Abstract
OBJECTIVE
To test the safety and efficacy of exenatide once weekly (EQW) compared with metformin (MET), pioglitazone (PIO), and sitagliptin (SITA) over 26 weeks, in suboptimally treated (diet and exercise) drug-naive patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Patients were randomized to subcutaneous (SC) EQW 2.0 mg + oral placebo (n = 248), MET 2,000 mg/day + SC placebo (n = 246), PIO 45 mg/day + SC placebo (n = 163), or SITA 100 mg/day + SC placebo (n = 163) for 26 weeks. MET and PIO therapies were increased to maximum-tolerated dosages. Injections with EQW or placebo were administered weekly, while oral medication or placebo was administered daily.
RESULTS
Baseline characteristics were as follows: 59% men, 67% Caucasian, mean age 54 years, HbA1c 8.5%, fasting serum glucose 9.9 mmol/L, body weight 87.0 kg, and diabetes duration 2.7 years. HbA1c reductions (%) at 26 weeks (least-squares means) with EQW versus MET, PIO, and SITA were −1.53 vs. −1.48 (P = 0.620), −1.63 (P = 0.328), and −1.15 (P < 0.001), respectively. Weight changes (kg) were −2.0 vs. −2.0 (P = 0.892), +1.5 (P < 0.001), and −0.8 (P < 0.001), respectively. Common adverse events were as follows: EQW, nausea (11.3%) and diarrhea (10.9%); MET, diarrhea (12.6%) and headache (12.2%); PIO, nasopharyngitis (8.6%) and headache (8.0%); and SIT, nasopharyngitis (9.8%) and headache (9.2%). Minor (confirmed) hypoglycemia was rarely reported. No major hypoglycemia occurred.
CONCLUSIONS
EQW was noninferior to MET but not PIO and superior to SITA with regard to HbA1c reduction at 26 weeks. Of the agents studied, EQW and MET provided similar improvements in glycemic control along with the benefit of weight reduction and no increased risk of hypoglycemia.
The 2009 consensus algorithm from the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) recommends lifestyle changes and metformin (MET) as the initial (tier one, well-validated) treatment at diagnosis of type 2 diabetes (1). Common adjunctive treatments not associated with an increased risk of hypoglycemia when used as monotherapy include the thiazolidinedione, pioglitazone (PIO), the dipeptidyl peptidase-4 inhibitor, sitagliptin (SITA), and the glucagon-like peptide-1 receptor agonist exenatide twice daily (BID) (2–5). PIO and exenatide BID are included in the second tier (less well-validated) of preferred agents in the ADA/EASD algorithm. SITA is not indentified, but may be an appropriate choice for selected patients (1). These agents, commonly used only in MET-intolerant patients as monotherapy, have unique risks, benefits, and mechanisms.
Effective and safe monotherapy treatment options for patients unable to tolerate MET are limited. In the United States, exenatide BID is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. A new formulation of exenatide (exenatide once weekly [EQW]) has been shown to result in greater improvements in glycemic control, with no increased risk of hypoglycemia and similar weight reduction compared with exenatide BID (6,7). This randomized trial (Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once-Weekly [DURATION-4]) directly compared the safety and efficacy of EQW monotherapy versus MET, PIO, and SITA monotherapy in patients with type 2 diabetes who were suboptimally treated with diet and exercise but naive to antihyperglycemic drugs.
RESEARCH DESIGN AND METHODS
Outcomes
The aim of this study was to assess the efficacy of EQW compared with MET, PIO, and SITA, as measured by change in HbA1c after 26 weeks. Secondary and exploratory measures included the proportion of patients achieving HbA1c <7.0 and ≤6.5%, changes in fasting serum glucose, seven-point self-monitoring of blood glucose (SMBG), weight, serum lipids, homeostasis model assessment of pancreatic β-cell function (HOMA-B) and insulin sensitivity (HOMA-S), and safety and tolerability. Patient-reported outcomes were collected, including Impact of Weight on Quality of Life Questionnaire-Lite (IWQOL-Lite), Binge Eating Scale (BES), the Diabetes Treatment Satisfaction Questionnaire (DTSQ), and EuroQol-5 dimensions (EQ-5D).
Safety end points were adverse events, clinical laboratory assessments, vital signs, hypoglycemia, and antibodies to exenatide. Treatment-emergent adverse events were defined as those occurring or worsening after the first dose of study drug. Minor hypoglycemia was defined as signs or symptoms associated with blood glucose <3.0 mmol/L (either self-treated or resolved independently). Major hypoglycemia was classified as symptoms resulting in loss of consciousness or seizure that showed prompt recovery after administration of glucose, or documented blood glucose <3.0 mmol/L that required the assistance of another person because of severe impairment in consciousness or behavior. A subset, defined as symptoms of hypoglycemia, was not confirmed by blood glucose measurement.
Sample size determination
The protocol specified that 822 patients be enrolled, with at least 740 patients (10% dropout before first HbA1c collection) available for primary analysis. A sample of 740 patients (222 EQW and MET, 148 PIO and SITA) would provide ∼90% power to detect true differences in HbA1c change of 0.4% (EQW vs. MET), 0.5% (EQW vs. PIO), and 0.5% (EQW vs. SITA), respectively (two-sided t test significance of 0.05 and common SD of 1.2%) (6). A predefined noninferiority margin of 0.3% and sample size of 444 patients would provide 74% power to test the noninferiority of EQW versus MET, and a sample size of 370 would provide 65% power to test the noninferiority of EQW versus PIO (and SITA).
Patients
A total of 820 patients in 22 countries participated between November 2008 and June 2010. Adults with type 2 diabetes met the following inclusion criteria: HbA1c 7.1–11.0%, BMI 23–45 kg/m2, and history of stable weight. Patients were excluded if treated with any antihyperglycemic drug for >7 days within 3 months of screening. Antihypertensive and lipid-lowering medication changes were only made if medically required.
Study design
Randomization was determined by computer-generated random sequence using an interactive voice response system. Treatment assignments were stratified by country. MET and PIO dosages were increased in weekly increments up to target doses of 2,000 and 45 mg/day, respectively. MET could be increased up to 2,500 mg/day based on glycemic control. Standard diet and exercise counseling was provided in each treatment group. Following 26 weeks, patients stopped study treatment and were allowed to follow diabetes regimens deemed appropriate by the investigator, with the exception of exenatide BID. Patients returned to the study sites 10 weeks after study end for collection of additional safety data.
Statistical analysis
Data were reported for randomized patients who received at least one dose of the study drug (intent-to-treat population). To control family-wise error within 0.05, the Bonferroni-Hommel gate-keeping procedure was used to test hypotheses. Three noninferiority hypotheses (EQW is inferior to MET, PIO, and SITA) were tested first using the Bonferroni test (noninferiority margin 0.3%, Bonferroni-adjusted significance of 0.0167). If any null hypothesis was rejected (i.e., noninferiority was established), the corresponding superiority hypothesis was tested using the Hommel test (8) and decision matrix algorithm (9). The nominal significance level based on Hommel adjustment was between 0.0167 and 0.05 depending on the noninferiority hypotheses rejected.
The primary end point was tested using a maximum likelihood-based mixed-model repeated-measures (MMRM) ANCOVA with change in HbA1c as the dependent variable; treatment, baseline HbA1c, country, week of visit, and treatment by week interaction as fixed effects; patient and error as random effects; and an unstructured variance/covariance matrix. All scheduled postbaseline measurements were included, with no imputation of missing data. Least-squares (LS) estimates and CIs of the treatment differences between EQW and the three oral comparators were presented. The differences were based on the estimate at 26 weeks of EQW minus the estimate for each comparator at 26 weeks. SEs were also presented. Secondary end points were tested using similar MMRM analyses. Analyses of patient-reported outcomes were performed without adjustment for multiple statistical testing.
The original primary analysis excluded unscheduled postbaseline observations for unexpected early discontinuations; therefore, a modified analysis was completed, including all postbaseline observations. This modified MMRM analysis was performed after database lock when it was determined that the original analysis excluded patients who discontinued before week 8. All statistical analyses were performed using SAS Version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patients
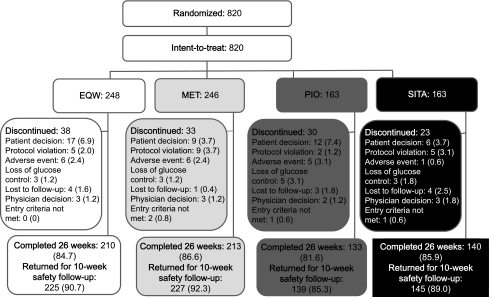
Of 820 intent-to-treat patients, 696 (84.9%) completed the 26-week treatment period and 736 (89.8%) returned for 10-week safety follow-up (Fig. 1). Patient characteristics at baseline were similar among the treatment groups (Supplementary Table 1). By week 12, 87% of patients taking MET and 75% taking PIO had been titrated to or above target doses for each agent (PIO 45 mg/day, MET 2,000 mg/day, respectively). At week 16–26, patients were on stable doses: PIO (≥45 mg/day) 88% and MET (≥2,000 mg/day) 76%.
Figure 1.
Disposition of all randomized patients. Data are presented as n or n (%) of patients.
Glycemic control and weight
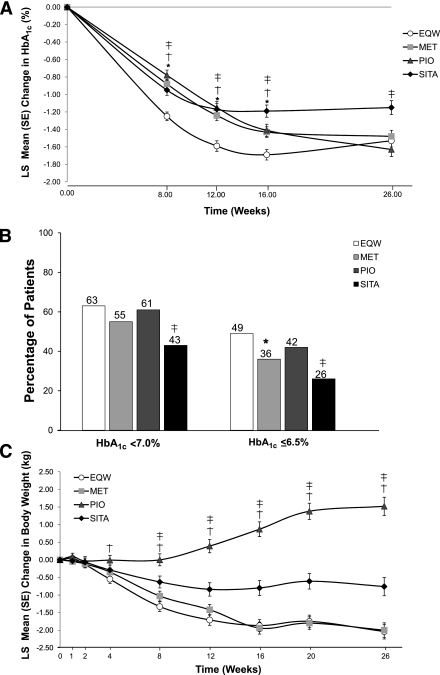
Mean baseline HbA1c values ranged from 8.4 to 8.6% across treatment groups. Figure 2A presents the time course of HbA1c change from modified MMRM analysis (intent-to-treat sample; all postbaseline observations included). This modified analysis demonstrated LS mean (SE) reductions in HbA1c at 26 weeks of −1.53% (0.07%) with EQW; −1.48% (0.07%) with MET (P = 0.620 vs. EQW; 98.3% CI was −0.26 to 0.17); −1.63% (0.08%) with PIO (P = 0.328 vs. EQW; 98.3% CI was −0.15 to 0.35); and −1.15% (0.08%) with SITA (P < 0.001 vs. EQW; 98.3% CI was −0.62 to −0.13).
Figure 2.
A: Changes in HbA1c over 26 weeks. *Significant difference between MET and EQW (all P ≤ 0.002). †Significant difference between PIO and EQW (all P ≤ 0.003). ‡Significant difference between SITA and EQW (all P < 0.001). B: Percentages of patients achieving HbA1c <7.0%, and ≤6.5% at end point (last observation carried forward). *Significant difference between MET and EQW (P = 0.004). ‡Significant difference between SITA and EQW (all P < 0.001). C: Changes in weight over 26 weeks. †Significant difference between PIO and EQW (all P ≤ 0.003). ‡Significant difference between SITA and EQW (all P ≤ 0.002).
The LS mean (SE) HbA1c at end point was 6.94 (0.07), 6.99 (0.07), 6.84 (0.08), and 7.32 (0.08) for EQW, MET, PIO, and SITA, respectively. The original primary analysis, which excluded post-baseline observations from patients who discontinued before week 8, showed LS mean (SE) reductions in HbA1c of −1.56% (0.07%) with EQW; −1.52% (0.06%) with MET (P = 0.609 vs. EQW; 98.3% CI was −0.26 to 0.17); −1.73% (0.08%) with PIO (P = 0.107 vs. EQW; 98.3% CI was −0.08 to 0.41); and −1.17% (0.08%) with SITA (P < 0.001 vs. EQW; 98.3% CI was −0.63 to −0.16). Findings from original and modified primary analyses were consistent and demonstrated that EQW was noninferior to MET and SITA, but did not reach the noninferiority measure with PIO. Subsequent hypothesis testing concluded that EQW was superior to SITA, but not to MET.
Similar percentages of EQW-, MET-, and PIO-treated patients reached a target HbA1c <7.0% (Fig. 2B). Significantly more patients treated with EQW versus SITA (P < 0.001) achieved HbA1c <7.0% after 26 weeks. Moreover, significantly more patients treated with EQW achieved HbA1c ≤6.5% after 26 weeks, versus MET- and SITA-treated patients (P = 0.004 and P < 0.001, respectively).
LS mean baseline fasting serum glucose values ranged from 9.7 to 9.9 mmol/L across treatment groups. Reductions in fasting serum glucose at 16 and 26 weeks were significantly greater in patients treated with EQW versus SITA (both P < 0.001). LS mean (SE) reductions in fasting serum glucose at 26 weeks were −2.3 mmol/L (0.1 mmol/L) in patients treated with EQW; −2.0 mmol/L (0.1 mmol/L) with MET (P = 0.155 vs. EQW); −2.6 mmol/L (0.2 mmol/L) with PIO (P = 0.153 vs. EQW); and −1.1 mmol/L (0.2 mmol/L) with SITA (P < 0.001 vs. EQW).
Mean seven-point SMBG profiles showed similar reductions in blood glucose from baseline to 26 weeks in EQW, MET, and PIO treatment groups (Supplementary Figs. 1A–C). There were no significant differences between EQW versus MET or PIO treatment groups with regard to changes from baseline for any of the seven time points or for daily mean SMBG; however, EQW was associated with greater mean reductions at all time points compared with SITA. Mean reductions in SMBG postmeal excursions after 26 weeks were similar among all treatment groups.
LS mean baseline weight values ranged from 85.9 to 88.6 kg. Weight decreased with EQW, MET, and SITA treatment, but increased with PIO treatment (Fig. 2C). Weight changes were significantly different between EQW versus PIO and SITA starting at 4 and 8 weeks, respectively, and continued through 26 weeks (all P ≤ 0.003). At 26 weeks, LS mean (SE) body weight changes were −2.0 kg (0.2 kg) with EQW; −2.0 kg (0.2 kg) with MET (P = 0.892 vs. EQW); +1.5 kg (0.3 kg) with PIO (P < 0.001 vs. EQW); and −0.8 kg (0.3 kg) with SITA (P < 0.001 vs. EQW). The percentage of patients with reductions in both body weight and HbA1c was similar for EQW and MET; percentages were comparably lower with PIO and SITA (Supplementary Figs. 2A–D).
Pancreatic β-cell function and insulin sensitivity
β-Cell function, as measured by geometric mean HOMA-B (C-peptide), at baseline ranged from 51.0 to 54.4%. Mean (SE) HOMA-B (ratio of end point [last observation carried forward] to baseline) was significantly (all P < 0.001) improved in patients treated with EQW [+1.8 (0.06)] compared with MET [+1.4 (0.04)], PIO [+1.3 (0.05)], and SITA [+1.3 (0.04)]. Insulin sensitivity, as measured by geometric mean HOMA-S (C-peptide), at baseline ranged from 36.4 to 39.5%. Mean (SE) HOMA-S (ratio of end point [last observation carried forward] to baseline) was significantly (both P < 0.001) improved in patients treated with MET [+1.3 (0.04)] and PIO [+1.5 (0.06)] compared with EQW [+1.0 (0.03)]; change with EQW was similar to SITA [+1.0 (0.04), P = 0.329].
Safety and tolerability
Serious adverse events were reported in 1.6% (4/248), 5.3% (13/246), 5.5% (9/163), and 1.8% (3/163) of EQW-, MET-, PIO-, and SITA-treated patients, respectively, during the treatment period. No serious adverse event was reported by more than one patient. One death, due to gastric adenocarcinoma, was reported during the 10-week follow-up in a patient receiving MET and was not considered by the investigator to be related to the study drug. One MET-treated patient experienced an adverse event of injection site nodule, considered serious for other medically significant reasons (i.e., psychological). The event was of mild severity and resolved without treatment.
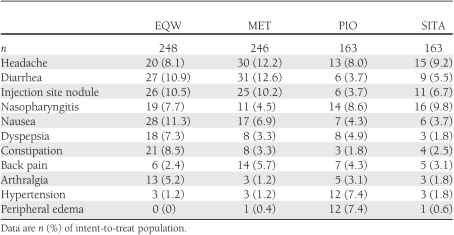
Treatment-emergent adverse events reported by ≥5% of patients in any group during the treatment period are shown in Table 1. All patients received injection, either with active EQW or placebo microsphere injection. Injection site nodules were more commonly reported with active EQW and placebo injection administered in the MET arm compared with placebo injection administered in the PIO and SITA arms. Nausea, the most common treatment-emergent adverse event with EQW, occurred in 11.3% of patients in this group. One EQW-treated patient withdrew because of nausea. Vomiting was reported in <5% of patients in all treatment groups, with incidences of 4.8% (12/248), 3.3% (8/246), 3.1% (5/163), and 1.8% (3/163) in EQW-, MET-, PIO-, and SITA-treated patients, respectively. Most treatment-emergent adverse events had resolved by the end of the 10-week follow-up period (Supplementary Table 2).
Table 1.
Treatment-emergent adverse events reported in ≥5% of patients in any treatment group
No patient had a major hypoglycemic episode during the treatment period of the study. The incidence of hypoglycemia unconfirmed by glucose measurement was low in all treatment groups: 5.2% (13/248), 4.1% (10/246), 3.7% (6/163), and 3.1% (5/163) of EQW-, MET-, PIO-, and SITA-treated patients, respectively. Minor (confirmed) hypoglycemia was limited to a small group of EQW-treated patients (5/248, 2.0%).
No clinically significant changes in fasting serum lipids were observed during the treatment period. LS mean (SE) systolic blood pressure reductions of −1.3 mmHg (0.8 mmHg), −1.7 mmHg (1.0 mmHg), and −1.8 mmHg (1.0 mmHg) were observed with EQW, PIO, and SITA treatment, respectively. Additionally, a reduction in diastolic blood pressure of −2.5 mmHg (0.6 mmHg) was observed with PIO treatment. Mean (SD) heart rate (bpm) increases were observed in the EQW [+1.5 (10.0)], MET [+0.3 (9.5)], and SITA [+0.5 (9.7)] groups; mean heart rate decreased in the PIO group [–1.7 (8.7)].
No clinically significant changes in mean amylase, lipase, or calcitonin were observed during the treatment period. One SITA-treated patient with elevated lipase at screening experienced moderate chronic pancreatitis after 8 days of treatment and discontinued from study treatment.
Lower-titer (<1/125) and higher-titer (≥1/625) antibodies to exenatide at end point were observed in 43.1% (107/248) and 11.3% (28/248) of EQW-treated patients, respectively. Mean HbA1c was reduced irrespective of antibody status: negative (−1.33%), positive lower (−1.51%), and positive higher (−1.02%) (Supplementary Table 3). Events with higher incidence in antibody-positive patients included injection site–related events (i.e., injection site erythema, injection site extravasation, injection site hematoma, injection site nodule, injection site pruritus, and injection site reaction). Injection site nodules are expected with microsphere injection and were not reported as an adverse event unless accompanied by symptoms such as pain, erythema, or pruritus.
Patients in all treatment groups had mean improvements from baseline in perceived treatment satisfaction, weight-related quality of life, and binge-eating behavior (Supplementary Tables 4–9). All treatments with the exception of PIO were associated with significant mean improvements in health status. Significant improvements in weight-related quality of life, binge-eating behavior, and health status were reported for patients treated with EQW compared with PIO.
CONCLUSIONS
Head-to-head comparative studies are needed to better inform treatment decisions for type 2 diabetes (1), a disease for which there are numerous treatment options. This study evaluated the efficacy and safety of EQW monotherapy, in comparison with MET, PIO, and SITA monotherapy, in drug-naive patients with type 2 diabetes. Treatment with EQW, MET, and PIO all resulted in improvements in glycemic control, achieving mean HbA1c concentrations at 26 weeks of <7%. EQW demonstrated superiority to SITA and noninferiority to MET but not to PIO with regard to HbA1c reduction at 26 weeks. The lack of noninferiority of EQW monotherapy compared with PIO monotherapy was unexpected based on DURATION-2 study results, where EQW was superior to PIO on a background of MET (10), and the fact that the HbA1c reduction with PIO was greater than that reported in other PIO studies with a similar baseline HbA1c (2,3,11–14). Several disparate factors could have contributed to the incongruent outcomes, including differences in background therapy and mean duration of type 2 diabetes.
SITA treatment led to comparatively lesser glycemic improvement than the other agents, which supports the contention that the increase in plasma glucagon-like peptide-1 associated with dipeptidyl peptidase-4 inhibition is not as effective as pharmacological concentrations achieved with receptor agonism (15,16). The similar effects obtained with EQW monotherapy and MET monotherapy in terms of HbA1c and body weight reductions support the appropriateness of considering MET as the first-line antihyperglycemic agent in type 2 diabetes. For example, the ADA/EASD recommends MET as a first-line agent; other antihyperglycemic agents are added in stepwise fashion with disease progression after MET failure (1). Findings from DURATION-4 demonstrate that both EQW and PIO offer similar improvements in glycemia as MET, with one key difference between these agents being the effect on body weight. Thus, health care practitioners have more treatment choices for drug-naive patients.
Clinicians are advised to consider multiple factors when treating patients with type 2 diabetes. Similar to previous studies evaluating PIO as monotherapy (3,13), patients treated with PIO in the current study gained weight. EQW and MET led to similar significant reductions in body weight after 26 weeks, whereas SITA led to a lesser reduction. Although variability based on study design and patient characteristics was expected, weight reduction observed in this study with EQW was on the lower end of the range (−2.0 to −3.7 kg) observed in previous DURATION studies (6,7,10,17). Conversely, reductions observed with SITA and MET in the current study tended to be greater than results from meta-analyses and recent monotherapy trials of similar duration (5,18–20). The potential for improvements in HbA1c and body weight in studies with placebo injection therapy has been noted in a previous study of exenatide therapy (21). These data show that EQW and MET provided the best profile in terms of improving glycemic control as well as reducing weight, without increased risk of hypoglycemia, particularly important for treatment of overweight or obese patients with type 2 diabetes.
The findings with respect to safety and tolerability were consistent with previous studies, including the observance of gastrointestinal adverse events in patients treated with EQW and MET, and hypertension and peripheral edema with PIO (3,5,6,10,17,20,22–24). Most notably, the safety profile of EQW in the current trial was consistent with previous DURATION studies (6,10,17), with low rates of confirmed minor hypoglycemia and few patients discontinuing treatment due to nausea (one patient) and vomiting (zero patients). Less nausea and vomiting with EQW have previously been reported in head-to-head studies versus exenatide BID. In general, hypoglycemia incidence and incidence of serious adverse events were low across all treatment groups during the current study. The small percentage (2%) of patients experiencing minor hypoglycemia with EQW monotherapy was similar to that observed with EQW adjunct to MET in previous clinical trials (0–3%) (6,7,10,17). Most treatment-emergent adverse events in this study had resolved by the end of the 10-week safety follow-up period, with few treatment-emergent adverse events starting during the safety follow-up.
There were several limitations to this study. First, patients were enrolled based on specific criteria and were followed according to the study schedule, which may not reflect real-world use. Second, no specific compliance data were collected; however, patient-reported outcomes indicated that both oral and injectable therapies were associated with increases in treatment satisfaction and quality of life in these previously drug-naive patients. Additionally, 26 weeks is too short a study duration to evaluate long-term glycemic control, weight loss, and β-cell preservation (25). For example, potential implications of the upward shift in HbA1c, observed in the EQW group between weeks 16 and 26 (Fig. 2A), cannot be assessed further without additional data points.
In conclusion, in these patients with type 2 diabetes who were naive to antihyperglycemic therapy, all four treatments resulted in improvements in HbA1c, and a majority of EQW-, MET-, and PIO-treated patients achieved a target HbA1c of <7.0%. This study recognizes that guidelines typically recommend MET as the first agent used, because it is inexpensive and supported by long-term data. Of the agents studied, EQW and MET provided similar improvements in glycemic control along with the benefit of weight reduction and no increased risk of hypoglycemia. There were no unexpected findings with regard to safety or tolerability. Based on these 26-week data, EQW is a once-weekly dosing option for initial therapy. Longer-term studies will be required to assess the durability of the observations in this study.
Supplementary Material
Acknowledgments
This study was funded by Amylin Pharmaceuticals (San Diego, CA) and Eli Lilly (Indianapolis, IN). D.R.-J. served as an investigator on clinical trials sponsored by Eli Lilly and Amylin Pharmaceuticals; is an advisory board member for Eli Lilly, Novo Nordisk, GlaxoSmithKline, and Takeda; and has received education grant support from Eli Lilly. R.M.C. has served as an investigator on clinical trials sponsored by Amylin, Abbott, Bayer, Calibra Medical, Daiichi-Sankyo, Dexcom, Edwards Lifesciences, Eli Lilly, Hygeia, Intarcia, Johnson & Johnson, MannKind, Medtronic, Merck, Novo Nordisk, Quotient Diagnostics, ResMed, Roche, sanofi-aventis, Schering-Plough, Takeda, and Valeritas. R.M.C. was an advisory board member for Abbott, Bayer, Eli Lilly, Novo Nordisk, and Roche and has received education grant support from Johnson & Johnson, Eli Lilly, Merck, Novartis, sanofi-aventis, and Schering-Plough. All honoraria, speaking fees, consulting fees, and research and educational support were paid directly to the nonprofit International Diabetes Center, of which R.M.C. was a salaried employee. In 2011, R.M.C. became an employee of sanofi-aventis. M.H. is an advisory board member for sanofi-aventis and Novartis and has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme Idea, Novartis, and sanofi-aventis. A.K. has received education grant support from Boeringher Ingelheim, Eli Lilly, Johnson & Johnson, Merck, Novo Nordisk, Spherix, and Takeda. A.K. has received speaking fees, honoraria, or travel support from Intas Pharmaceuticals, Ltd., Novo Nordisk, Merck, and USV. M.C., A.M.W., and M.K.B. are employees and/or shareholders of Eli Lilly. No other potential conflicts of interest relevant to this article were reported.
D.R.-J., R.M.C., M.H., A.K., and J.G.G. were principal clinical investigators for this trial. M.C. was the lead statistician. A.M.W. was the project manager, performed literature searches, and drafted sections of the manuscript. M.K.B. was involved in the study design and protocol development. All coauthors contributed to the interpretation of data, critically revised the manuscript for important intellectual content, and approved the version to be published. M.K.B. is the designated guarantor for this publication.
Parts of this study were presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, CA, 24–28 June 2011.
The authors thank Michael Trautmann, MD (Eli Lilly), Justin Northrup, MPT (Eli Lilly), Matt Reaney, MSc (Eli Lilly), Jaret Malloy, PhD (Amylin Pharmaceuticals), and Dr. Luis de Teresa (Clínica Mediterránea de Neurociencias, Medicina Innovadora, Alicante, Spain), who provided medical, scientific, and writing support for this article. The authors thank the patients, investigators, and staffs at the 124 sites in the 22 participating countries (Argentina, Belgium, Brazil, Canada, France, Germany, Hungary, India, Israel, Italy, South Korea, Mexico, Poland, Romania, the Russian Federation, Slovakia, South Africa, Spain, Taiwan, Turkey, the United Kingdom, and the United States).
Footnotes
Clinical trial reg. no. NCT00676338, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1107/-/DC1.
*A complete list of study group participants can be found in the Supplementary Data.
References
- 1.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 10.2337/dc08-9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P; Quartet [corrected] Study Group Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab 2004;89:6068–6076 10.1210/jc.2003-030861 [DOI] [PubMed] [Google Scholar]
- 3.Pavo I, Jermendy G, Varkonyi TT, et al. Effect of pioglitazone compared with metformin on glycemic control and indicators of insulin sensitivity in recently diagnosed patients with type 2 diabetes. J Clin Endocrinol Metab 2003;88:1637–1645 10.1210/jc.2002-021786 [DOI] [PubMed] [Google Scholar]
- 4.Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008;30:1448–1460 10.1016/j.clinthera.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Aschner P, Katzeff HL, Guo H, et al. Efficacy and safety of monotherapy of sitagliptin compared with metformin in patients with type 2 diabetes. Diabetes Obes Metab 2010;12:252–261 10.1111/j.1463-1326.2009.01187.x [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 10.1016/S0140-6736(08)61206-4 [DOI] [PubMed] [Google Scholar]
- 7.Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:1301–1310 10.1210/jc.2010-2081 [DOI] [PubMed] [Google Scholar]
- 8.Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 1988;75:383–386 10.1093/biomet/75.2.383 [DOI] [Google Scholar]
- 9.Dmitrienko A, Molenberghs G, Chuang-Stein C, Offen W. Analysis of Clinical Trials Using SAS: A Practical Guide. Cary, NC, SAS Institute Inc, 2005 [Google Scholar]
- 10.Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431–439 10.1016/S0140-6736(10)60590-9 [DOI] [PubMed] [Google Scholar]
- 11.Tan MH, Johns D, Strand J, et al. Sustained effects of pioglitazone vs. glibenclamide on insulin sensitivity, glycaemic control, and lipid profiles in patients with type 2 diabetes. Diabet Med 2004;21:859–866 10.1111/j.1464-5491.2004.01258.x [DOI] [PubMed] [Google Scholar]
- 12.Tan MH, Baksi A, Krahulec B, et al. Comparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care 2005;28:544–550 10.2337/diacare.28.3.544 [DOI] [PubMed] [Google Scholar]
- 13.Rosenstock J, Kim SW, Baron MA, et al. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 2007;9:175–185 10.1111/j.1463-1326.2006.00698.x [DOI] [PubMed] [Google Scholar]
- 14.Charbonnel BH, Matthews DR, Schernthaner G, Hanefeld M, Brunetti P; QUARTET Study Group A long-term comparison of pioglitazone and gliclazide in patients with type 2 diabetes mellitus: a randomized, double-blind, parallel-group comparison trial. Diabet Med 2005;22:399–405 10.1111/j.1464-5491.2004.01426.x [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008;24:2943–2952 10.1185/03007990802418851 [DOI] [PubMed] [Google Scholar]
- 16.Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–1456 10.1016/S0140-6736(10)60307-8 [DOI] [PubMed] [Google Scholar]
- 17.Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 2010;375:2234–2243 10.1016/S0140-6736(10)60406-0 [DOI] [PubMed] [Google Scholar]
- 18.Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with type 2 diabetes. Diabet Med 2007;24:955–961 10.1111/j.1464-5491.2007.02191.x [DOI] [PubMed] [Google Scholar]
- 19.Johansen K. Efficacy of metformin in the treatment of NIDDM: meta-analysis. Diabetes Care 1999;22:33–37 10.2337/diacare.22.1.33 [DOI] [PubMed] [Google Scholar]
- 20.Jadzinsky M, Pfützner A, Paz-Pacheco E, et al. Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009;11:611–622 10.1111/j.1463-1326.2009.01056.x [DOI] [PubMed] [Google Scholar]
- 21.Liutkus J, Rosas Guzman J, Norwood P, et al. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin. Diabetes Obes Metab 2010;12:1058–1065 10.1111/j.1463-1326.2010.01251.x [DOI] [PubMed] [Google Scholar]
- 22.Schweizer A, Dejager S, Bosi E. Comparison of vildagliptin and metformin monotherapy in elderly patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Obes Metab 2009;11:804–812 10.1111/j.1463-1326.2009.01051.x [DOI] [PubMed] [Google Scholar]
- 23.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE; Sitagliptin 036 Study Group Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007;30:1979–1987 10.2337/dc07-0627 [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Goodman AM; The Multicenter Metformin Study Group Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1995;333:541–549 10.1056/NEJM199508313330902 [DOI] [PubMed] [Google Scholar]
- 25.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 10.2337/dc11-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.