Abstract
OBJECTIVE
The study objective was to examine the associations among visceral fat (VF), all-cause mortality, and obesity-related mortality.
RESEARCH DESIGN AND METHODS
A total of 733 Japanese Americans were followed for 16.9 years. Hazard ratios (HRs) per interquartile range increase in VF were calculated using time-dependent Cox proportional hazard models censored at age 82 years, with age as the time axis adjusted for sex and smoking.
RESULTS
Higher VF was associated with all-cause mortality (HR 1.39 [95% CI 1.11–1.75] 107 deaths) and obesity-related mortality (1.39 [1.04–1.85], 68 deaths from cardiovascular disease, diabetes, or obesity-related cancer). After further adjustment for waist circumference, VF remained significantly associated with all-cause mortality (1.41 [1.04–1.92]) but not with obesity-related mortality. The associations between mortality and VF were not independent of BMI.
CONCLUSIONS
VF was associated with all-cause mortality and obesity-related mortality in Japanese Americans. VF did not significantly improve mortality risk assessment beyond that of BMI.
To date, only one study of 291 men, followed for 2.2 years, has examined the association between visceral fat (VF) and mortality (1). The purpose of this study was to assess the associations between VF and other measures of obesity, mortality, and cause of death in a cohort of 733 Japanese Americans.
RESEARCH DESIGN AND METHODS
Recruitment for the Japanese Community Diabetes Study has been described (2). Staggered enrollment of 735 second-generation (Nisei) and third-generation (Sansei) men and women of 100% Japanese ancestry aged 34 to 74 years occurred between 1983 and 1998 with follow-up visits every 2.5 to 5 years. The last possible visit was 10–11 years (N = 658) or 2.5 years (N = 77) after baseline. Most participants (71%) attended ≥3 visits. Two participants had no computed tomography (CT) measurements, leaving 733 participants for this analysis. All participants provided written informed consent. This study was approved by the University of Washington Human Subjects Division.
At each visit, the following measures were obtained: smoking habits, medical history, waist circumference at the umbilicus, height, weight, VF and subcutaneous abdominal fat (SAF) areas from a 10-mm CT slice at the umbilicus (3), and plasma insulin and glucose levels measured after a 12-h fast. Type 2 diabetes was defined as the presence of any of the following: fasting glucose ≥7.0 mmol/L, 2-h glucose ≥11.1 mmol/L during a 75-g oral glucose tolerance test, or diabetes medication use (4).
The National Death Index provided date and cause of death from death certificates through 31 December 2007 (5). Cardiovascular disease (CVD) included the following diagnoses (ICD-9/ICD-10 codes): hypertensive or ischemic heart disease (402/ I11; 404/I13; 410–414, 429.2/I20–I25); heart failure (428/I50); cerebrovascular disease or stroke (430–434, 436–438/I60–I69); atherosclerosis or peripheral vascular disease (440/I70); and aortic aneurysm (441/I171). Deaths from CVD, diabetes, hypertensive renal disease, or obesity-related cancers were considered obesity related (6). Obesity-related cancers included colon, breast, esophageal, kidney, ovarian, and pancreatic cancers.
Mortality risk was analyzed using time-dependent Cox proportional hazard models, using all available longitudinal values for independent variables. The enrollment date had no clinical significance, so age was used as the time axis and was not included as a covariate (7,8). To account for temporal changes in mortality risk, models were stratified by three categories of birth cohort: 1910–1922, 1923–1940, and 1941–1954 (9). All analyses were adjusted for sex and smoking (<1, 1–9.99, 10–19.99, 20–29.99, >30 pack-years). Proportional hazards assumptions for each covariate were assessed graphically and using the test for proportional hazards based on Schoenfeld residuals (9). The hazard for VF was nonproportional across age. VF was positively associated with mortality at younger ages and negatively at older ages; the association changed direction at approximately age 82 years. To avoid violating assumptions of proportional hazards, age at death was censored at age 82 years. Hazard ratios (HRs) were estimated as the risk associated with an increase equivalent to the magnitude of the interquartile range (25th to 75th percentile) for the adiposity variable of interest (10) with 95% CI using Wald-based standard errors and P values based on likelihood ratio tests. Analyses were conducted using R 2.9.1 software (11).
RESULTS
As of 31 December 2007, 161 participants were deceased; 54 deaths occurred after age 82 years, leaving 107 deaths for this analysis. The average duration of follow-up was 16.9 years. Most deaths (95.3%) occurred ≥3 years after baseline. There were 68 obesity-related deaths, 46 of which were from CVD. The Pearson correlation coefficients between baseline VF and SAF, BMI, and waist circumference were 0.41, 0.66, and 0.73, respectively.
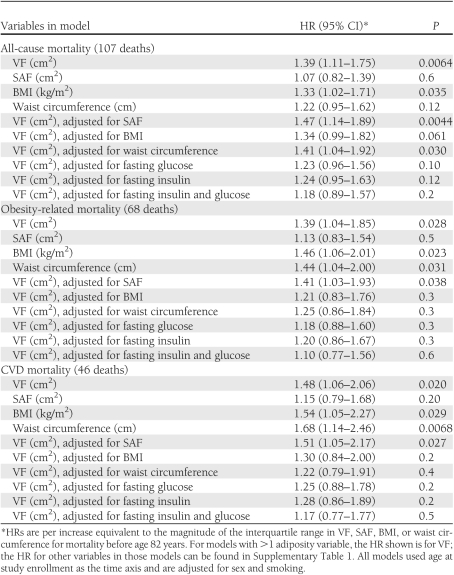
Results of all-cause mortality, obesity-related mortality, and CVD mortality analyses are shown in Table 1. For models where VF was adjusted for SAF, BMI, waist circumference, insulin, or glucose, the partial HR values for non-VF variables are shown in Supplementary Table 1. The association between all-cause mortality and VF did not differ by sex or diabetes status (addition of a sex × VF or diabetes × VF interaction term did not improve the fit of the model, P > 0.05). Age at death censored at 80 years instead of 82 years yielded similar results. When four participants with missing smoking data were coded as being in the highest instead of the lowest smoking category, results were also similar.
Table 1.
HRs for mortality associated with various measures of adiposity adjusted for sex and smoking over an average follow-up of 16.9 years
CONCLUSIONS
Our results confirm an age-adjusted association between VF and total mortality reported in men by Kuk et al. (1), and extend those findings through additional analyses in men and women. VF was significantly associated with all-cause mortality, obesity-related mortality, and CVD mortality in men and women before achieving age 82 years after taking into account age, sex, and smoking. After further adjustment for waist circumference, VF remained significantly associated with all-cause mortality but not with obesity-related mortality. After further adjustment for BMI in separate models, VF was no longer significantly associated with all-cause mortality, obesity-related mortality, or CVD mortality. These results demonstrate that a direct measure of VF is a better predictor of all-cause mortality than a surface measurement (waist circumference). However, VF does not improve mortality risk assessment beyond that provided by BMI. The association between VF and mortality was also attenuated by adjustment for fasting glucose or fasting insulin, suggesting that glycemia or insulinemia may partially mediate this association.
Limitations to this study include a relatively small number of deaths. We cannot exclude the possibility that VF may be associated with mortality independently of BMI in larger studies. VF was measured using single-slice CT at the level of the umbilicus. Irlbeck et al. (12) showed that single-slice CT at L3–4 correlates closely with total VF and SAF volumes. The umbilicus is typically at the L3–4 level (13). We could not account for effects of comorbid conditions. The study was limited to Japanese Americans, and the results may not be generalizable to other populations.
In summary, during an average follow-up period of 16.9 years, higher VF was associated with all-cause mortality, CVD mortality, and obesity-related mortality in Japanese Americans. VF predicted all-cause mortality independently of waist circumference but did not significantly improve mortality risk assessment beyond that of BMI.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grants DK-77745, DK-31170, HL-49293, and DK-02654) and by facilities and services provided by the Diabetes and Endocrinology Research Center (DK-17047), Clinical Nutrition Research Unit (DK-35816), and General Clinical Research Center (RR-00037) at the University of Washington. VA Puget Sound provided support for Dr. Boyko’s involvement in this research.
No potential conflicts of interest relevant to this article were reported.
Preliminary results of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
M.J.M wrote the manuscript, researched data, contributed to the discussion, reviewed and edited the manuscript, and takes responsibility for the contents of the article. J.B.S. wrote the manuscript, researched data (including statistical analyses), contributed to the discussion, and reviewed and edited the manuscript. D.L.L., W.Y.F., and E.J.B. researched data, contributed to the discussion, and reviewed and edited the manuscript.
The authors thank the King County Japanese-American Community for support and cooperation.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1193/-/DC1.
References
- 1.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14:336–341 [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto WY, Leonetti DL, Kinyoun JL, et al. Prevalence of diabetes mellitus and impaired glucose tolerance among second-generation Japanese-American men. Diabetes 1987;36:721–729 [DOI] [PubMed] [Google Scholar]
- 3.Shuman WP, Morris LL, Leonetti DL, et al. Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol 1986;21:483–487 [DOI] [PubMed] [Google Scholar]
- 4.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 5.The National Death Index [website], 2011. Available from http://www.cdc.gov/nchs/ndi.htm Accessed 22 June 2011
- 6.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007;298:2028–2037 [DOI] [PubMed] [Google Scholar]
- 7.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997;145:72–80 [DOI] [PubMed] [Google Scholar]
- 8.Cheung YB, Gao F, Khoo KS. Age at diagnosis and the choice of survival analysis methods in cancer epidemiology. J Clin Epidemiol 2003;56:38–43 [DOI] [PubMed] [Google Scholar]
- 9.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model New York, Springer-Verlag, 2000 [Google Scholar]
- 10.Harrell FE., Jr Regression Modeling Strategies: With Application to Linear Models, Logistic Regression, and Survival Analysis. New York, Springer-Verlag, 2001 [Google Scholar]
- 11.R: A language and environment for statistical computing [article online], 2009. Available from http://www.R-project.org Accessed 22 June 2011
- 12.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 2010;34:781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoppenfeld S. Physical Examination of the Spine and Extremities Norwalk, CT, Appleton-Century-Crofts, 1976 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.