Abstract
OBJECTIVE
Gain-of-function ABCC8/sulfonylurea (SU) receptor 1 mutations cause neonatal diabetes mellitus (NDM) or late-onset diabetes in adult relatives. Given the effectiveness of SU treatment in ABCC8-NDM patients, we further characterized late-onset ABCC8-associated diabetes.
RESEARCH DESIGN AND METHODS
Seven adult subjects from three NDM families and one family with type 2 diabetes were studied. Insulin secretion and insulin sensitivity were assessed using clamp techniques. We screened 139 type 2 diabetic patients who were well controlled by SU for ABCC8 mutations.
RESULTS
ABCC8 mutation carriers exhibited glucose intolerance, frank diabetes, or insulin-requiring diabetes since diagnosis. HbA1c improved in five SU-treated patients. Insulin secretion capacity was impaired in three patients compared with adult control subjects but was restored after a 4-week SU trial in two patients. Cohort screening revealed four SU-treated patients with ABCC8 mutations, two of which are likely causal.
CONCLUSIONS
Although of rare occurrence, recognition of adult-onset ABCC8-associated diabetes may help in targeting patients for SU therapy.
Activating ABCC8 mutations are responsible of neonatal diabetes mellitus (NDM) (1–5) and late-onset diabetes with variable clinical phenotypes (2,4,6,7). Sulfonylurea (SU) drugs bind the high-affinity pancreatic β-cell–expressed sulfonylurea receptor (SUR 1) (encoded by ABCC8) of the ATP-sensitive K+ channels (KATP channels) and close them, subsequently stimulating insulin secretion (8). SU treatment may successfully replace insulin to control diabetes during the neonatal period (2,9,10).
Here, we report detailed clinical and metabolic investigations in seven adult carriers of gain-of-function ABCC8 mutations (2,7,11), of whom five developed late-onset diabetes diagnosed between the ages of 14 and 39 years. We also screened an adult outpatient cohort with type 2 diabetes well controlled by SU for ABCC8 mutations.
RESEARCH DESIGN AND METHODS
Two populations were studied. A family-based study included four patients with adulthood developing-onset diabetes, who were relatives of a child with NDM because of a previously identified ABCC8 mutation (2,11), and one adult patient and his two children who carry the same ABCC8 mutation (7) (Table 1). From an adult type 2 diabetic outpatient cohort, 139 patients who were well controlled with SU were analyzed for ABCC8 sequencing (Supplementary Data). Written, informed consent was obtained from all patients
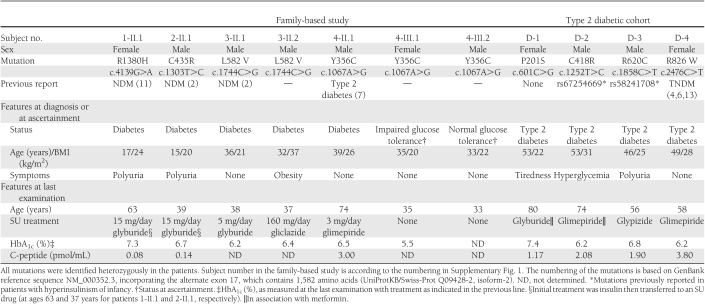
Table 1.
Main characteristics of the adult carriers of an ABCC8 mutation identified from the family-based study and from a type 2 diabetic patient cohort
ABCC8 gene sequencing
All ABCC8 exons and flanking intron-exon boundaries were amplified from a genomic DNA sample and directly sequenced by double-stranded Sanger sequencing, as previously described (2). Each mutation identified from the reference sequence, NM_000352.3, was confirmed by resequencing the original sample. The frequency of two mutations was assessed in a French general population by using the high-resolution melting genotyping method (Supplementary Data).
Metabolic studies
Insulin secretion was evaluated during oral or intravenous glucose tolerance tests and by graded glucose infusion and arginine tests (12). Insulin sensitivity was assessed using a euglycemic-hyperinsulinemic clamp (Supplementary Data).
RESULTS
Seven subjects from four families (Supplementary Fig. 1) were studied: five patients with diabetes, of whom two were successfully switched from insulin to SU, one subject presenting with glucose intolerance and one with normal glucose tolerance. Their clinical and genetic characteristics are shown in Table 1.
During the graded glucose infusion, the four investigated patients had a low insulin secretion rate (ISR) at the 10 mmol/L plateau; at the 15 mmol/L glucose level, two patients remained in the low to normal range, whereas two other subjects exhibited a normal ISR (Supplementary Fig. 2A). Three of four patients studied responded normally to the arginine test (Supplementary Table 1). In two patients, early insulin response (EIR) after the oral glucose tolerance test was blunted or low compared with control subjects. In two other patients who underwent the intravenous glucose tolerance test, the acute insulin response to glucose was blunted, whereas after a 4-week SU treatment (7.5 and 15 mg/day glyburide, respectively), it increased by 2.5- and 22-fold, respectively (Supplementary Fig. 2B). The glucose disposal rate (or M value) was low in two patients, and the derived insulin sensitivity index was comparable with control subjects, except in one obese patient (Supplementary Table 1). Finally, the disposition index was low in the glucose-intolerant patient compared with control subjects (Supplementary Table 1).
Four patients with adult-onset type 2 diabetes from the cohort study carried a heterozygote missense ABCC8 mutation (Table 1). Three mutations were previously reported (c.2476C>T/p.R826W in transient NDM [TNDM] (3,4,13), c.1252T>C/p.C418R and c.1858C>T/p.R620C in congenital hyperinsulinism [CHI] (14,15)), and one mutation is novel (c.601C>G/p.P201S). No relatives of these patients were available for mutation testing. The P201S mutation that lies at a highly conserved residue across mammalian species, in the L0-linker loop of the TMD0-L0 gatekeeper module of SUR1 (16), was not present in 330 normoglycemic French subjects. TMD0-L0 was proposed to transduce Mg-nucleotide stimulation and high-affinity SU inhibition from the SUR1 core to the Kir6.2 pore (17). Genotyping of C418R and R620C mutations in >4,000 normoglycemic individuals from the French Data from an Epidemiological Study on the Insulin Resistance Syndrome (D.E.S.I.R.) cohort (18) identified five and one carrier, respectively, suggesting that they may represent rare variants (population carrier frequency of 0.06 and 0.012%) likely not related to disease.
CONCLUSIONS
Our study strengthens the findings that ABCC8 mutations cause variable clinical phenotypes with glucose intolerance, overt diabetes, or insulin-requiring diabetes from a young age to adulthood. Several factors may explain this variability, also seen within families, such as the type and location of the mutation itself or other modifier genetic and superimposed environmental factors (2,6). Impaired insulin secretion in response to glucose is a major metabolic feature in adult ABCC8 mutation carriers, whereas insulin secretion in response to arginine is relatively preserved in keeping with KATP channel–independent mechanisms for insulin exocytosis. As expected, SU treatment as a monotherapy provided good glycemic control in five adult diabetic patients, in accordance with previous reports in children (2,9,10).
A pathogenic role for three mutations (C435R, L582V, and R1380H) is very likely, because NDM was diagnosed in family relatives (this study) or reported by other studies (2,4,11). The previously published Y356C and R826W mutations (4,6,7,13) were functionally demonstrated to alter MgATP sensitivity or ATPase activity of SUR1, respectively, leading to KATP channel activation with increased resting whole-cell currents (7,13). We cannot exclude that two rare variants (C418R and R620C), as previously reported in CHI (14,15) and found here in two diabetic patients, are nonfunctional (i.e., nondeleterious). An earlier study reported a dominant form of CHI attributed to the E1507K-ABCC8 mutation, which is responsible for decreased insulin secretory capacity and diabetes in middle age (19).
Identifying an ABCC8 mutation in adult relatives of NDM patients will have significant clinical implications; insulin therapy may be successfully replaced by oral SU in idiopathic type 1 diabetes, and type 2 diabetic patients could be better treated by SU rather than other drugs such as incretins. However, from our study, the occurrence of likely causal ABCC8 mutations in adult-onset type 2 diabetes well-controlled by SU is low (~1–1.5%). Another important issue is genetic counseling in the rest of the family.
Translation of these results into medical practice would be to search for ABCC8 mutations in adult patients with a family history of NDM or hyperinsulinism of infancy and in type 2 diabetic patients who are not overweight but have a dominant pattern of diabetes inheritance (patients with unexplained maturity-onset diabetes of the young). With the progress of targeted next-generation sequencing, the recognition of a diabetes genetic subtype is of great interest for genuine personalized medicine.
Supplementary Material
Acknowledgments
The study was supported by an ANR-MRAR (Agence Nationale de la Recherche-Maladies Rares) Research Program (grant no. ANR-07-MRAR-000; to M.P. and M.V.), by a transnational European Research Grant on rare diseases (grant no. ERANET 09 RARE 005; to M.V. and M.P.), by the CERITD (centre d'études et de recherches pour l'intensification du traitement du diabète, Corbeil-Essonnes, France), and by a CPER (Contrat Plan Etat Region) Research Grant from the Region Nord-Pas-de-Calais in France (to M.V. and P.F.).
No potential conflicts of interest relevant to this article were reported.
J.-P.R. and M.V. contributed to the conception and design of the study; acquired, analyzed, and interpreted the data; wrote, reviewed, and edited the manuscript; and are guarantors of this article. E.R. acquired, analyzed, and interpreted the data and reviewed the manuscript. Y.R. contributed to the conception of the study; acquired, analyzed, and interpreted the data; and reviewed the manuscript. S.F., J.P., and A.D. acquired, analyzed, and interpreted the data. A.H. and M.P. contributed to the discussion and reviewed the manuscript. C.P. and G.C. contributed diabetic patients and clinical data and reviewed the manuscript. J.-F.G. contributed healthy control participants, analyzed and interpreted the data, and reviewed the manuscript. P.F. contributed funding and to the discussion of the data and reviewed and edited the manuscript.
The authors thank Marianne Deweirder, Frédéric Allegaert, and Emmanuel Vaillant for DNA biobank management and their technical assistance (all at the Unité Mixte de Recherche 8199, Centre National de la Recherche Scientifique, Lille, France). The authors are grateful to the patients and their families for participating in the study, as well as to Pr. Jean-Jacques Robert (René Descartes Paris University, Necker Enfants Malades Hospital, Paris, France) and Dr. Gilberto Velho (INSERM, Research Unit 695, Paris, France), who contributed to the metabolic explorations performed in the control population, and to Dr. Beverley Balkau (Centre for Research in Epidemiology and Population Health, Villejuif, and Paris-Sud 11 University, Orsay, France), Dr. Jean Tichet (Regional Institute for Health, Tours, France), and Pr. Michel Marre (Department of Endocrinology, Diabetology, and Nutrition, Bichat-Claude Bernard University Hospital, Paris, France) for access to the D.E.S.I.R. samples and prospective data. The authors would also like to thank Pr. José Timsit from the Department of Endocrinology-Diabetology at the Assistance Publique-Hôpitaux de Paris, Cochin Hospital, Paris, France, for his helpful advice and guidance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1469/-/DC1.
References
- 1.Greeley SA, Tucker SE, Naylor RN, Bell GI, Philipson LH. Neonatal diabetes mellitus: a model for personalized medicine. Trends Endocrinol Metab 2010;21:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babenko AP, Polak M, Cavé H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 2006;355:456–466 [DOI] [PubMed] [Google Scholar]
- 3.Vaxillaire M, Dechaume A, Busiah K, et al. ; SUR1-Neonatal Diabetes Study Group New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes 2007;56:1737–1741 [DOI] [PubMed] [Google Scholar]
- 4.Flanagan SE, Patch AM, Mackay DJ, et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 2007;56:1930–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellard S, Flanagan SE, Girard CA, et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet 2007;81:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klupa T, Kowalska I, Wyka K, et al. Mutations in the ABCC8 (SUR1 subunit of the K(ATP) channel) gene are associated with a variable clinical phenotype. Clin Endocrinol (Oxf) 2009;71:358–362 [DOI] [PubMed] [Google Scholar]
- 7.Tarasov AI, Nicolson TJ, Riveline JP, et al. A rare mutation in ABCC8/SUR1 leading to altered ATP-sensitive K+ channel activity and β-cell glucose sensing is associated with type 2 diabetes in adults. Diabetes 2008;57:1595–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 2006;440:470–476 [DOI] [PubMed] [Google Scholar]
- 9.Pearson ER, Flechtner I, Njølstad PR, et al. ; Neonatal Diabetes International Collaborative Group Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 2006;355:467–477 [DOI] [PubMed] [Google Scholar]
- 10.Ashcroft FM. New uses for old drugs: neonatal diabetes and sulphonylureas. Cell Metab 2010;11:179–181 [DOI] [PubMed] [Google Scholar]
- 11.Hartemann-Heurtier A, Simon A, Bellanné-Chantelot C, et al. Mutations in the ABCC8 gene can cause autoantibody-negative insulin-dependent diabetes. Diabetes Metab 2009;35:233–235 [DOI] [PubMed] [Google Scholar]
- 12.Sobngwi E, Boudou P, Mauvais-Jarvis F, et al. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet 2003;361:1861–1865 [DOI] [PubMed] [Google Scholar]
- 13.de Wet H, Proks P, Lafond M, et al. A mutation (R826W) in nucleotide-binding domain 1 of ABCC8 reduces ATPase activity and causes transient neonatal diabetes. EMBO Rep 2008;9:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 1999;20:101–135 [DOI] [PubMed] [Google Scholar]
- 15.Otonkoski T, Näntö-Salonen K, Seppänen M, et al. Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes 2006;55:13–18 [PubMed] [Google Scholar]
- 16.Babenko AP, Bryan J. Sur domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem 2003;278:41577–41580 [DOI] [PubMed] [Google Scholar]
- 17.Bryan J, Vila-Carriles WH, Zhao G, Babenko AP, Aguilar-Bryan L. Toward linking structure with function in ATP-sensitive K+ channels. Diabetes 2004;53(Suppl. 3):S104–S112 [DOI] [PubMed] [Google Scholar]
- 18.Vaxillaire M, Veslot J, Dina C, et al. ; DESIR Study Group Impact of common type 2 diabetes risk polymorphisms in the DESIR prospective study. Diabetes 2008;57:244–254 [DOI] [PubMed] [Google Scholar]
- 19.Huopio H, Otonkoski T, Vauhkonen I, Reimann F, Ashcroft FM, Laakso M. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet 2003;361:301–307 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.