Abstract
OBJECTIVE
To evaluate the changes in circulating endotoxin after a high–saturated fat meal to determine whether these effects depend on metabolic disease state.
RESEARCH DESIGN AND METHODS
Subjects (n = 54) were given a high-fat meal (75 g fat, 5 g carbohydrate, 6 g protein) after an overnight fast (nonobese control [NOC]: age 39.9 ± 11.8 years [mean ± SD], BMI 24.9 ± 3.2 kg/m2, n = 9; obese: age 43.8 ± 9.5 years, BMI 33.3 ± 2.5 kg/m2, n = 15; impaired glucose tolerance [IGT]: age 41.7 ± 11.3 years, BMI 32.0 ± 4.5 kg/m2, n = 12; type 2 diabetic: age 45.4 ± 10.1 years, BMI 30.3 ± 4.5 kg/m2, n = 18). Blood was collected before (0 h) and after the meal (1–4 h) for analysis.
RESULTS
Baseline endotoxin was significantly higher in the type 2 diabetic and IGT subjects than in NOC subjects, with baseline circulating endotoxin levels 60.6% higher in type 2 diabetic subjects than in NOC subjects (P < 0.05). Ingestion of a high-fat meal led to a significant rise in endotoxin levels in type 2 diabetic, IGT, and obese subjects over the 4-h time period (P < 0.05). These findings also showed that, at 4 h after a meal, type 2 diabetic subjects had higher circulating endotoxin levels (125.4%↑) than NOC subjects (P < 0.05).
CONCLUSIONS
These studies have highlighted that exposure to a high-fat meal elevates circulating endotoxin irrespective of metabolic state, as early as 1 h after a meal. However, this increase is substantial in IGT and type 2 diabetic subjects, suggesting that metabolic endotoxinemia is exacerbated after high fat intake. In conclusion, our data suggest that, in a compromised metabolic state such as type 2 diabetes, a continual snacking routine will cumulatively promote their condition more rapidly than in other individuals because of the greater exposure to endotoxin.
Studies examining the interrelationships between adipose tissue, inflammation, and insulin resistance appear key to understanding type 2 diabetes risk (1,2). It is known that low-grade chronic systemic inflammation contributes to this risk, which appears altered by several factors such as increasing age, sex, ethnicity, genetics, and dietary influences. However, systemic inflammation appears to persist in type 2 diabetic subjects, despite medication, while the mechanisms and mediators of this continual inflammation appear less clear. Evidently, adipose tissue accumulation has a significant impact on disease risk and inflammation in type 2 diabetes but may merely act in response to systemic primary insults (3–9).
One potential cellular mechanism for increased inflammation may arise through activation of the innate immune system in human adipose tissue (10–13). Previous studies have shown that increased activation of the innate immune pathway may arise through excess circulating gut-derived bacteria, known as lipopolysaccharide (LPS) or endotoxin, which represents the outer cell wall membrane of gram-negative bacteria (10,11,14–17). Our previous work has shown that endotoxin has an immediate impact on the innate immune pathway in human adipose tissue, acting via key receptors known as the Toll-like receptors, which recognize antigens, such as the LPS component, to initiate an acute-phase response to infection (8,10). Stimulation of the Toll-like receptors leads to intracellular activation of nuclear factor-κB (NF-κB), a key transcription factor in the inflammatory cascade that regulates the transcription of numerous proinflammatory adipokines (9,10). Therefore, in vitro endotoxin may act as a mediator of inflammation through activation of NF-κB, leading to a rapid response within adipose tissue that may be exacerbated by increased adipose tissue mass (18–22).
However, clinical studies have also implicated gut-derived endotoxin as a “primary insult” to activate the inflammatory state, contributing to metabolic disease, with current cross-sectional data showing elevated systemic endotoxin levels in conditions of obesity, type 2 diabetes, coronary artery disease, and fatty liver disease (8,10,11,14–17). Within these studies, circulating endotoxin is observed to be positively associated with waist circumference, waist-to-hip ratio, insulin levels, inflammatory cytokines and lipids, including total cholesterol, triglycerides (TGs), and LDL cholesterol, and negatively associated with HDL cholesterol (8,10,11,14–17). The combined importance of dietary lipids and LPS in determining inflammatory risk may arise, since endotoxin has a strong affinity for chylomicrons (lipoproteins that transport dietary long-chain saturated fatty acids [SFAs] through the gut wall) as endotoxin crosses the gastrointestinal mucosa (23–25). As such, atherogenic and inflammatory risk may arise through a combination of dietary lipoprotein patterns and an increase in circulating endotoxin, exacerbated by feeding patterns (26,27). Therefore, altering the lipid profile through dietary intervention may reduce endotoxin and the arising inflammatory response. Recent human studies have explored dietary effects of a high-SFA, high-carbohydrate meal on circulating endotoxin levels in healthy individuals. The findings showed a substantial increase in circulating endotoxin, in subjects given a high-fat meal, in conjunction with markers of inflammation (as noted from mononuclear blood cells) (13,28). Murine studies have also identified an association between endotoxin and insulin resistance, through infusion of endotoxin, with the same effect also noted by a high-fat diet (12), with insulin resistance and weight gain both affecting gut permeability (11,17,28). In studies to date, using either infused endotoxin as a bolus or derived from the gut because of dietary changes, both methods suggest endotoxin has the capacity to affect the inflammatory pathways (28,29). However, it remains to be established whether diets in different metabolic states affect absorption of endotoxin. Also, do such postprandial circulating endotoxin levels correlate with systemic lipid changes postprandially, being compounded in more insulin-resistant states? Therefore, these studies sought to establish whether a high-fat meal increased circulating endotoxin and whether this is altered in different metabolic disease states.
RESEARCH DESIGN AND METHODS
The study consisted of healthy control subjects (n = 9), obese subjects (n = 15), and patients with impaired glucose tolerance (IGT) (n = 12) and type 2 diabetes (n = 18). All subjects with type 2 diabetes were of South Asian origin except one subject, who was Afro-Caribbean. Similarly, all healthy control subjects were South Asian in origin except one, who was Afro-Caribbean.
All subjects were nonsmokers. Screening blood tests were performed for both baseline measurements to qualify for the study, as well as to assess glucose control. Routine blood tests included renal function, glucose, HbA1c, and full cholesterol profile. All subjects had their height, weight, abdominal circumference, and BMI measurements taken using standard equipment. Blood samples were taken either from the right or left antecubital vein in a sitting position. Blood pressure was checked with a blood pressure monitor on the left arm and rechecked after 2 min. Finally, a 12-lead electrocardiogram was performed using the same machine for the duration of the study. Subjects included in the study had a normal resting electrocardiogram, normal renal function tests and blood pressure, and no history of vascular disease. Detailed medical drug histories were taken on medications, and those subjects on medication considered to lead to a change in inflammatory status were excluded, including the thiazolidinediones. Ethical approval was obtained from the local research ethics committee, and all patients gave written consent.
All research subjects (n = 54) with and without type 2 diabetes were given a high-fat meal (standardized meal: 75 g fat, 5 g carbohydrate, 6 g protein) after an overnight fast of 12–14 h, as previously described (30). The cohort consisted of nonobese control (NOC) subjects (age 39.9 ± 11.8 years [mean ± SD], BMI 24.9 ± 3.2 kg/m2, n = 9) obese subjects (age 43.8 ± 9.5 years, BMI 33.3 ± 2.5 kg/m2, n = 15) subjects with IGT (age 41.7 ± 11.3 years, BMI 32.0 ± 4.5 kg/m2, n = 12), and type 2 diabetic subjects (age 45.4 ± 10.1 years, BMI 30.3 ± 4.5 kg/m2, n = 18). Blood samples were drawn at baseline (0 h) and postprandially (1, 2, 3, and 4 h), and endotoxin and lipid levels were measured.
In vivo assessment of the biochemical profile
On the assigned date, fasting blood samples were collected from participating subjects, and lipid profiles and fasting plasma glucose were determined using routine laboratory methods undertaken in the biochemistry laboratory at University Hospital Coventry and Warwickshire. In brief, the routine blood tests included renal function, glucose, HbA1c, and a full cholesterol profile (TGs, HDL, and LDL), as noted in Table 2. Insulin measurements were performed by a solid-phase enzyme amplified sensitivity multiplex immunoassay (Millipore, Hertfordshire, U.K.), and glucose was measured by a glucose oxidase method (YSL 200 STAT plus). Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated for all patients using the HOMA formula: HOMA-IR = fasting insulin (mU/L) × plasma glucose (mmol/L)/22.5.
Table 2.
Variable data for the different cohorts
Analysis of circulating endotoxin levels
Serum endotoxin was analyzed using a commercially available QCL-1000 LAL End Point Assay (Lonza, Allendale, NJ). The assay, and the values given by the manufacturer for intra-assay coefficient of variation (CV) (3.9 ± 0.46) and interassay CV (9.6 ± 0.75), have been validated in our laboratory, as detailed previously (10).
Statistical analysis
For assessment of the different variables, statistical analysis was undertaken using a paired Student t test, for intra-comparison of hourly time points versus baseline, and an unpaired Student t test for inter-comparisons. The threshold for significance was P < 0.05. Data in the text and figures are presented as means ± SD or means ± SEM. Correlations were determined with a Pearson correlation. Variables with a non-Gaussian distribution were logarithmically or square root transformed, as deemed appropriate, before statistical analysis. All statistics were performed on SPSS version 17.0.
RESULTS
Baseline characteristics across groups
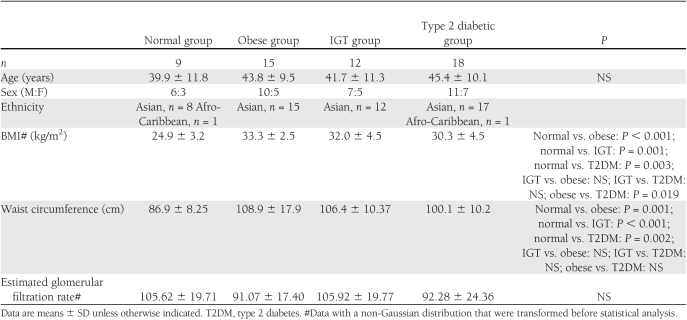
Table 1 shows the anthropometric data. Table 2 shows the metabolic baseline and hour time point characteristics of the four groups analyzed in this study. Age did not differ significantly between the groups, whereas BMI was altered across the groups, with the NOC group possessing the lowest BMI (24.9 ± 3.2 kg/m2) (Table 1). The other three groups (obese: 33.3 ± 2.5 kg/m2***↑ (increase); IGT: 32.0 ± 4.5Kgm2***↑, and type 2 diabetic: 30.3 ± 4.5 kg/m2**↑ (increase) subjects; P value; ***P < 0.001, **P < 0.01) differed significantly compared with NOC subjects, whereas the type 2 diabetic group exhibited a significantly lower BMI than the obese cohort (P < 0.05). Waist circumference followed a similar pattern to the BMI data. NOC subjects’ waist circumference was 86.9 ± 8.25 cm versus obese (108.9 ± 17.9 cm***↑), IGT (106.4 ± 10.37 cm***↑), and type 2 diabetic (100.1 ± 10.2 cm**↑) subjects (Table 1). The mean systolic blood pressure (SBP) levels of the groups were similar across all groups and did not change significantly over the 4-h duration, which was also noted for the diastolic blood pressure (DBP) levels (NOC subjects: SBP 128.2 ± 9.7 mmHg, DBP 72.3 ± 10.0 mmHg; obese: SBP 128.2 ± 10.7 mmHg, DBP 3.4 ± 7.8 mmHg; IGT: SBP 127.3 ± 10.1 mmHg, DBP 75.1 ± 8.8 mmHg; and type 2 diabetic: SBP 129.1 ± 10.1 mmHg, DBP 74.5 ± 6.9 mmHg).
Table 1.
Anthropometric data for the different cohorts
As anticipated, the baseline NOC group had significantly lower fasting plasma glucose levels than the type 2 diabetic group, while showing similar glucose levels to the obese and IGT cohorts (NOC: 4.7 ± 0.69 mmol/L vs. type 2 diabetic: 8.1 ± 1.8 mmol/L***↑; IGT: 5.6 ± 1.2 mmol/L; obese IGT: 4.9 ± 0.93 mmol/L; ***P < 0.001). HbA1c was similar in the obese and NOC groups but was significantly higher in the IGT and type 2 diabetic groups (NOC: 5.9 ± 0.31% vs. type 2 diabetic: 7.5 ± 1.12%***↑; IGT: 6.3 ± 0.47%*↑; obese IGT: 5.9 ± 0.49%; ***P < 0.001, *P < 0.05). Within each cohort, glucose levels were not significantly altered over the 4-h postprandial time period.
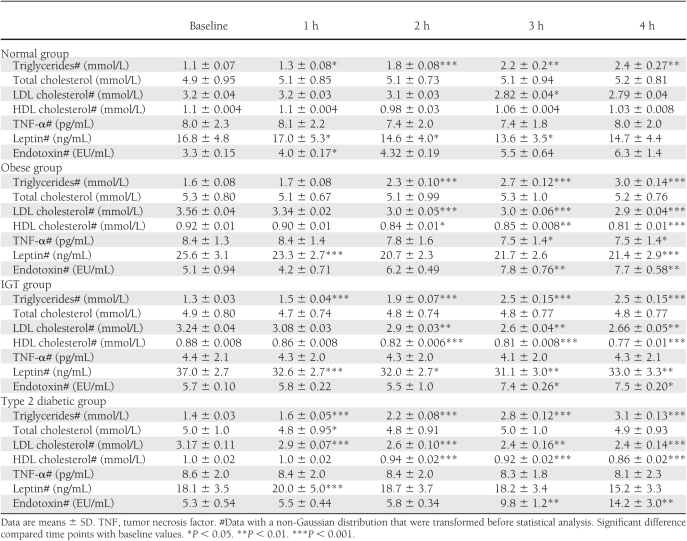
The baseline lipid profile across the groups was comparable. Serum endotoxin levels were significantly lower in the baseline NOC group compared with the IGT and type 2 diabetic groups (NOC: 3.3 ± 0.15 endotoxin unit/mL [EU/mL] vs. obese: 5.1 ± 0.94 EU/mL↑; IGT 5.7 ± 0.10 EU/mL**↑; type 2 diabetic: 5.3 ± 0.54 EU/mL*↑; **P < 0.01, *P < 0.05; Fig. 1A, Table 2).
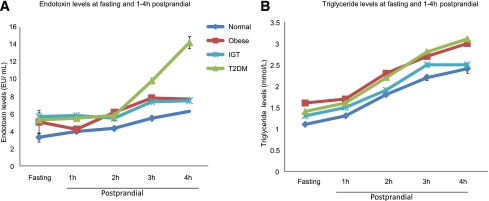
Figure 1.
Changes in circulating endotoxin levels (A) and triglyceride levels (B) in NOC, IGT, obese, and type 2 diabetic (T2DM) subjects. Endotoxin and triglyceride levels were measured at baseline and then, after a high-SFA meal, at each hour postprandially over a 4-h duration. Each point on the graph represents the mean value for each cohort (± SEM).
Postprandial change in endotoxin levels over time in individual groups
Postprandial exposure to a high-fat meal led to a significant rise in endotoxin levels in obese subjects (baseline: 5.1 ± 0.94 EU/mL; 1 h: 4.2 ± 0.71 EU/mL; 2 h: 6.2 ± 0.49* EU/mL; 3 h: 7.8 ± 0.76** EU/mL; 4 h: 7.7 ± 0.58** EU/mL; **P < 0.01, *P < 0.05; Fig. 1A, Table 2); IGT (IGT: baseline: 5.7 ± 0.10 EU/mL; 1 h: 5.8 ± 0.22 EU/mL; 2 h: 5.5 ± 1.0 EU/mL; 3 h: 7.4 ± 0.26* EU/mL; 4 h: 7.5 ± 0.20* EU/mL; *P < 0.05, Fig. 1A, Table 2), and type 2 diabetic subjects (baseline: 5.3 ± 0.54 EU/mL; 1 h: 5.5 ± 0.44 EU/mL; 2 h: 5.8 ± 0.34 EU/mL; 3 h: 9.8 ± 1.2** EU/mL; 4 h: 14.2 ± 3.0** EU/mL; **P < 0.01, Fig. 1A, Table 2) over the 4-h time period. In the NOC group, whereas there was a rise in circulating endotoxin over the 4-h period, this trend did not reach significance past 1 h (Fig. 1A, Table 2). Fasting endotoxin levels showed a positive correlation with fasting TG levels in the whole cohort (r = 0.303, P = 0.026). Further examination of this relationship postprandially identified the positive correlation strengthened over time, with the strongest relationship between endotoxin and TG noted at 2 and 3 h, respectively (2-h time point: r = 0.531, P < 0.001; 3-h time point: r = 0.498, P < 0.001) with a decline by 4 h after feeding (r = 0.434, P = 0.001). No further correlations with any other parameters were observed, and those noted were not influenced by age or sex.
Postprandial change in endotoxin levels between groups
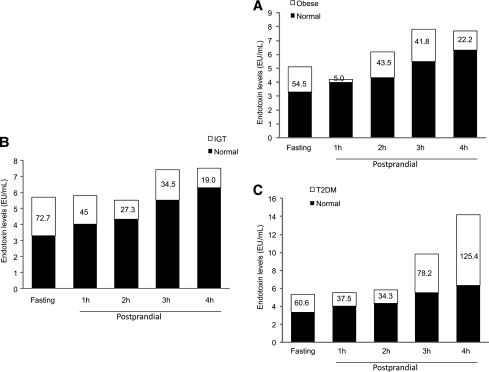
Fasting endotoxin levels were significantly higher in IGT and type 2 diabetic subjects than in NOC subjects (72.7%** and 60.6%* increase, respectively; **P < 0.01, *P < 0.05, Fig. 2B and C). However, during the postprandial 4-h time period, the circulating endotoxin levels in both obese and IGT subjects diminished to ∼20% higher than that of the NOC (4 h, Fig. 2A and B), whereas circulating endotoxin levels were sustained at significantly higher levels in the type 2 diabetic subjects than in the NOC subjects (4 h, Fig. 2C, P < 0.05).
Figure 2.
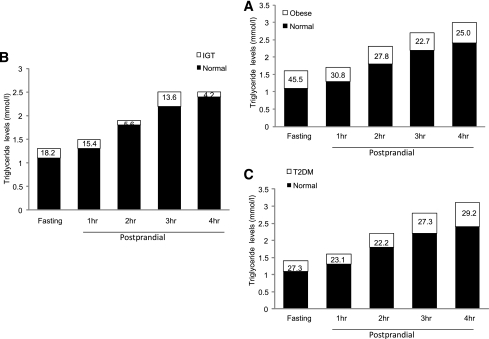
Increase in endotoxin levels between the NOC subjects and the obese (A), IGT (B), and type 2 diabetic (T2DM) (C) subjects from baseline to 4 h after a high-fat meal. Endotoxin is measured in EU/mL, and the percentage increase compared with NOC is also shown.
Postprandial changes in lipids over time in individual groups
Postprandial exposure to a high-fat meal led to a significant rise in TG levels in NOC, IGT, and type 2 diabetic subjects after 1 h (P < 0.05, Fig. 1B, Table 2). Although the obese subjects followed the same trend, TGs levels were only significantly altered at 2-h post-feeding (P < 0.05; Fig. 3, Table 2).
Total cholesterol remained relevantly unaltered over the 4-h period within all four groups (Table 2). In addition, no change was noted in LDL cholesterol and HDL cholesterol for the NOC subjects over the 4-h period (Table 2). LDL cholesterol and HDL cholesterol in the other three groups did show significant individual group changes over time. For all metabolic states, LDL and HDL cholesterol significantly changed (increased and reduced, respectively; P < 0.05; Table 2), whereas levels in NOC subjects were not altered.
Postprandial changes in lipid levels between groups
Fasting total cholesterol, TG, LDL cholesterol, and HDL cholesterol levels were comparable at baseline within the four groups and did not differ significantly throughout the 4-h duration (Figs. 1B and 3; Table 2).
CONCLUSIONS
This is the first study to examine the comparative and differential changes in circulating endotoxin after a SFA meal from subjects with and without type 2 diabetes, obesity, or IGT. The novel data highlight that a SFA meal increases circulating endotoxin levels in all subjects irrespective of their metabolic status, although circulating endotoxin shows dramatic postprandial changes in the high–metabolic risk groups. More specific comparative analysis of NOC subjects versus subjects with type 2 diabetes at 4-h postprandial identified that the latter had a mean endotoxin level 125.4% higher than that of NOC subjects. Cumulative data derived from the fasting state and the SFA postprandial state indicate that type 2 diabetic subjects are subjected to 336% more circulating endotoxin than NOC subjects over the 4-h duration. In comparison to other metabolic states, the obese and IGT subjects were still subjected to 167 and 198.5% more circulating endotoxin than NOC subjects. As such, endotoxin, which is considered a potential mediator of chronic low-grade inflammation, is considerably higher in the state of type 2 diabetes, with implications for a continual inflammatory state, as other articles have observed (15,16,28,29).
While our previous studies have shown significant associations in the fasted state among circulating endotoxin, lipoprotein patterns, and anthropometric data (8,10,11,14–17), these current studies have sought to establish whether endotoxin acutely changes postprandially and whether this is altered by differing metabolic states. By undertaking this, our current studies have highlighted subtle but significant differences in how endotoxin levels change in the postprandial period. After a SFA meal, the NOC endotoxin levels rose over the 4-h duration, but circulating levels did not increase significantly. In contrast, in the obese and IGT groups, there was a significant rise in endotoxin, which appeared to plateau by 4 h. However, at the 4-h time point, both the IGT and obese groups’ endotoxin levels were much lower than those of the type 2 diabetic subjects, since the levels of endotoxin in the type 2 diabetic subjects appeared to still be rising 4 h after a SFA meal. Circulating endotoxin in the type 2 diabetic group, after 4 h, did not appear to normalize, which suggests the cumulative exposure to endotoxin after a high-SFA meal is disproportionately high compared with any other group. Furthermore, in the type 2 diabetic subjects, the rising endotoxin levels may be further compounded by the refeeding stage. These data appear to indicate that a person eating three high-SFA meals each day may encounter endotoxin levels that remain perpetually high, since refeeding may increase the levels. As such, fasted endotoxin data, while important, may appear to miss the daily variation, as feeding data appear to show. The type of meal is clearly important, since previous studies highlight that dietary changes alter circulating endotoxin and influence inflammation, even in healthy subjects (13,28). In addition, recent studies have reported that the simultaneous ingestion of certain “healthy” food groups with saturated fat can negate an increase in circulating endotoxin and the customary inflammatory response (29). Because it is acknowledged that obese and type 2 diabetic subjects tend to eat high SFA without correspondingly high levels of fruit or healthy foods, this diet would clearly affect their endotoxin and inflammatory status (28,31,32). Therefore, a high-SFA intake could represent a continual inflammatory insult for type 2 diabetic subjects, daily.
In the obese and IGT groups, the postprandial 4-h endotoxin levels appear to plateau, while still remaining high compared with NOC subjects. Subsequently, another SFA meal may compound the circulating endotoxin levels further within the obese and IGT groups; therefore, the type and frequency of meals may significantly affect the metabolic risk. In addition to the type of meal, the food intake frequency is also relevant, although currently, there are few studies examining the importance of this. Previous studies indicate no difference between a diet based on three meals a day or a diet comprising smaller meals and snacks, with regard to the long-term effects on glucose, lipid, or insulin responses; although the unknown acute postprandial effects on the inflammatory status may have a more profound long-term impact (32,33). In addition, previous studies have often stressed the division of food intake should be based on individual preference, with no clear recommendations on pattern of food intake. Within type 2 diabetes clinics, the recommendation for patients is to consume five smaller meals per day. This step may reduce the potentially overwhelming orexigenic effects patients might experience with only three meals a day, as well as the potential spikes in insulin, although the data do not necessarily give clear insight into these benefits (32,33). Based on these current studies, more frequent saturated fat exposure may exacerbate both endotoxin and inflammation further. Furthermore, smaller more frequent meals have the potential to allow endotoxin to spike several times a day, thus activating the innate immune system within adipose tissue without desensitization (9,10,28,29). As such, the resulting downstream production of diabetogenic cytokines would be in continuous production, as previous in vivo and in vitro studies have demonstrated (9,10,17,28,29). The TG levels did not differ significantly across the four groups of subjects at any of the time points; however, the TG levels did increase from baseline to 4 h within each group, in a similar pattern to circulating endotoxin, but most significantly in the metabolic risk subjects (obese, IGT, and type 2 diabetic), while also demonstrating an association with endotoxin, over the 4-h period (10,16,17). The three different metabolic states showed significantly higher fasting TG levels than NOC subjects, which postprandially became further exacerbated in the obese and type 2 diabetic subjects.
Unsurprisingly, postprandial TG levels increased in a similar pattern to circulating endotoxin, while also demonstrating an association over the 4-h period (10,16,17). The three different metabolic states showed no significant differences in TG levels compared with levels in NOC subjects. However, the significant correlation between fasting TG and endotoxin levels confirms previous studies in which an association between these two metabolic parameters had been observed (10,16,17). Our data indicated that the association between TGs and circulating endotoxin became stronger in the postprandial state each hour over the 4-h duration, substantiating previous evidence that lipids mediate the transfer of endotoxin from the gastrointestinal tract into the circulation (9,11).
Concurrent with postprandial changes in TGs, the LDL/HDL ratio reduced compared with baseline measurements. Specifically, HDL was significantly reduced at time points postprandially within all except the NOC group, potentially due to parallel elevations in chylomicrons and VLDL, as noted in other studies (34–36). Whereas it is established that obese type 2 diabetic patients suffer from a syndrome of high serum TG and low HDL (37), low levels of HDL are also associated with low levels of sCD14 (soluble CD14) (38). This result corresponds with the data that endotoxin has been demonstrated to bind to HDL in the presence of sCD14 and LPS binding protein (39,40), an enzyme involved in presentation of endotoxin to sCD14. This outcome supports a role for HDL in the immunological response to endotoxin. Therefore, a reduction in HDL would reduce the removal of endotoxin further and exacerbate the inflammatory status, further compounded by higher circulating levels of endotoxin in the obese, IGT, and type 2 diabetic subject groups.
Figure 3.
Increase in triglyceride levels between the NOC subjects and the obese (A), IGT (B), and type 2 diabetic (T2DM) (C) subjects from baseline to 4 h after a high-fat meal. Triglyceride levels are measured in mmol/L, and the percentage increase compared with NOC is also shown.
Whereas our studies have highlighted the impact of metabolic disease status on circulating endotoxin, it is important to recognize the limitations of the study. In all research, it is always preferable to increase the subject numbers that comprise each cohort. In the present studies, increased numbers might have noted different postprandial responses to the high-fat meal within each cohort, if the groups were further subdivided. However, in light of this being a cross-sectional study, in which intra- and inter-comparisons can be made, the numbers do not detract from the findings. Consistent and significant trends were observed within the subjects over the 4-h postprandial duration, and differences between the cohorts were duly noted. We also recognize that the research subjects were given a very high-fat meal (75 g), roughly equivalent to their total daily intake of fat, which some observers might argue is an excessive (nonphysiological) amount of fat. However, despite the fat load, there was no significant change in endotoxin, cholesterol, LDL, or HDL levels postprandially in the NOC subjects in contrast to the other groups examined; the fat load administered was based on previous studies (30). Furthermore, administration of 75 g glucose could also be considered high and would far exceed normal intake of glucose in one sitting, yet this is standard clinical practice for assessment of insulin sensitivity, whereas the fat load is only currently used as a research tool. No ill effects were noted in any of the patients during or after the study.
In summary, our current data shed new light on our understanding of metabolic endotoxinemia in the postprandial state in metabolic disease. Our findings suggest that circulating endotoxin levels change depending on whether you are prediabetic, are nonobese, are obese, have IGT, or have type 2 diabetes. Further, circulating endotoxin levels noted in subjects with type 2 diabetes, at 4-h postprandial high-fat meal, far exceed our previous understanding based on other feeding studies in healthy subjects or the fasted state in type 2 diabetic subjects. Therefore, our 4-h data suggest a much higher inflammatory risk than previous studies have indicated. These findings highlight the point that requesting patients to eat smaller, more frequent meals may actually increase their inflammatory risk further, especially in subjects with type 2 diabetes (who tend to favor high-fat foods) (32). Finally, while the most obvious solution to metabolic endotoxinemia appears to be to reduce saturated fat intake, the Western diet is not conducive to this mode of action, and it is difficult for patients to comply with this request. Therefore, we need to understand the complexity of diet, meal frequency, and its acute effects on inflammatory risk and give more guidelines to particular subject groups, since leaving food intake to “individual preferences” appears not to represent a beneficial solution to reduce the inflammatory state in metabolic at-risk subjects.
Supplementary Material
Acknowledgments
M.C.V. was funded by a British Medical Institute fellowship. The authors thank Birmingham Science City for supporting this research and the British Heart Foundation for the Intermediate Fellowship for funding A.L.H.
No potential conflicts of interest relevant to this article were reported.
A.L.H. performed design, endotoxin experiments, and statistical analysis and drafted the manuscript. M.C.V. conducted the in vivo experiments and acquired all of the samples and anthropometric data. G.T. drafted and revised the manuscript. K.C.M. carried out the adipokine assays. N.M.A.-D. and O.S.A.-A. performed the lipid analysis. S.S. performed statistical analysis and interpretation of data. J.P.O., A.C., and P.S. provided the concept, interpreted data, and provided intellectual input. S.K. and P.G.M. provided design and concept, developed the manuscript, and performed the final revision of the manuscript. P.G.M. is also the guarantor of the article.
The authors thank Dr. Martin Been (University Hospital Coventry and Warwickshire [UHCW]) and the Cardiology Department, Nuclear Physics and Radiology Department, and all the departments and teams based at both UHCW and St George’s Hospital London for their contributions. The authors acknowledge and thank Mr. Saim Ulhaq Quddusi, Biomarkers Research Programme, for his input to the statistical analysis.
References
- 1.Laaksonen DE, Niskanen L, Nyyssönen K, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia 2004;47:1403–1410 [DOI] [PubMed] [Google Scholar]
- 2.Tuttle HA, Davis-Gorman G, Goldman S, Copeland JG, McDonagh PF. Proinflammatory cytokines are increased in type 2 diabetic women with cardiovascular disease. J Diabetes Complications 2004;18:343–351 [DOI] [PubMed] [Google Scholar]
- 3.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956;4:20–34 [DOI] [PubMed] [Google Scholar]
- 4.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab 2003;284:E1065–E1071 [DOI] [PubMed] [Google Scholar]
- 5.Fisher FM, McTernan PG, Valsamakis G, et al. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm Metab Res 2002;34:650–654 [DOI] [PubMed] [Google Scholar]
- 6.Hill MJ, Metcalfe D, McTernan PG. Obesity and diabetes: lipids, ‘nowhere to run to’ (Review). Clin Sci (Lond) 2009;116:113–123 [DOI] [PubMed] [Google Scholar]
- 7.Tabák AG, Brunner EJ, Miller MA, et al. Low serum adiponectin predicts 10-year risk of type 2 diabetes and HbA1c independently of obesity, lipids, and inflammation: Whitehall II study. Horm Metab Res 2009;41:626–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker AR, Harte AL, Howell N, et al. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab 2009;94:261–267 [DOI] [PubMed] [Google Scholar]
- 9.Youssef-Elabd EM, McGee KC, Tripathi G, et al. Acute and chronic saturated fatty acid treatment as a key instigator of the TLR-mediated inflammatory response in human adipose tissue, in vitro. J Nutr Biochem 2011 Mar 16. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 10.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292:E740–E747 [DOI] [PubMed] [Google Scholar]
- 11.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 2007;292:G518–G525 [DOI] [PubMed] [Google Scholar]
- 12.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007;50:2374–2383 [DOI] [PubMed] [Google Scholar]
- 13.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 14.Dixon AN, Valsamakis G, Hanif MW, et al. Effect of the orlistat on serum endotoxin lipopolysaccharide and adipocytokines in South Asian individuals with impaired glucose tolerance. Int J Clin Pract 2008;62:1124–1129 [DOI] [PubMed] [Google Scholar]
- 15.Al-Attas OS, Al-Daghri NM, Al-Rubeaan K, et al. Changes in endotoxin levels in type 2 diabetes mellitus subjects on anti-diabetic therapies. Cardiovasc Diabetol 2009;8:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MA, McTernan PG, Harte AL, et al. Ethnic and sex differences in circulating endotoxin levels: a novel marker of atherosclerotic and cardiovascular risk in a British multi-ethnic population. Atherosclerosis 2009;203:494–502 [DOI] [PubMed] [Google Scholar]
- 17.Harte AL, da Silva NF, Creely SJ, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15 [DOI] [PMC free article] [PubMed]
- 18.Lin YH, Lee H, Berg AH, Lisanti MP, Shapiro L, Scherer PE. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem 2000;275:24255–24263 [DOI] [PubMed] [Google Scholar]
- 19.Kopp A, Buechler C, Neumeier M, et al. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 2009;17:648–656 [DOI] [PubMed] [Google Scholar]
- 20.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 2006;346:739–745 [DOI] [PubMed]
- 21.Shoelson SE, Goldfine AB. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med 2009;15:373–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 2009;50:90–97 [DOI] [PubMed] [Google Scholar]
- 24.Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008;87:1219–1223 [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Navarrete JM, Manco M, Ibáñez J, et al. Metabolic endotoxemia and saturated fat contribute to circulating NGAL concentrations in subjects with insulin resistance. Int J Obes (Lond) 2010;34:240–249 [DOI] [PubMed] [Google Scholar]
- 26.Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev 2009;22:18–38 [DOI] [PubMed] [Google Scholar]
- 27.Wyness L. Understanding the role of diet in type 2 diabetes prevention. Br J Community Nurs 2009;14:374–379 [DOI] [PubMed] [Google Scholar]
- 28.Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009;32:2281–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deopurkar R, Ghanim H, Friedman J, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 2010;33:991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceriello A, Assaloni R, Da Ros R, et al. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation 2005;111:2518–2524 [DOI] [PubMed] [Google Scholar]
- 31.Vitolins MZ, Anderson AM, Delahanty L, et al. Action for Health in Diabetes (Look AHEAD) trial: baseline evaluation of selected nutrients and food group intake. J Am Diet Assoc 2009;109:1367–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold L, Mann JI, Ball MJ. Metabolic effects of alterations in meal frequency in type 2 diabetes. Diabetes Care 1997;20:1651–1654 [DOI] [PubMed] [Google Scholar]
- 33.Beebe CA, Van Cauter E, Shapiro ET, et al. Effect of temporal distribution of calories on diurnal patterns of glucose levels and insulin secretion in NIDDM. Diabetes Care 1990;13:748–755 [DOI] [PubMed] [Google Scholar]
- 34.Hanwell HE, Kay CD, Lampe JW, Holub BJ, Duncan AM. Acute fish oil and soy isoflavone supplementation increase postprandial serum (n-3) polyunsaturated fatty acids and isoflavones but do not affect triacylglycerols or biomarkers of oxidative stress in overweight and obese hypertriglyceridemic men. J Nutr 2009;139:1128–1134 [DOI] [PubMed] [Google Scholar]
- 35.Callow J, Summers LK, Bradshaw H, Frayn KN. Changes in LDL particle composition after the consumption of meals containing different amounts and types of fat. Am J Clin Nutr 2002;76:345–350 [DOI] [PubMed] [Google Scholar]
- 36.Thomsen C, Storm H, Holst JJ, Hermansen K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr 2003;77:605–611 [DOI] [PubMed] [Google Scholar]
- 37.Laakso M, Pyörälä K. Adverse effects of obesity on lipid and lipoprotein levels in insulin-dependent and non-insulin-dependent diabetes. Metabolism 1990;39:117–122 [DOI] [PubMed] [Google Scholar]
- 38.Eggesbø JB, Hjermann I, Lund PK, Joø GB, Ovstebø R, Kierulf P. LPS-induced release of IL-1 beta, IL-6, IL-8, TNF-alpha and sCD14 in whole blood and PBMC from persons with high or low levels of HDL-lipoprotein. Cytokine 1994;6:521–529 [DOI] [PubMed] [Google Scholar]
- 39.Wurfel MM, Hailman E, Wright SD. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med 1995;181:1743–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker TS, Levine DM, Chang JC, Laxer J, Coffin CC, Rubin AL. Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect Immun 1995;63:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.