central nervous system abnormalities associated with T1D, thus confirming that diabetes complications are not irreversible.
RESEARCH DESIGN AND METHODS
We enrolled 38 patients with T1D, admitted to our center for posttransplantation routine analysis and with at least 3 years of follow-up, to be scanned via MRI and MRS. Fifteen patients were on hemodialysis (ESRD plus T1D), 9 had received a KD transplantation, and 14 had a simultaneous KP transplantation. Eight normal volunteers of similar age and sex (control subjects) were studied as well. Infectious diseases, septic states, elevated inflammatory markers, systemic immunologic conditions, and history of cerebrovascular disease (either transitory ischemic attack or stroke) were considered exclusion criteria for participation in the study. The general characteristics of our participants, divided by group, are shown in Table 1.
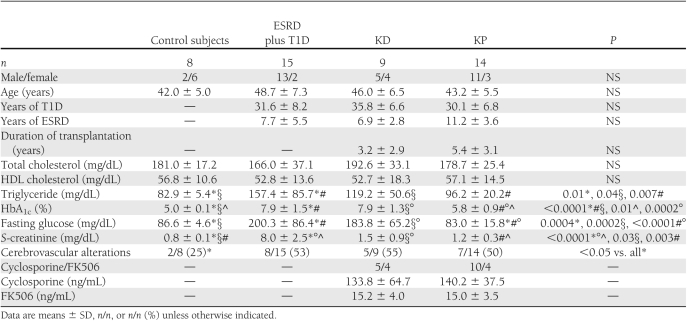
Table 1.
Demographic and metabolic characteristics of our patients
Immunosuppression
Organs for transplantation were obtained from cadaveric donors through the organ procurement agency Nord Italia Transplant. The KD and KP groups were similar for human leukocyte antigen matches, pretransplant panels of reactive antibodies, cold ischemia time, rates of kidney rejection, cytomegalovirus infection, donor age, and rate of lymphoproliferative disease posttransplant. Patients received the following immunosuppressive treatment: antithymoglobulin [IMTIX SANGSTAT (UK)], cyclosporine (target blood trough levels between 100 and 250 ng/mL), or FK506 (target blood levels between 10 and 15 ng/mL), mofetilmycophenolate (0.5–2 mg/day), and prednisone (5–10 mg/day). Steroids were tapered and then withdrawn within 6 months after transplant.
Magnetic resonance protocols
MRI and 1H MRS were performed using a 1.5T scanner (Vision; Siemens, Erlangen, Germany), with a head-dedicated coil. During a single session, the following sequences were obtained:
Axial dual-echo turbo-spin echo proton density and T2-weighted images (repetition time [TR] 2,600 ms, echo time [TE] 14/96 ms, flip angle 180°, 20 contiguous 5-mm thick axial slices, 200 × 512 matrix, field of view (FOV) 240 mm, two acquisitions, scan time 3 min and 34 s).
Axial fluid-attenuated inversion recovery images (TR 9,999 ms, TE 105 ms, flip angle 180°, 20 slices, 5-mm slice thickness, 0.30 cm gap, 182 × 356 matrix, FOV 240 mm, one acquisition, scan time 4 min and 29 s).
Axial spin echo T1-weighted images (TR 600 ms, TE 14 ms, flip angle 70°, 20 slices, 5-mm slice thickness, 0.20 cm gap, 173 × 256 matrix, FOV 240 mm, one acquisition, scan time 1 min and 47 s).
Coronal spin echo T1-weighted images (TR 600 ms, TE 14 ms, flip angle 70°, 20 slices, 5-mm slice thickness, 0.20 cm gap, 173 × 256 matrix, FOV 240 mm, one acquisition, scan time 1 min and 47 s).
The entire pool of images was analyzed by an experienced neuroradiologist to detect the presence of white matter lesions suggesting a process of leukoaraiosis on the basis of micro- and macroangiopathy during the clinical course of diabetes (18). A diagnosis of cerebrovascular disease was formulated according to the literature (12). T1 axial images of each participant were transferred to an off-line workstation (Sun Sparcstation; Sun Microsystem, Mountain View, CA) for brain volume assessment performed by a single observer, who was unaware of the identity and group of the study participants. This procedure used a segmentation technique based on a signal intensity thresholding and characterized by high intrarater reproducibility (13).
Brain volume was measured from T1-weighted images via use of a seed-growing technique for brain tissue segmentation. This method is based on signal-intensity thresholding. A seed point was positioned in different parts of cerebral tissue, and from this seed, a region of interest (ROI) was grown. This ROI contained all connected pixels within two given signal-intensity values. The upper and lower signal intensity for seed growing could be interactively changed on a section-by-section basis. If the ROI crossed the border of interest, a manual boundary was drawn to limit the seed growing. This procedure was particularly useful in some anatomical regions such as in the frontal-basal regions or in the polar portion of temporal lobes where brain tissue is close to the bone or when the exclusion of ventricular plexus and cranial nerves is needed (e.g., optic nerves and optic tracts, which are close to the parenchyma). The process was repeated for each slice until the entire brain volume was covered from the bulb to the vertex. At the end of the segmentation process, tissue volume was calculated by multiplying the number of pixels included in the ROI for the voxel size. All brain-volume measurements were done by the same operator, reducing intraindividual error and giving good accuracy (13).
Single-voxel spectroscopy magnetic resonance was performed positioning a single voxel in the bifrontal cortex, in its first circumvolution, including gray and white matter, with its posterior border at the level of the precentral gyrus. The dimensions of the voxel were 40 × 30 × 20 mm (anteroposterior × right-left × craniocaudal) (Fig. 1B), with TE 135 ms, 256 acquisitions, and scan time 6 min and 31 s. The position of the voxel was case by case adjusted by the operator, giving particular attention to avoid as much as possible contamination by cerebrospinal fluid. Spectra were separately analyzed on the magnetic resonance scanner toolbox by the same operator regardless of to whom they belonged. Cho, creatinine, and NAA peaks were calculated and then their integral function was solved by automatic and manual measurement. Results were expressed as ratios: NAA to creatinine, Cho to creatinine, and NAA to Cho.
Figure 1.
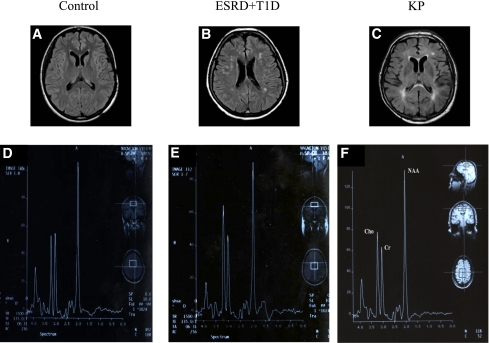
Axial fluid-attenuated inversion recovery acquisitions showing hyperintense lesions suggestive of chronic cerebrovascular disease in a control subject (A), an ESRD plus T1D patient (B), and a KP-transplanted patient (C). Single-voxel 1H MRS brain examination shows Cho, total creatine (Cr), and NAA spectra in a control subject (D), in an ESRD plus T1D patient (E), and in a KP patient (F). (A high-quality color representation of this figure is available in the online issue.)
WBNAA spectroscopy was performed using the following parameters: TR 10,000 ms, TE 15 ms, FOV 240 mm, 8 acquisitions, and acquisition time 2 min and 41 s. Five consecutive measurements were obtained from each subject in order to get the best quality spectra, as previously described by Gonen and Grossman (14). Consequently, the whole scanning time of not-localized MRS became 13 min and 25 s. Spectra were sent to an off-line workstation and analyzed by the same operator to avoid interindividual variability, regardless of to whom they belonged. IDL 5.3 WBNAA software (Research Systems, Boulder, CO) was used to process the spectra and calculate NAA peak. To get an absolute amount of the entire cerebral NAA, it was necessary to scale NAA peak integral against the one of a standard referee. The latter is represented by a plexiglass phantom of spherical shape, filled with water in which a solution of a known concentration of NAA (15 mmol/L, corresponding to 2.63 g) and NaCl (130 mmol/L, corresponding to 7.6 g) is solved. As the acquisition of the whole group of transplanted and untransplanted ESRD plus T1D and control subjects took a long period and corruption of the phantom was possible, more than one phantom was obtained and measured. Each pathologic patient and each control subject were then paired with the closest in-time phantom measurement. It was then possible to calculate the absolute NAA concentration (AbsNAA) using the following equation, as stated by Gonen and Grossman: AbsNAA (millimoles per liter) = 15 × [(Voltp/Voltf) × (Sp/Sf)], where Voltp and Voltf, respectively, are patient/subject and phantom voltage and Sp and Sf, respectively, are patient/subject and phantom NAA peak integral. To correct for interindividual variations in brain size, AbsNAA of each patient and healthy subject was divided by brain volume, obtained, as described above, using a seed-growing technique based on signal-intensity thresholding. The following equation was used to get an absolute brain NAA concentration ([NAA]): [NAA] (millimoles per liter) = AbsNAA/Vp, where Vp is brain volume.
Psychological and neuropsychological tests
Psychological assessment focused on emotional patterns and quality of life (QoL) of patients. The Profile of Mood State (POMS) was used for measuring present mood states and disturbance symptoms, dissecting six dimensions of mood including tension-anxiety (POMS-T), depression-dejection (POMS-D), anger-hostility (POMS-A), vigor-activity (POMS-V), fatigue-inertia (POMS-S), and confusion-bewilderment (POMS-C) (19). The questionnaire consisted of 65 adjectives on a five-point scale, ranging from “not at all” to “extremely,” with which the patients indicated their mood over the past week. To assess the QoL of patients, the LEIPAD test was administered (20). Forty-nine self-assessment items, 31 of which were grouped into seven subscales such as physical function, self-care, depression and anxiety, cognitive functioning, social functioning, sexual functioning, and life satisfaction, were administered to the patients; the remaining 18 items served as moderators for assessing the influence of social desirability factors and personality characteristics on the seven scores obtained previously.
Neuropsychological evaluation was carried out, in one session, examining childhood intellectual failures, global cognitive efficiency, language, attention, reasoning, and verbal/visuospatial memory. History of psychiatric disorders and childhood intellectual failures were considered exclusion criteria for joining the study, and an intelligence test was used to exclude primary cognitive deficiencies. The Mini Mental State Examination was used for analyzing patients’ global cognitive abilities (21). Language functioning was evaluated with token and word fluency tests (22). Attention abilities were analyzed with the Stroop test, which evaluated selective attention and inhibitory ability, and the Paced Auditory Serial Addition Test (PASAT), which evaluated sustained attention and the speed of information processing (23,24). Verbal and visual short-term memory function was assessed through verbal span and Corsi span test, while verbal long-term memory was assessed with short story recall (25). Finally, abstract reasoning and categorization abilities were evaluated with the Wisconsin card-sorting test (48-card form) (26).
Statistical analysis
Continuous variables are presented as means ± SD for variables with a normal distribution and as median (interquartile range) for variables without a normal distribution. Categorical variables are presented as proportions (95% CI). Continuous variables with a normal distribution were compared using the two-tail Student t test for unpaired data (two groups) or one-way ANOVA (three or more groups). Continuous variables without a normal distribution were compared using the Mann-Whitney U test (two groups) or the Kruskall-Wallis test (three or more groups). Categorical variables were compared using the χ2 test if the number of observations in each cell was five or more or Fisher’s exact test if the number of observations in any cell was less than five. A P value <0.05 was considered statistically significant. CI was estimated using the exact binomial. Data analysis was performed using Stata, version 11.1 (StataCorp, College Station, TX).
RESULTS
Characteristics of study participants
The general characteristics of our participants, divided by group, are shown in Table 1. The four groups were similar for sex and age, and no major differences in T1D and ESRD duration were found. KD and KP patients had similar follow-up time after organ transplantation and were not different for antihypertensive or antidislipidemic therapies (Table 1) (data not shown). ESRD plus T1D and KD groups were on chronic insulin therapy, while the KP group conserved an optimal glucometabolic control (Table 1) (data not shown). As expected on the basis of the selection criteria, fasting glucose (control subjects 86.6 ± 4.6 mg/dL, ESRD plus T1D 200.3 ± 86.4 mg/dL, KD 183.8 ± 65.2 mg/dL, and KP 83.0 ± 15.8 mg/dL; control subjects vs. ESRD plus T1D/KD, P < 0.001; KP vs. ESRD plus T1D/KD, P < 0.0001) and HbA1c (control subjects 5.0 ± 0.1%, ESRD plus T1D 7.9 ± 1.5%, KD 7.9 ± 1.3%, and KP 5.8 ± 0.9%; control subjects vs. all, P ≤ 0.01; KP vs. ESRD plus T1D/KD, P < 0.001) levels were significantly lower in the control subjects and KP group than in the ESRD plus T1D and KD groups (Table 1). Serum creatinine was significantly higher in the ESRD plus T1D group than in the control, KD, and KP groups (control subjects 0.8 ± 0.1 mg/dL, ESRD plus T1D 8.0 ± 2.5 mg/dL, KD 1.5 ± 0.9 mg/dL, and KP 1.2 ± 0.3 mg/dL; ESRD plus T1D vs. all, P < 0.0001; control subjects vs. KD/KP, P < 0.05) and triglycerides were significantly higher in the ESRD plus T1D group than in the control, KD, and KP groups (control subjects 82.9 ± 5.4 mg/dL, ESRD plus T1D 157.4 ± 85.7 mg/dL, KD 119.2 ± 50.6 mg/dL, and KP 96.2 ± 20.2 mg/dL; control subjects vs. ESRD plus T1D/KD, P < 0.05; ESRD plus T1D vs. KP, P = 0.007) (Table 1).
Conventional MRI
Cerebral volume.
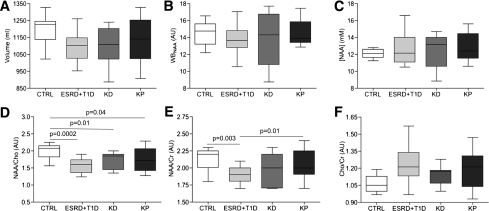
Mean cerebral volume was similar between the four groups of participants, although slightly higher brain volumes were found in the KP-transplanted subjects compared with ESRD plus T1D and KD patients (control subjects 1,197.0 ± 98.2 mL, ESRD plus T1D 1,099.0 ± 88.2 mL, KD 1,096.0 ± 111.6, and KP 1,135.0 ± 128.3 mL; not significant) (Figs. 1A–C and 2A).
Figure 2.
MRI and 1H MRS evaluation were performed in the four groups of patients. No differences between groups were evident for brain volume (A), WBNAA (B), and [NAA] (C). NAA/Cho (D) and NAA/creatinine (Cr) (E) were significantly lower in ESRD plus T1D patients compared with control (CTRL) subjects and were near normalized in KP- but not KD-transplanted patients. The Cho-to-creatinine ratio is shown as well (F).
Chronic cerebrovascular disease.
On the basis of the conventional MRI, among patients with diabetes 20 of 38 had manifestations related to chronic cerebrovascular disease compared with 2 of 8 control subjects (ESRD plus T1D 53% [95% CI 36–69] vs. control subjects 25% [3–6]; P = 0.04) (Fig. 1A–C and Table 1), without major differences between ESRD plus T1D and KD/KP (Table 1).
MRS
Localized MRS–single-voxel spectroscopy.
A low NAA-to-Cho ratio (which is an index of neuroaxonal loss or damage associated with gliosis) (27,28) was found among ESRD plus T1D, KD, and KP groups compared with control subjects (control subjects 2.0 ± 0.2 arbitrary units [AU], ESRD plus T1D 1.6 ± 0.6 AU, KD 1.7 ± 0.2 AU, and KP 1.7 ± 0.3 AU; control subjects vs. all, P < 0.05) (Figs. 1D–F and 2D). The NAA-to-creatinine ratio, suggesting neuronal loss or damage when decreased (29), was significantly lower in the ESRD plus T1D group compared with the control subjects and KP group (control subjects 2.1 ± 0.2 AU, ESRD plus T1D 1.9 ± 0.1 AU, KD 1.9 ± 0.2 AU, and KP 2.0 ± 0.2 AU; ESRD plus T1D vs. control subjects/KP, P ≤ 0.01) (Figs. 1D–F and 2E). Finally, the Cho-to-creatinine ratio, which has previously been found to be higher in patients with diabetes-related brain gliosis (30), was similar between groups (Figs. 1D–F and 2F). An inverse linear correlation between HbA1c and NAA/creatinine regardless of kidney function was found (r = −0.353, P < 0.03) (data not shown).
Nonlocalized MRS (WBNAA spectroscopy).
NAA is a neuronal marker, and its total concentration decrease indicates neuroaxonal loss/impairment (15,16). In our sample, WBNAA mean content was similar in all groups (control subjects 14.5 ± 1.5 AU, ESRD plus T1D 13.8 ± 1.6 AU, KD 13.8 ± 3.2 AU, and KP 14.5 ± 1.4 AU; not significant) (Figs. 1D–F and 2B). AbsNAA was similar in the four groups of participants (data not shown), and overlapping results were found as well for [NAA] when AbsNAA values were corrected for subjects’ brain volume to adjust for interindividual variations (control subjects 12.1 ± 0.6 AU, ESRD plus T1D 12.6 ± 1.8 AU, KD 12.5 ± 2.0 AU, and KP 12.9 ± 1.6 AU; not significant) (Figs. 1D–F and 2C).
Psychological and neuropsychological assessment
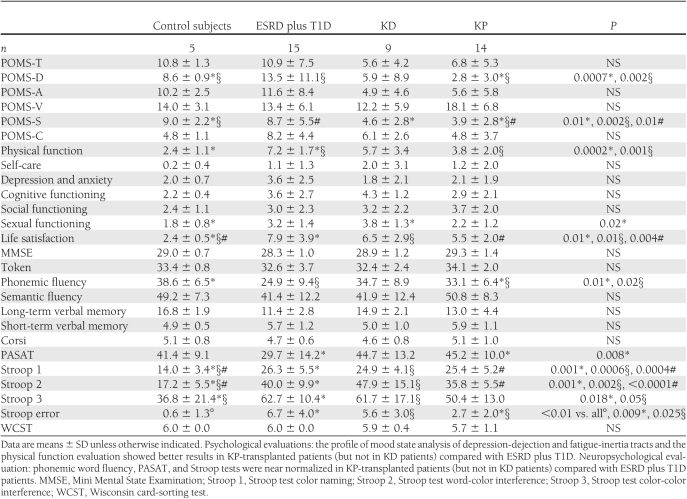
The psychological assessment evaluated the emotional axis and QoL of patients through POMS and LEIPAD tests, respectively (19,20). The POMS test, examining six different dimensions of mood, revealed a significant higher score for POMS-D between control subjects and KD patients and a significantly lower score in ESRD plus T1D compared with KP subjects (control subjects 8.6 ± 0.9, ESRD plus T1D 13.5 ± 11.1, KD 5.9 ± 8.9, and KP 2.8 ± 3.0; control subjects vs. KD, P = 0.0007; ESRD plus T1D vs. KP, P = 0.002) (Table 2). POMS-S showed a significant difference among control subjects and KD and KP patients and in ESRD plus T1D compared with KP subjects (control subjects 9.0 ± 2.2, ESRD plus T1D 8.7 ± 5.5, KD 4.6 ± 2.8, and KP 3.9 ± 2.8; control subjects vs. KD/KP, P < 0.01; ESRD plus T1D vs. KP, P = 0.01). No differences in POMS-T, -A, -V and -C were detected in our sample (Table 2). The LEIPAD test revealed a significantly lower score (where lower is better) for physical function in control subjects compared with ESRD plus T1D subjects, while transplanted patients (KD and KP) showed a lower score compared with ESRD plus T1D patients (control subjects 2.4 ± 1.1, ESRD plus T1D 7.2 ± 1.7, KD 5.7 ± 3.4, and KP 3.8 ± 2.0; ESRD plus T1D vs. control subjects/KP, P ≤ 0.001) (Table 2). The sexual function index was found to be significantly lower (where higher is better) in control subjects compared with KD patients (control subjects 1.8 ± 0.8, ESRD plus T1D 3.2 ± 1.4, KD 3.8 ± 1.3, and KP 2.2 ± 1.2; control subjects vs. KD, P = 0.02) (Table 2). Finally, life satisfaction score was significantly lower (where lower is better) in control subjects compared with ESRD, KD, and KP patients (control subjects 2.4 ± 0.5, ESRD plus T1D 7.9 ± 3.9, KD 6.5 ± 2.9, and KP 5.5 ± 2.0; control subjects vs. all, P < 0.01) (Table 2). Other scores showed a general better trend in KP-transplanted patients compared with ESRD plus T1D and KD patients (Table 2). Patients' neuropsychological evaluation confirmed that KP and KD subjects experienced benefits from transplantation compared with ESRD plus T1D patients in almost the whole pool of tests (Table 2). Particularly, KP (but not KD) patients obtained better results in phonemic word fluency and the PASAT test, respectively, compared with results in ESRD plus T1D patients (control subjects 38.6 ± 6.5, ESRD plus T1D 24.9 ± 9.4, KD 34.7 ± 8.9, and KP 33.1 ± 6.4; ESRD plus T1D vs. control subjects/KP, P < 0.05; control subjects 41.4 ± 9.1, ESRD plus T1D 29.7 ± 14.2, KD 44.7 ± 13.2, and KP 45.2 ± 10.0; ESRD plus T1D vs. KP, P = 0.008) (Table 2). The color naming (control subjects 14.0 ± 3.4, ESRD plus T1D 26.3 ± 5.5, KD 24.9 ± 4.1, and KP 25.4 ± 5.2; control subjects vs. all, P ≤ 0.001) and the word-color interference variants of the Stroop test (control subjects 17.2 ± 5.5, ESRD plus T1D 40.0 ± 9.9, KD 47.9 ± 15.1, and KP 35.8 ± 5.5; control subjects vs. all; P < 0.01) were higher (i.e., worse) in ESRD plus T1D, KD, and KP patients compared with control subjects (Table 2). The color-color interference Stroop test showed significantly lower scores (where lower is worse) in ESRD plus T1D and KD patients compared with control subjects (control subjects 36.8 ± 21.4, ESRD plus T1D 62.7 ± 10.4, KD 61.7 ± 17.1, and KP 50.4 ± 13.0; control subjects vs. ESRD plus T1D/KD, P ≤ 0.05) (Table 2). A valuation of the number of errors made by the patients while performing the three sections of the Stroop test showed a significantly lower number of errors in control and KP subjects compared with ESRD plus T1D and KD subjects (control subjects 0.6 ± 1.3, ESRD plus T1D 6.7 ± 4.0, KD 5.6 ± 3.0, and KP 2.7 ± 2.0; control subjects vs. all, P < 0.01; ESRD plus T1D vs. KP, P = 0.009; KD vs. KP, P = 0.025) (Table 2).
Table 2.
Psychological and neuropsychological evaluations
Correlations
A linear correlation between HbA1c and NAA/creatinine was evident in the entire cohort of patients regardless of kidney function (r = −0.353, P < 0.03) (Supplementary Fig. 1, available in the Supplementary Data).
CONCLUSIONS
The pathogenesis of cerebral disorders in T1D is still unclear but is thought to be multifactorial, involving chronic hyperglycemia and recurrent hypoglycemia, which has been proved to cause neuronal loss (31). In ESRD plus T1D patients, KP transplantation is an effective treatment in increasing the QoL and in reducing diabetes-related mortality and morbidity (17,27,28). We analyzed morphological, metabolic, and functional features of the central nervous system in 15 ESRD plus T1D, 9 KD, and 14 KP patients and 8 control subjects. First, we performed MRIs, showing the higher prevalence of chronic cerebrovascular disease in the population of patients with ESRD plus T1D. No major differences were observed from a morphological point of view between transplanted patients and ESRD plus T1D patients as percentage of vasculopathy or brain volume. However, through analysis of the relative levels of NAA, Cho, and creatinine and their ratios through localized single-voxel spectroscopy, NAA-to-Cho and NAA-to-creatinine ratios were shown to be significantly lower in ESRD plus T1D and transplanted patients compared with control subjects. A reduced NAA-to-Cho ratio, as we found in our diabetic population, which results from the presence of elevated Cho-containing compounds or a reduced NAA resonance, could be interpreted as an index of neuroaxonal loss/impairment associated with diabetes-related gliosis (29,30). The NAA-to-creatinine ratio is an index of neuronal loss or damage (29,30). Interestingly, only KP patients—not KD patients—had NAA-to-creatinine values higher than ESRD plus T1D patients, suggesting a potential reversible effect or role exerted by combined KP transplantation in improving cerebral metabolic function in the clinical course of diabetes-related encephalopathy. Finally, we verified that our spectroscopic findings were correlated with signs of psychological and neuropsychological impairment. We found a significant improvement in mood profile, physical function, phonemic fluency, and attention abilities in KP, but not KD, subjects compared with ESRD plus T1D patients. Moreover, control subjects and KP-transplanted patients showed a generally more conserved psychological and neuropsychological attitude compared with KD-transplanted and ESRD plus T1D patients. Diabetes and hyperglycemia are implicated in the genesis of early cognitive decline and possibly dementia owing to multiple factors such as severe hypoglycemia episodes, glucometabolic disorders, blood-brain barrier damage, and sustained ketoacidotic episodes, being able to reduce NAA/creatinine cerebral content and myelination (9,32,33). Interestingly, HbA1c correlated with the NAA-to-creatinine ratio, a good marker of axonal/neuronal loss, confirming the close relationship between glycometabolic control and brain metabolic abnormalities (19,20). Additionally, ESRD causes the accumulation of uremic toxins and induces encephalopathy and cognitive impairment, with symptoms ranging from mild confusional states and sleep disorders to deep coma (34). Hemodyalitic therapy should be considered a cause as well, being able to induce chronic dialysis encephalopathy (35). By contrast, successful KP transplantation has been shown to provide normoglycemia in the long term and to halt the progression of diabetes complications, reducing the extent of diabetic retinopathy, nephropathy, and encephalopathy (18). Blood pressure, cardiac function, and cholesterol are positively affected by KP transplantation as well (36). Interestingly, we and others have shown that these results were not fully obtained with KD transplantation, confirming that normalizing hyperglycemia is as important as the removal of ESRD. The reason for the reduced life expectancy in patients with poorly controlled T1D and in ESRD plus T1D patients has been studied extensively. Previous work has shown that severe platelet functional abnormalities are evident in patients with ESRD plus T1D patients (37), and this may cause a propensity for cardiovascular and cerebrovascular diseases. Uremia is also characterized by prolonged bleeding time (38) and accelerated vascular calcification that clearly predisposes uremic patients to vascular pathologic events (39). It is interesting to observe that KP transplantation, despite longer dialysis duration, had the most dramatic improvement in brain structure/function. Finally, it is noteworthy that patients who no longer need to inject insulin (e.g., who are using insulin pumps) and have good metabolic control have been shown to have improved mood (40); thus, our observations could partially reflect this rather than a real functional effect on the brain.
Despite the cross-sectional limitation of our study, which renders our observations preliminary and worthy of further confirmation, our results suggest that the brain is another organ damaged by diabetes from a morphologic, metabolic, and functional point of view and that KP transplantation is an effective approach to ameliorating metabolic abnormalities. 1H MRS is a valuable tool for monitoring the morphological and metabolic aspects of the central nervous system in patients with diabetes.
Supplementary Material
Acknowledgments
P.F. is the recipient of a Juvenile Diabetes Research Foundation Career Development Award and an American Society of Nephrology Career Development Award. P.F. acknowledges the support from a pilot and feasibility award from the Boston Area Diabetes Endocrinology Research Center (5P30DK57521) and a Ministero dell'Istruzione, dell'Università e della Ricerca grant (“Staminali” RF-FSR-2008-1213704).
No potential conflicts of interest relevant to this article were reported.
P.F. designed and performed research, analyzed data, wrote the manuscript. P.V., C.G., M.F., F.D., A.V., E.A., R.C., and M.S. performed research. R.B. performed research, wrote the manuscript, and analyzed data. L.C. and A.M. collected data and performed research. G.M. designed and performed research. A.F. designed research. A.S. designed research and edited the manuscript. P.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1697/-/DC1.
References
- 1.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 2.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 3.Jolivalt CG, Hurford R, Lee CA, Dumaop W, Rockenstein E, Masliah E. Type 1 diabetes exaggerates features of Alzheimer’s disease in APP transgenic mice. Exp Neurol 2010;223:422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sima AA. Encephalopathies: the emerging diabetic complications. Acta Diabetol 2010;47:279–293 [DOI] [PubMed] [Google Scholar]
- 5.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004;61:661–666 [DOI] [PubMed] [Google Scholar]
- 6.Franceschi M, Cecchetto R, Minicucci F, Smizne S, Baio G, Canal N. Cognitive processes in insulin-dependent diabetes. Diabetes Care 1984;7:228–231 [DOI] [PubMed] [Google Scholar]
- 7.Ryan C, Vega A, Longstreet C, Drash A. Neuropsychological changes in adolescents with insulin-dependent diabetes. J Consult Clin Psychol 1984;52:335–342 [DOI] [PubMed] [Google Scholar]
- 8.Pozzessere G, Rizzo PA, Valle E, et al. Early detection of neurological involvement in IDDM and NIDDM. Multimodal evoked potentials versus metabolic control. Diabetes Care 1988;11:473–480 [DOI] [PubMed] [Google Scholar]
- 9.Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol 2002;441:1–14 [DOI] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 11.O’Brien JT, Paling S, Barber R, et al. Progressive brain atrophy on serial MRI in dementia with Lewy bodies, AD, and vascular dementia. Neurology 2001;56:1386–1388 [DOI] [PubMed] [Google Scholar]
- 12.Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995;26:1171–1177 [DOI] [PubMed] [Google Scholar]
- 13.Rovaris M, Filippi M, Calori G, et al. Intra-observer reproducibility in measuring new putative MR markers of demyelination and axonal loss in multiple sclerosis: a comparison with conventional T2-weighted images. J Neurol 1997;244:266–270 [DOI] [PubMed] [Google Scholar]
- 14.Gonen O, Grossman RI. The accuracy of whole brain N-acetylaspartate quantification. Magn Reson Imaging 2000;18:1255–1258 [DOI] [PubMed] [Google Scholar]
- 15.Ross B, Michaelis T. Clinical applications of magnetic resonance spectroscopy. Magn Reson Q 1994;10:191–247 [PubMed] [Google Scholar]
- 16.Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 1991;4:47–52 [DOI] [PubMed] [Google Scholar]
- 17.Fiorina P, Perseghin G, De Cobelli F, et al. Altered kidney graft high-energy phosphate metabolism in kidney-transplanted end-stage renal disease type 1 diabetic patients: a cross-sectional analysis of the effect of kidney alone and kidney-pancreas transplantation. Diabetes Care 2007;30:597–603 [DOI] [PubMed] [Google Scholar]
- 18.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998;339:69–75 [DOI] [PubMed] [Google Scholar]
- 19.Albrecht RR, Ewing SJ. Standardizing the administration of the Profile of Mood States (POMS): development of alternative word lists. J Pers Assess 1989;53:31–39 [DOI] [PubMed] [Google Scholar]
- 20.De Leo D, Diekstra RF, Lonnqvist J, et al. LEIPAD, an internationally applicable instrument to assess quality of life in the elderly. Behav Med 1998;24:17–27 [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 22.DeRenzi E. The Token Test: a sensitive test to detect receptive disturbance in aphasia. Brain 1962;65:665–678 [DOI] [PubMed] [Google Scholar]
- 23.Cohn NB, Dustman RE, Bradford DC. Age-related decrements in Stroop Color Test performance. J Clin Psychol 1984;40:1244–1250 [DOI] [PubMed] [Google Scholar]
- 24.Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch Clin Neuropsychol 2006;21:53–76 [DOI] [PubMed] [Google Scholar]
- 25.White RF, Gerr F, Cohen RF, et al. Criteria for progressive modification of neurobehavioral batteries. Neurotoxicol Teratol 1994;16:511–524 [DOI] [PubMed] [Google Scholar]
- 26.Feldstein SN, Keller FR, Portman RE, Durham RL, Klebe KJ, Davis HP. A comparison of computerized and standard versions of the Wisconsin Card Sorting Test. Clin Neuropsychol 1999;13:303–313 [DOI] [PubMed] [Google Scholar]
- 27.Fiorina P, Folli F, D’Angelo A, et al. Normalization of multiple hemostatic abnormalities in uremic type 1 diabetic patients after kidney-pancreas transplantation. Diabetes 2004;53:2291–2300 [DOI] [PubMed] [Google Scholar]
- 28.Larsen JL, Stratta RJ, Ozaki CF, Taylor RJ, Miller SA, Duckworth WC. Lipid status after pancreas-kidney transplantation. Diabetes Care 1992;15:35–42 [DOI] [PubMed] [Google Scholar]
- 29.Lai PH, Chen PC, Chang MH, et al. In vivo proton MR spectroscopy of chorea-ballismus in diabetes mellitus. Neuroradiology 2001;43:525–531 [DOI] [PubMed] [Google Scholar]
- 30.Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes 2010;59:236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCall AL. Diabetes mellitus and the central nervous system. Int Rev Neurobiol 2002;51:415–453 [DOI] [PubMed] [Google Scholar]
- 32.Wootton-Gorges SL, Buonocore MH, Caltagirone RA, Kuppermann N, Glaser NS. Progressive decrease in N-acetylaspartate/Creatine ratio in a teenager with type 1 diabetes and repeated episodes of ketoacidosis without clinically apparent cerebral edema: evidence for permanent brain injury. AJNR Am J Neuroradiol 2010;31:780–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlassara H, Brownlee M, Cerami A. Excessive nonenzymatic glycosylation of peripheral and central nervous system myelin components in diabetic rats. Diabetes 1983;32:670–674 [DOI] [PubMed] [Google Scholar]
- 34.Seifter JL, Samuels MA. Uremic encephalopathy and other brain disorders associated with renal failure. Semin Neurol 2011;31:139–143 [DOI] [PubMed] [Google Scholar]
- 35.Geissler A, Fründ R, Kohler S, Eichhorn HM, Krämer BK, Feuerbach S. Cerebral metabolite patterns in dialysis patients: evaluation with H-1 MR spectroscopy. Radiology 1995;194:693–697 [DOI] [PubMed] [Google Scholar]
- 36.Luan FL, Miles CD, Cibrik DM, Ojo AO. Impact of simultaneous pancreas and kidney transplantation on cardiovascular risk factors in patients with type 1 diabetes mellitus. Transplantation 2007;84:541–544 [DOI] [PubMed] [Google Scholar]
- 37.Vicari AM, Taglietti MV, Pellegatta F, et al. Deranged platelet calcium homeostasis in diabetic patients with end-stage renal failure. A possible link to increased cardiovascular mortality? Diabetes Care 1996;19:1062–1066 [DOI] [PubMed] [Google Scholar]
- 38.Mannucci PM, Remuzzi G, Pusineri F, et al. Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med 1983;308:8–12 [DOI] [PubMed] [Google Scholar]
- 39.Brancaccio D, Bellasi A, Cozzolino M, Galassi A, Gallieni M. Arterial accelerated aging in dialysis patients: the clinical impact of vascular calcification. Curr Vasc Pharmacol 2009;7:374–380 [DOI] [PubMed] [Google Scholar]
- 40.Wolf FM, Jacober SJ, Wolf LL, Cornell RG, Floyd JC., Jr Quality of life activities associated with adherence to insulin infusion pump therapy in the treatment of insulin dependent diabetes mellitus. J Clin Epidemiol 1989;42:1129–1136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.