Abstract
OBJECTIVE
To investigate whether a difference in the risk for diabetes exists in Japanese workers with regard to sleep duration/quality and the presence or absence of a family history of diabetes (FHD).
RESEARCH DESIGN AND METHODS
The researchers conducted a prospective, occupational-based study of local government employees in Sapporo, Japan. Between April 2003 and March 2004, 3,570 nondiabetic participants, aged 35–55 years, underwent annual health checkups and completed a self-administered questionnaire that included information on sleep duration/quality and FHD at baseline. Having diabetes was defined as taking medication for diabetes or a fasting plasma glucose level of ≥126 mg/dL at follow-up (2007–2008).
RESULTS
A total of 121 (3.4%) new cases of diabetes were reported. In multivariate logistic regression models of workers without an FHD, and after adjustment for potential confounding factors, the odds ratio (95% CI) for developing diabetes was 5.37 (1.38–20.91) in those with a sleep duration of ≤5 h compared with those with a sleep duration of >7 h. Other risk factors were awakening during the night (5.03 [1.43–17.64]), self-perceived insufficient sleep duration (6.76 [2.09–21.87]), and unsatisfactory overall quality of sleep (3.71 [1.37–10.07]). In subjects with an FHD, these associations were either absent or weaker.
CONCLUSIONS
The current study shows that poor sleep is associated with a higher risk of developing diabetes in workers without an FHD. Promoting healthy sleeping habits may be effective for preventing the development of diabetes in people without an FHD.
Modern society encourages late-night activities, such as watching television, using the computer or Internet, round-the-clock entertainment, as well as demanding shift work or night work that further promotes such activities. According to a survey by the National Sleep Foundation (2010), approximately one-fourth of participants stated that their current work schedule prevented them from getting enough sleep (1). One study reported that the average sleep duration for Finnish people had decreased by ~18 min during a period of 33 years (1972–2005), whereas sleep complaints, such as difficulty in falling asleep or awakening during the night, had increased, especially among the employed middle-aged population (2). According to a 2006 survey of Japanese adults on “Time Use and Leisure Activities,” average sleep duration was the shortest it has been for the past two decades (3). Furthermore, another report showed that one-fifth of Japanese adults habitually used alcohol or medicine to help them fall asleep (4).
It has been reported that poor sleep is associated with higher HbA1c levels in subjects with type 2 diabetes (5,6). Some prospective studies have reported that extreme sleep duration (7–10) and poor sleep quality, such as difficulty in sleep initiation (11–13) and sleep maintenance (12,14,15), are associated with a higher risk of impaired glucose tolerance or developing type 2 diabetes. However, those at greatest risk of developing diabetes from poor sleep remains to be elucidated. A recent meta-analysis indicated that no significant difference existed between men and women with regard to sleep duration/quality and diabetes, with the exception of short sleep duration in women (16). One multiethnic cohort study found that the risk in non-Hispanic whites and Hispanics differed from that in African Americans (10). However, the sleep-diabetes association in Asians is controversial, with few published studies (12,13).
Type 2 diabetes is well known as a disease involving a complex combination of genetic, environmental, and behavioral factors. In 2004, the U.S. Centers for Disease Control and Prevention developed Family Healthware, a family history screening tool, to prevent common chronic diseases, including diabetes. They subsequently reported that a family history of diabetes (FHD) was associated with a two- to sixfold risk of developing diabetes compared with no FHD. Three academic centers now are trying to determine whether personalized prevention messages tailored to familial risk will motivate people at risk to change their lifestyles or screening behavior (17). FHD is considered to be an indicator for intervention to prevent the development of diabetes in a high-risk population. However, it is not clear whether the sleep-diabetes association also should be an indicator for intervention. Furthermore, whether people without an FHD have any specific risks for developing diabetes according to their daily lifestyle has not been ascertained. The aim of this article was to investigate whether a difference in risk for diabetes exists in Japanese workers with regard to sleep duration/quality and the presence or absence of an FHD.
RESEARCH DESIGN AND METHODS
This is a prospective occupational-based study that took place in Sapporo, Hokkaido prefecture, Japan. We contacted a total of 10,423 (8,229 men and 2,194 women) local government employees, aged between 35 and 60 years, who were to undergo their annual health checkup between April 2003 and March 2004. We then excluded participants aged ≥56 years, because they were due to retire during the follow-up period. The total number of respondents was 4,195 (3,073 men and 1,122 women). After completing their checkup, participants submitted a self-administered questionnaire that they had received previously, which included questions on sleep duration/quality, medical history, family history, lifestyle, and potential diabetes risk factors, as well as working conditions. A total of 208 participants with a previous diagnosis of diabetes and/or a fasting plasma glucose (FPG) level of ≥126 mg/dL at baseline, 7 who had no information on FPG, and 21 who failed to complete all questions on sleep were excluded. Follow-up data for 3,576 participants (90.3%) who had undergone their health checkup and completed an additional questionnaire on medical history and FHD between April 2007 and March 2008 were obtained. Of these, six participants without an FPG were excluded from the final analysis.
The ethical committee for medicine at Hokkaido University approved recruitment of the participants, as well as consent and field procedures prior to the survey. Written informed consent was obtained from all participants before participation in the study.
Outcome and exposures
The development of diabetes was defined as having been prescribed medication for diabetes after the first health checkup and/or an FPG ≥126 mg/dL at the follow-up health checkup. Information on sleep was obtained from a self-administered questionnaire. Sleep duration was assessed by the question “How long on average, in hours and minutes, do you normally sleep?” It then was further categorized into ≤5, 5–6, 6–7, and >7 h. Because only 69 (1.9%) participants slept for >8 h, they were combined into the >7-h category to increase statistical power. Sleep quality was assessed by the Athens Insomnia Scale (AIS), the consistency, reliability, and validity of which has been ascertained as a screening or diagnosis tool for insomnia (18). We used the AIS to assess sleep conditions. Another major sleep questionnaire is the Pittsburgh Sleep Quality Index (PSQL), but we selected the AIS because it has fewer questions and is quicker to answer. A recent study on sleep quality reported similar results between the PSQL and the AIS (19). The AIS consists of eight items: the first five of which pertain to sleep induction, awakening during the night, final awakening earlier than desired, total sleep duration (not quantity but perceived sufficiency), and overall quality of sleep (sleep satisfaction); the subsequent three items refer to the sense of well being, functioning, and sleeping during the day. Participants were requested to score on a scale of 0–3, with a score of 0 signifying no problem at all, a score of 1 signifying a minor or slight problem, a score of 2 signifying marked or considerable problems, and a score of 3 signifying serious problems or being unable to sleep at all. Answers were based on any sleep difficulties participants may have experienced at least three times a week during the previous month. For the analyses, we used the total score obtained from all items to assess the severity of comprehensive sleep quality. A higher score expressed a more aggravated sleep quality. We also investigated each of the five main items to determine the extent of the risk of diabetes for each item separately. Because few participants (<1% for each symptom) had serious problems (a score of 3), results were transformed into dichotomous variables, where a score of 0 or 1 represented “no difficulty,” and a score of 2 or 3 represented “difficulty or suffering” in order to increase statistical power.
FHD was obtained from the self-reported questionnaire. FHD was defined as having (or having had) a first-degree relative with diabetes. We did not ask for specific information on diabetes type, because it was possible for participants to be mistaken. Type 1 diabetes is rare in Japan (childhood incidence: 2.1–3.5 per 100,000 per year), whereas type 2 diabetes is common (adult prevalence: 4–11%) (20).
Potential confounding factors
Many potential confounders existed. BMI was calculated by height and weight via the health checkup data of participants at baseline. Smoking was categorized into never, past, and current smoker. Drinking was categorized into no (never or rarely) or yes (often or regularly). Physical exercise was categorized into ≥150 min/week or <150 min/week. Education was grouped into more than high school or less. Sedentary work was grouped into the following categories: <25, 25–50, 50–75, and ≥75% and then transformed into dichotomous variables of ≥75 or <75%. Occupational stress, which is significantly associated with insomnia, was assessed using two major job-stress models: the demand-control model (DCM) and the effort-reward imbalance model (ERI). Descriptions of the association between insomnia and occupational stress assessed by both models are detailed in a previous study (21). For the analyses, the DCM was redefined as the demand-to-control ratio; demand scores and control scores were summed separately, after which the demand score was divided by the control score. An alternative model, the ERI was redefined as the effort-reward rate; effort scores and reward scores were summed separately, after which the effort score was divided by the reward score for analyses. In both models, high scores meant a more stressful job situation for the individual.
Analysis
The Mann-Whitney U test and Fisher exact test were used to compare participants who did and not develop diabetes, along with characteristics and sleep duration/qualities. All analyses were performed separately for participants without or with an FHD. To examine how the association of sleep duration/quality and the development of diabetes was affected by other factors, we calculated the risk (odds ratio [OR] [95% CI]) in different logistic regression models. Model 1 was adjusted for age, sex, and FPG. Model 2 included additional adjustments for the following established lifestyle risk factors for diabetes: BMI, smoking, drinking, and physical exercise. Model 3 included additional adjustments for education and the following occupational factors: working hours per week, shift work, rate of sedentary work, DCM, and ERI. The risk of developing diabetes was calculated separately in these models, along with sleep duration, total AIS score, and each of the AIS’s five main items in participants without/with an FHD. P < 0.05 was considered statistically significant. All analyses were performed using PASW Statistics 18 (SPSS, Chicago, IL).
RESULTS
The number of participants without an FHD was 2,862 (80.2%). Having an FHD was significantly associated with approximately twice the risk of developing diabetes after adjustment for age, sex, FPG, and lifestyle and occupational factors (OR 1.94 [95% CI 1.19–3.16]; P < 0.01]. The interaction between an FHD and sleep duration/quality in the multivariate logistic model was not statistically significant.
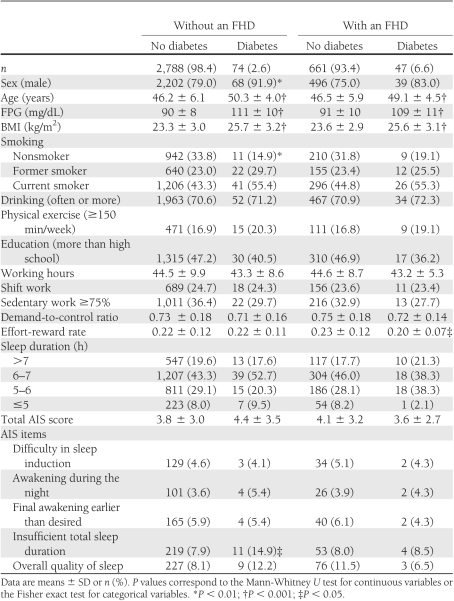
Table 1 shows the comparison between participants who did and did not develop diabetes, with regard to characteristics and sleep duration/quality at baseline. Those with and without an FHD were analyzed separately. In both groups, participants who developed diabetes were older, with a higher mean FPG and higher mean BMI. In participants without an FHD, those who developed diabetes were significantly more often male, smokers, and more likely to have perceived insufficient total sleep duration than those who did not. In participants with an FHD, those who developed diabetes had a significantly lower mean ERI than those who did not.
Table 1.
Baseline characteristics and sleep duration/quality for the risk of diabetes according to FHD
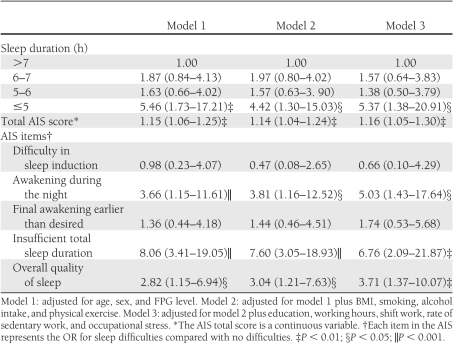
Table 2 shows the ORs for developing diabetes in participants without an FHD in each regression model, along with sleep duration/quality. Sleep duration of ≤5 h was significantly associated with a higher risk for diabetes compared with the reference of >7 h. However, additional adjustment for diabetes-related lifestyle factors reduced this risk; however, after additional adjustment for education and occupational factors, the risk increased once more. Total AIS score was unequivocally associated with a significant increase in risk in all models. For each of the five main AIS items, awaking during the night, insufficient total sleep duration, and unsatisfactorily overall quality of sleep were significantly associated with an increased risk of diabetes, but difficulty in sleep induction and final awakening earlier than desired were not. The OR for awakening during the night was slightly higher in model 2 but significantly higher in model 3. Perceived insufficient total sleep duration had the highest ORs among the five items in all models. Even after additional adjustment for self-reported sleep duration, the OR decreased only slightly, and the significance remained (5.18 [95% CI 1.50–17.85]; P < 0.01). The ORs for unsatisfactory quality of sleep were similar in all models.
Table 2.
ORs (95% CIs) for diabetes according to sleep duration/quality (participants without an FHD)
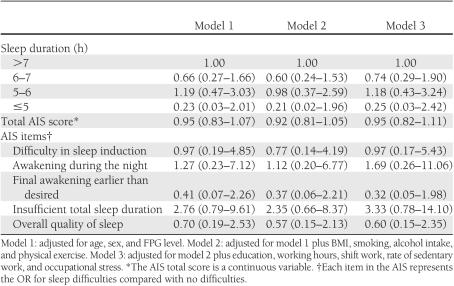
In contrast, in those participants with an FHD (Table 3), neither sleep duration nor sleep quality were significantly associated with risk of diabetes. Overall, ORs were smaller than for participants without an FHD.
Table 3.
ORs (95% CIs) for diabetes according to sleep duration/quality (participants with an FHD)
In the current study, the OR for diabetes according to sleep duration/quality increased in subjects without an FHD (Table 2) after adjustment because many potential confounders influenced the results. In particular, a difference in the distribution of FPG in sleep duration and quality was observed. Because only 14 women developed diabetes, the number was not sufficient for statistical analysis to be performed on sex differences.
CONCLUSIONS
This study is one of few to document differences with regard to an FHD and the association between sleep and diabetes. It presents new findings in that short sleep duration and poor sleep quality are significantly associated with an increased risk of diabetes in Japanese workers without an FHD, but not in those with an FHD. This discrepancy in the risk of developing diabetes may be explained by studies on the pathogenesis of type 2 diabetes in people with familial risks. Cusi (22) noted that insulin resistance in both muscle and hepatic tissue are genetically determined and fully established early in life in those with an FHD. Participants with an FHD in the current study already may have lapsed into insulin resistance by the time they reached the age of 35–55 years, and poor sleep duration/quality would not alter or diminish the risk of diabetes. On the basis of this hypothesis, short sleep duration/poor quality of sleep might be more influential in the early phase of the process of impaired glucose metabolism or developing diabetes, before obvious clinical abnormalities appear.
In the current study, sleep duration ≤5 h was significantly associated with a higher risk of developing diabetes only in those participants without an FHD, after adjusting for potential confounders. In an earlier study of 6,509 Japanese workers aged 19–69 years, no significant association between short sleep and developing diabetes was observed (13). In the current study as well, the association was not seen in all participants, including those with an FHD (OR 1.64 [95% CI 0.58–4.61]; P = 0.35). One epidemiological study from Sweden (15) and three from the U.S. (8–10) have shown an association between short sleep and higher risk for developing diabetes. One possible explanation of the discrepancy between these results and our results is that the effect of short sleep on risk differs with ethnicity. One multiethnic study demonstrated that the risk in non-Hispanic whites and Hispanics differed with that in African Americans (10). Another possible explanation is the duration of follow-up. One meta-analysis demonstrated that the risk of developing diabetes with regard to poor sleep tended to increase with the duration of follow-up (16). The average duration of 4.2 years in a previous Japanese study (13) and 4 years in the current study might be shorter than that necessary to reach a significant association. Some studies have reported that long sleep duration also was associated with increased risk (7–10). We did not analyze the risk regarding long sleep duration, because few workers in our study slept for >8 h.
An increase in total AIS score was significantly associated with an increased risk of developing diabetes. One cross-sectional population-based study reported that insomnia combined with objectively measured sleep duration of ≤5 h was associated with a higher risk of diabetes compared with normal sleep of >6 h, but only insomnia, poor sleep quality, or short sleep duration alone were not statistically significant (23). However, in our prospective study, the OR (95% CI) of the total AIS score after adjusting for additional self-reported sleep duration remained significant (1.14 [1.02–1.28]; P = 0.02) in those without an FHD. This means that increasingly poor sleep quality may be associated with an increased risk of developing diabetes independent of sleep duration in those without an FHD.
A significant association between difficulty initiating sleep and a higher risk of diabetes has been reported in some studies (11–13); however, two studies also found no such association (14,15). The current study supports the latter, irrespective of an FHD. The discrepancy in these results may be explained by the differences in the measurement of difficulty initiating sleep: frequency (never, sometimes, or often), yes/no, or degree of severity in difficulty initiating sleep. All studies that had a positive result measured the frequency of or whether there were symptoms. The AIS measures the degree in severity of symptoms at least three times a week. Frequency of difficulty initiating sleep might be a more predictable risk than the degree of symptoms.
Difficulty maintaining sleep also has been reported to be a significant risk factor for developing diabetes (12,14,15), and the association is likely to be consistent with either the measurement of frequency or degree. The mechanism involved in this association has not been fully explained. One recent laboratory study demonstrated that all-night selective suppression of falling into a deep sleep without change of sleep duration led to a 25% decrease in insulin sensitivity (24). The present results support the theory that difficulty in maintaining sleep or having persistently shallow sleep could result in the induction of insulin resistance and consequent the development of diabetes.
Perceived insufficient total sleep duration had the highest OR for diabetes even in those with an FHD without reaching statistical significance. Mallon et al. (15) reported that of 38 men, 3 with a sleep duration ≤5 h had sufficient individual sleep duration and did not develop diabetes during the 12-year follow-up. One cross-sectional study in African Americans with type 2 diabetes found that a perceived sleep debt was more strongly correlated with higher HbA1c levels compared with sleep duration in patients without complications, but this association was not seen in those with complications or in those taking insulin (5). This, combined with our results, suggests that perceived sufficiency of sleep duration is an important factor for predicting future aggravation of glucose metabolism in both healthy subjects and those with incipient diabetes. Unsatisfactory overall quality of sleep was associated with a significantly higher risk in those without an FHD. Thus, individual optimum sleep duration may exist with perceived sufficiency or satisfaction, which prevents the development of diabetes.
There are several limitations to our study. One limitation is that participants were relatively healthy local government employees, who were mostly men aged between 35 and 55 years working in a city, with a relatively homogeneous socioeconomic status. Consequently, our results are not applicable to the general population. Another limitation is the lack of objective sleep measurement with polysomnography or actigraphy, so some misclassifications may have occurred. A report described a moderate correlation between self-reported sleep duration and measured sleep duration using wrist actigraphy (r = 0.47); the former was more likely to overestimate the latter by 0.8 h on average, and this overestimate escalated particularly with shorter sleep duration (25). Therefore, the risk of diabetes by short sleep duration might be overestimated. However, using the AIS as a measurement of sleep quality already has been validated as an invaluable tool in sleep research and clinical practice (18). We did not obtain information on sleep disorders, such as apnea, which has been associated with a higher prevalence of type 2 diabetes in adults (26). FHD was gained by a self-reported questionnaire. Regarding self-reported family history of diabetes, one Japanese study confirmed the validity of self-reporting in Japanese subjects with <5% discordance (27); however, we must not overlook the potential for familial diabetes to also develop in the future. Another limitation is that we could not control for diet in the individuals. Taheri et al. (28) found that participants with short sleep duration had reduced leptin levels and elevated ghrelin, both of which are related to an increase in appetite and consequently an increasing BMI. Additional studies are needed to clear the association between quality of sleep, calorific intake and weight, and the contribution of dietary constituents to the sleep-diabetes relationship in an FHD.
In conclusion, sleep duration ≤5 h, awakening during the night, perceived insufficient total sleep duration, and unsatisfactorily overall quality of sleep were each found to be associated with a future independent risk of diabetes in Japanese workers without an FHD. It is well known that people with an FHD are more likely to develop diabetes than those who do not and, from our observations so far, seem to be more conscious of their risk and thus take active measures to prevent the disease. However, those without an FHD, also should be made aware that they have their own risks for diabetes, such as poor sleep, and take active measures to reduce this risk. Consequently, the current study proposes that public health strategies for diabetes prevention need to consider the presence or absence of an FHD.
Acknowledgments
This work was supported in part by a grant-in-aid for young scientists from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and a grant-in-aid for scientific research from the Ministry of Health, Labor, and Welfare, Japan.
No potential conflicts of interest relevant to this article were reported.
T.K. is the guarantor of this work, wrote the manuscript, and researched data. E.Y. researched data, contributed to the discussion, and reviewed and edited the manuscript. H.S. contributed to the discussion and reviewed and edited the manuscript. Y.S. and M.K. researched data and contributed to the discussion. E.O. contributed to the discussion. R.K. researched data and reviewed and edited the manuscript.
The authors thank Hiroyuki Arizuka, Chisato Watanabe, Chizuko Sato, Manabu Shojiguchi, Masahiro Kawamura, Naoto Sasaki, Shiho Kimura, Takanori Mogi, Dr. Takehito Nakabayashi, Takeshi Tsuda, Toyoko Enomoto, Tomoko Arihara, Dr. Toshiyuki Hayashi, and Yoshinori Hirata, of the Mutual Aid Association of Public Workers in Sapporo, Japan, for their excellent assistance with data collection. The authors appreciate the advice and expertise of Dr. H. Tamashiro, Professor of the Department of Public Health Sciences, Hokkaido University Graduate School of Medicine. The authors also are grateful to Sharon Hanley of the Hokkaido University Graduate School of Medicine for her help in proofreading the English manuscript.
References
- 1.National Sleep Foundation. 2010 sleep and ethnicity [article online], 2010 Available from http://www.sleepfoundation.org/article/sleep-america-polls/2010-sleep-and-ethnicity Accessed 18 August 2010
- 2.Kronholm E, Partonen T, Laatikainen T, et al. Trends in self-reported sleep duration and insomnia-related symptoms in Finland from 1972 to 2005: a comparative review and re-analysis of Finnish population samples. J Sleep Res 2008;17:54–62 [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Public Management Home Affairs Posts and Communications in Japan. 2006 survey on time use and leisure activities [article online], 2006. Available from http://www.stat.go.jp/english/data/shakai/index.htm Accessed 2 October 2011
- 4.Ministry of Health Labor and Welfare in Japan. Outline for the results of the National Health and Nutrition Survey Japan, 2007 [article online], 2007. Available from http://www.nih.go.jp/eiken/english/research/project_nhns.html Accessed 12 August 2011
- 5.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 2006;166:1768–1774 [DOI] [PubMed] [Google Scholar]
- 6.Tsai YW, Kann NH, Tung TH, et al. Impact of subjective sleep quality on glycemic control in type 2 diabetes mellitus. Fam Pract 2011;0:1–6 [DOI] [PubMed] [Google Scholar]
- 7.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003;26:380–384 [DOI] [PubMed] [Google Scholar]
- 8.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006;29:657–661 [DOI] [PubMed] [Google Scholar]
- 9.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007;30:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol 2009;19:351–357 [DOI] [PubMed] [Google Scholar]
- 11.Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 2004;27:2464–2469 [DOI] [PubMed] [Google Scholar]
- 12.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care 2004;27:282–283 [DOI] [PubMed] [Google Scholar]
- 13.Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H; HIPOP-OHP Research group Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health 2007;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meisinger C, Heier M, Loewel H; MONICA/KORA Augsburg Cohort Study Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia 2005;48:235–241 [DOI] [PubMed] [Google Scholar]
- 15.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care 2005;28:2762–2767 [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2010;33:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon PW, Scheuner MT, Jorgensen C, Khoury MJ. Developing Family Healthware, a family history screening tool to prevent common chronic diseases. Prev Chronic Dis 2009;1:A33. [PMC free article] [PubMed] [Google Scholar]
- 18.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 2000;48:555–560 [DOI] [PubMed] [Google Scholar]
- 19.Szymczak RK, Sitek EJ, Sławek JW, Basiński A, Siemiński M, Wieczorek D. Subjective sleep quality alterations at high altitude. Wilderness Environ Med 2009;20:305–310 [DOI] [PubMed] [Google Scholar]
- 20.Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci 2006;1084:1–29 [DOI] [PubMed] [Google Scholar]
- 21.Utsugi M, Saijo Y, Yoshioka E, et al. Relationships of occupational stress to insomnia and short sleep in Japanese workers. Sleep 2005;28:728–735 [DOI] [PubMed] [Google Scholar]
- 22.Cusi K. Lessons learned from studying families genetically predisposed to type 2 diabetes mellitus. Curr Diab Rep 2009;9:200–207 [DOI] [PubMed] [Google Scholar]
- 23.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care 2009;32:1980–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 2008;105:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology 2008;19:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, Casal E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract 2007;13:355–362 [DOI] [PubMed] [Google Scholar]
- 27.Saito T, Nanri S, Saito I. Reliability of family history of lifestyle-related diseases on questionnaire. Pediatr Int 2009;51:514–519 [DOI] [PubMed] [Google Scholar]
- 28.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004;3:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]