Abstract
OBJECTIVE
To describe the long-term metabolic outcome of children with congenital hyperinsulinism after near-total or partial elective pancreatectomy.
RESEARCH DESIGN AND METHODS
Patients (n = 105: 58 diffuse and 47 focal congenital hyperinsulinism) received operations between 1984 and 2006. Follow-up consisted of periodic measurements of pre- and postprandial plasma glucose over 24 h, OGTT, and IVGTT. Cumulative incidence of hypo- or hyperglycemia/insulin treatment was estimated by Kaplan-Meier analysis.
RESULTS
After near-total pancreatectomy, 59% of children with diffuse congenital hyperinsulinism still presented mild or asymptomatic hypoglycemia that responded to medical treatments and disappeared within 5 years. One-third of the patients had both preprandial hypoglycemia and postprandial hyperglycemia. Hyperglycemia was found in 53% of the patients immediately after surgery; its incidence increased regularly to 100% at 13 years. The cumulative incidence of insulin-treated patients was 42% at 8 years and reached 91% at 14 years, but the progression to insulin dependence was very variable among the patients. Plasma insulin responses to IVGTT and OGTT correlated well with glycemic alterations. In focal congenital hyperinsulinism, hypoglycemia or hyperglycemia were rare, mild, and transient.
CONCLUSIONS
Patients with focal congenital hyperinsulinism are cured of hypoglycemia after limited surgery, while the outcome of diffuse congenital hyperinsulinism is very variable after near-total pancreatectomy. The incidence of insulin-dependent diabetes is very high in early adolescence.
Congenital hyperinsulinism is the most common cause of persistent severe hypoglycemia at birth or in early infancy, requiring early intensive multidisciplinary management to prevent brain damage (1,2). The inappropriate secretion of insulin by the β-cells has heterogeneous genetic origins. In the neonatal forms of congenital hyperinsulinism, the most common genetic mutations (in ABCC8 and KCNJ11 genes) cause alterations of the ATP-dependent K+ channels (SUR1 and Kir6.2 subunits) in the β-cells, which play a critical role in the regulation of insulin secretion (3).
The frequent resistance of congenital hyperinsulinism to medical treatments often leads to surgery, which has for a long time consisted of near-total pancreatectomy, with a high risk of insulin-dependent diabetes (4–7). However, the differentiation between two forms—the diffuse form with no histological anomalies but signs of β-cell hyperfunction and the adenomatous localized hyperplasia—has considerably changed the surgical treatment and the prognosis of the disease (8,9). The focal forms can indeed be definitively cured of hypoglycemia with partial elective pancreatectomy (10). The preoperative distinction between the two forms was initially established using selective pancreatic venous catherization (11,12), which has recently been abandoned for the less invasive positron emission tomography with [18F]fluoro-l-DOPA (13).
The long-term outcome of patients suffering from congenital hyperinsulinism has not been well described (4,5,14–17). Few studies have clearly differentiated diffuse and focal forms of insulin hypersecretion. If the incidence of postoperative recurrent hypoglycemia was often documented, the follow-up was often short and data upon glucose tolerance and insulin secretion were rarely available, making it difficult to evaluate the incidence of postsurgical diabetes, although the risk seems to be high before the second decade of life after near-total pancreatectomy (4,5,16).
We report the long-term follow-up study of glucose and insulin metabolism in 105 children who underwent surgery for either a focal or a diffuse form of congenital hyperinsulinism. The study clearly confirms the contrast between the evolution of the two forms but also shows the great variability in the incidence of hypoglycemia and hyperglycemia within the diffuse form and contributes to characterizing insulin secretion and sensitivity and to determining the investigations needed for an optimal follow-up.
RESEARCH DESIGN AND METHODS
From 1984 to 2006, 105 patients received operations for congenital hyperinsulinism at Hôpital Necker–Enfants Malades and were followed over the long term (Table 1). The first symptoms occurred in 87 neonates (<1 week), and after 1 month (to 1 year) of age in 18 cases. The patients presented with severe (<0.3 mmol/L) pre- and postprandial hypoglycemias, with no ketones, concomitant with relatively high plasma insulin levels (>3 mU/L) and a glycemic response to subcutaneous glucagon. Syndromic or hyperammonemic hyperinsulinisms and atypical histological forms of hyperinsulinism were not included in the study. In most patients, the distinction between diffuse and focal hyperinsulinism was based on transhepatic portal catheterization (11,15) or on results of a positron emission tomography scan with [18F]fluoro-L-DOPA (13).
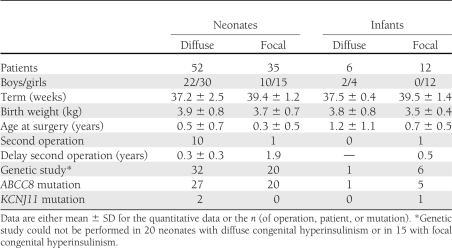
Table 1.
Characteristics of 105 children who underwent near-total or partial elective pancreatectomy for congenital hyperinsulinism
Surgery was indicated in all patients with a focal form and in those with a diffused form of congenital hyperinsulinism that resisted medical treatments where resection was always identical. For the children who required a second surgery (persistent hypoglycemia), the evaluation of follow-up started from the second intervention.
During surgery, biopsies were taken in various areas of the pancreas and analyzed extemporaneously to confirm the form, localize the focal lesion, and determine the extent of the pancreatectomy (8,18,19). For focal lesions, surgery was limited to the head of the pancreas in 13 patients, the body in 6, and the tail in 5; the head and body of the pancreas in 4 patients, the head and tail in 4, and the body and tail in 4; and two-thirds of the pancreas in 3 (not specified in 3 cases).
If a sample was taken, pancreatic DNA was extracted to search for the specific loss of allele in the 11p15 region in the focal lesion. When samples were available, mutations of the ABCC8, KCNJ11, and the glucokinase genes were searched for on blood leukocytes of the patients and their parents, using PCR and direct sequencing (Table 1).
Glucose metabolism and insulin secretion were evaluated immediately after surgery and then periodically for a mean duration of 6.3 ± 2.1 years (range 1–19) in the diffuse forms and 2.6 ± 4.1 years (0–17) in the focal forms. The following tests were used: HbA1c, plasma glucose, insulin and C-peptide concentrations over 24 h (before and after each meal and at 0 and 4 h; 168 24-h cycles in 49 children with diffuse and 67 cycles in 39 children with focal congenital hyperinsulinism), oral glucose tolerance test (OGTT) (77 tests in 23 children with diffuse and 50 in 27 children with focal congenital hyperinsulinism), and intravenous glucose tolerance test (IVGTT) (41 tests in 15 children with diffuse and 21 in 11 children with focal congenital hyperinsulinism). Hypoglycemia was defined as a value <3 mmol/L during the 24-h cycle (1). Hyperglycemia was defined as an abnormal OGTT (World Health Organization criteria), a plasma glucose level >11 mmol/L during the 24-h cycle, or HbA1c >6%. Insulin treatment was generally initiated based on two main criteria: permanent hyperglycemia (pre- and postprandial) and elevated HbA1c (>7%). Plasma glucose was measured by a glucose oxidase technique and plasma insulin by radioimmunoassay (Bi-Insulin IRMA Ciis-Bio International; Gif-sur-Yvette, France). HbA1c was determined by high-pressure liquid chromatography (Tosoh Bioscience A1C 2.2) (National Glycohemoglobin Standardization Program: normal values 4.2–5.6%).
Insulin secretion indices were computed as the ratios of area under the curve (AUC)insulin to AUCglucose and of Δinsulin30–0 min to glucose30 min during OGTT and as the sum of insulin levels at 1′ and 3′ during IVGTT (20). Insulin sensitivity was assessed by the composite insulin sensitivity index (21). Homeostasis model assessment (HOMA) indices of β-cell function and insulin sensitivity were computed from baseline fasting levels of glucose and insulin and expressed as percentage of average values found in young fit subjects (22).
Statistical analysis
Data are given as means ± SD unless otherwise indicated. The evolution of hypoglycemia and hyperglycemia or initiation of insulin treatment was estimated using Kaplan–Meier analysis. Given the small variations in the age of surgery, it was considered more appropriate to present these results in relation to age rather than time after surgery.
Average values of glycemic control, glucose tolerance, insulin secretion, and insulin sensitivity throughout the follow-up were compared between children with focal or diffuse forms of congenital hyperinsulinism. To take into account that a different number of tests was performed in different individuals and in order to use all the available data to maximize results, we compared data between groups as standard least squares computed in mixed regression models with random effects, including as covariables sex, the duration of follow-up when the parameter was measured, and the subject identification number. Statistics were performed with JMP software (SAS Institute, Inc., Cary, NC).
RESULTS
Focal hyperinsulinism
Among 47 children who underwent operations for focal congenital hyperinsulinism, none received any antidiabetes treatment. Four children presented persisting but asymptomatic hypoglycemias (three neonatal and one infant form) during the 24-h cycles immediately after surgery. In only one case, asymptomatic hypoglycemias were also found at the age of 4 years, but this patient showed no abnormalities at 3 or 5 years of age.
As for hyperglycemia, minor and transitory anomalies were found. A child had a plasma glucose of 8.8 mmol/L at 120 min of the OGTT at age 4 years, but the OGTT was normal at age 6 years. Four children showed plasma glucose values between 11.5 and 14.4 mmol/L at 30 or 60 min of OGTTs postoperatively and at 2, 4, and 10 years of age, but they showed normal 24-h glucose cycles and no abnormalities at all other ages. In these five patients, HbA1c remained in normal range for the whole duration of the follow-up.
Diffuse hyperinsulinism
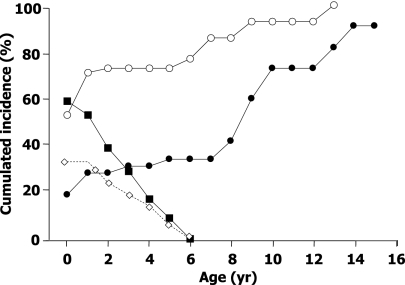
Of 58 children who underwent near-total pancreatectomy for diffuse congenital hyperinsulinism, 59% were still suffering from hypoglycemias immediately after surgery (after first surgery, 48 children; after second surgery, 10 children). However, hypoglycemias did not have the severity of those before surgery and they occurred principally in the preprandial periods, especially at the end of the night. They could be controlled by dietetic adjustments (raw corn starch at bedtime in 16 children) and medical treatments (in 15 patients: diazoxide in 10, octreotide in 6, oral steroids in 11, and nifedipine in 2 and diazoxide-octreotide, diazoxide-steroids, octreotide-steroids, or diazoxide-nifedipine in 2–3 patients each). The incidence of hypoglycemia decreased slowly, in some cases persisting until 5 years of age (Fig. 1).
Figure 1.
Cumulative incidence of hypoglycemia (■), hyperglycemia (○), association hypohyperglycemia (◇), and insulin therapy (●) in 58 patients pancreatectomized for diffuse congenital hyperinsulinism. Patients with both hypo- and hyperglycemia are a subset of both hypo- and hyperglycemia groups. yr, years.
A very peculiar and unusual situation was observed in 19 patients who presented fasting persisting hypoglycemia (value <3 mmol/L on the 24-h cycle) associated with postprandial hyperglycemia (>11 mmol/L on the 24-h cycle or impaired glucose tolerance or diabetes at the OGTT). This association, in 35% of the children after surgery, could persist until 5 years of age (Fig. 1).
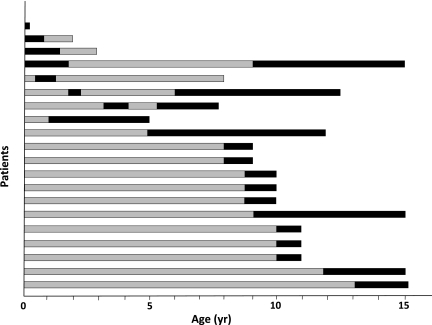
One-half of the patients (53%) with diffuse congenital hyperinsulinism presented with hyperglycemia in the days after surgery. The incidence of hyperglycemia increased regularly with age, reaching 100% at the age of 13 years (Fig. 1). The incidence of insulin therapy increased from 19% postoperatively to 42% at 8 years and then more rapidly to reach 91% at 14 years of age. However, it does not depict the great variability of the postoperative progression toward definitive need for insulin in the 33 treated patients. Insulin therapy was started immediately after surgery and definitively (follow-up 5.4 ± 3.8 years [range 0.5–11]) in eight children. It was initiated after a delay, between 5 months after surgery and 14.8 years of age (age 8.4 ± 4.2 years), and definitively in 13 patients (Fig. 2). For the last 12 patients, the evolution was more erratic. Five patients required insulin immediately after surgery but for a short period of time (1–16 days); four of these five children remained hyperglycemic but untreated (follow-up 5.5 ± 4.1 years [1–11]), while the last one did not show any abnormality in glucose tolerance up to 6 years of age. Four patients received insulin immediately after surgery for a longer period of time, 2–21 months (Fig. 2), but it had to be interrupted for recurrent hypoglycemia; the first three remained hyperglycemic but untreated until 1–3 years of age, while the fourth again received insulin when 9 years old. For the last three patients, insulin was started several months after surgery, but it had to be stopped after 8–11 months for recurrent hypoglycemia; one child remained untreated until 7.9 years of age, while two were again treated with insulin before 6 years of age.
Figure 2.
Illustration of changes over time of insulin requirements in 20 of the 33 patients pancreatectomized for diffuse congenital hyperinsulinism. Four upper bars: Patients with insulin immediately after surgery but transiently. The three following bars: Patients with insulin after a delay but transiently. Thirteen lower bars: Patients with insulin after a delay and definitively. The other patients are not presented: eight with insulin immediately after surgery and definitively, with no insulin treatment (including five who received insulin immediately after surgery for 1–16 days). Dark bars, time of insulin therapy; gray bars, time of follow-up, with hyperglycemia but without insulin. yr, years.
Insulin secretion and sensitivity
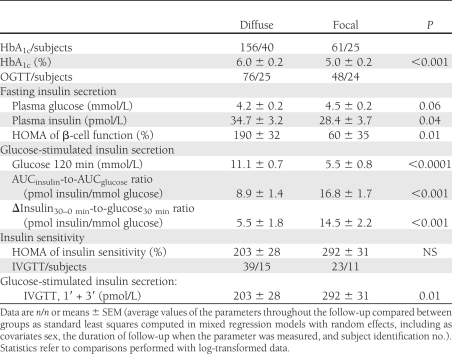
In the fasting state, plasma insulin was significantly higher in the diffuse than in the focal forms, and this was associated with slightly lower plasma glucose values. By contrast, all indices of glucose-stimulated insulin secretion were significantly lower, which was associated with postprandial (or postglucose) hyperglycemia in the diffuse forms. Indices of insulin sensitivity did not differ between the two forms (Table 2).
Table 2.
Insulin secretion and sensitivity in children who underwent near-total or partial elective pancreatectomy for congenital hyperinsulinism
Metabolic follow-up after near-total pancreatectomy
Comparisons were made between results of the OGTT, the 24-h glucose cycle, and HbA1c in the evaluation of the metabolic status in diffuse hyperinsulinism, when at least two tests were performed on the same day: 72 pairs with OGTT and HbA1c, 95 with the 24-h cycle and HbA1c, and 56 with OGTT and the 24-h cycle. When the OGTT was normal, the other tests were also normal in most cases. When the OGTT showed criteria of diabetes, hyperglycemia was also found during the 24-h cycle in 81% of the patients and an HbA1c >5.6% in 58% of the patients. With criteria of impaired glucose tolerance at the OGTT, hyperglycemia was found in 18% and an HbA1c >5.6% in 36% of the patients. When the 24-h cycle showed hyperglycemia, HbA1c was >5.6% in 56% of the patients. Relative to that of the OGTT, the sensitivity of the 24-h glucose cycle was greater than that of HbA1c (81 vs. 58%, respectively) for diabetes criteria, but it was very low with both tests (18 and 36%) for impaired glucose tolerance. The specificity varied from 69% (HbA1c) to 80% (24-h cycle) for diabetes and from 79 to 75% for impaired glucose tolerance.
CONCLUSIONS
The aim of this work was to extend the description of the metabolic evolution of patients who were pancreatectomized for congenital hyperinsulinism. We previously reported the general evolution of 52 neonatal forms of congenital hyperinsulinism after near-total or partial elective pancreatectomy (15). The greater number of patients followed for a longer period of time after surgical treatment based on well-identified lesions makes these updated results of interest.
The present results illustrate the dramatic contrast in metabolic outcome between the two forms of congenital hyperinsulinism. The follow-up of 47 focal forms of hyperinsulinism strongly confirms that the children who underwent partial elective pancreatectomy were cured of hypoglycemia. Our previous results and those of other teams led to a 2000 European consensus on the management of persistent congenital hyperinsulinism, which recommended a conservative surgical attitude in the focal forms (1). The development of the positron emission tomography scan with [18F]fluoro-l-DOPA (23), a less invasive alternative to the transhepatic pancreatic catheterization, should help to generalize these recommendations and prevent patients from undergoing an unnecessary extended pancreatectomy leading to a progression toward insulin-dependent diabetes. However, one must keep in mind that the existence of rare multifocal and complex focal forms (23) of hyperinsulinism justifies the systematic and regular evaluation of the surgery-treated patients until complete disappearance of hypoglycemia. Even in the present series, two patients required a second surgery because all the hypersecreting hyperplasia had not been resected. In contrast, although the rare and mild hyperglycemia observed during follow-up in some patients did not show any predictive value of later deterioration of the metabolic status, the ablation of a limited but significant part of the pancreas could add to later aggravating factors, such as pregnancy, obesity, or aging, and confer on these subjects a risk of developing long-term anomalies in glucose tolerance. A regular and long-term follow-up, with periodic evaluation of glucose tolerance, is thus strongly recommended for these patients.
For the 58 operated diffuse forms, the cumulative incidence of hyperglycemia was 100% at 13 years of age and the incidence of insulin therapy reached 91% a year later. As opposed to this inevitable evolution, the very slow progression toward insulin-requiring diabetes is astonishing, such as the case of one 19-year-old patient, who was free of insulin with normal HbA1c and OGTT after a 95% pancreatectomy in the first months of life. Indeed, this study clearly shows that postoperative evolution is unpredictable and sometimes complex, with a recurrence of both hypo- and hyperglycemia. The age at institution of insulin also varied greatly. These characteristics are noteworthy, as they may seem unexpected in view of the extent of the pancreatic resection, but also unusual compared with what is known of the development of any other type of diabetes. However, whatever the variability of this evolution, the final result is insulin therapy that was started before puberty in more than one-half of the cases. These results thus confirm previous reports in the literature, with 33–40% of recurrent hypoglycemia risk, and the risk of being treated with insulin was estimated to be between 14 and 69% in studies enrolling enough patients to appreciate the incidence of this complication (4,5,16). However, they allow a nice detailed description of this evolution based on a clear definition of the histological form of the disease.
Such unpredictable evolution toward diabetes seems to reflect the persistent abnormal function of the pancreatic residual tissue and, indeed, renders even more complex the postoperative metabolic evaluation of the patients. Pancreatic endocrine function of these patients, using metabolic tests, has previously been evaluated in one study, showing the altered but variable insulin response to an IVGTT and its poor correlation with later evolution (24). Results in our patients illustrate that in some cases, the dramatic reduction of β-cell mass cannot compensate for the excessive insulin secretion of the remaining cells. Remarkably, dysfunction of the residual pancreatic tissue associates in most cases an excessive fasting secretion as a sequel of the β-cells dysfunction and an expected lower insulin secretory capacity in response to glucose as a sequel of the extended resection. This clearly explains the very novel association of fasting hypoglycemia and postprandial hyperglycemia observed in a number of these patients in the early years after surgery.
Mechanisms of progression toward diabetes cannot be definitely stated from this study. Some authors have suggested that mutation of the ATP-dependent K+ channels could increase β-cell apoptosis (25). However, the few data available report a lower incidence of diabetes in adulthood in diffuse congenital hyperinsulinism medically treated than that reported here. If involution of the β-cells in the remaining tissue of the patients could contribute to the progressive decline of insulin secretion and the development of diabetes, the removal of 95% of the pancreas is the most probable cause of developing diabetes.
These results also have implications for the various tests used to follow the patients (1). The 24-h glucose cycle is necessary to screen for hypoglycemia and must thus be used in all focal and diffuse forms as long as there is no certainty that there is no more risk of hypoglycemia. This is generally the case during the first months in the focal forms—the time to make sure that there is not a rare complex or multifocal form. This can last up to 5 years in the diffuse forms at an annual or biannual rate. For hyperglycemia, the OGTT remains the most sensitive test to detect early impairment of glucose tolerance and should be done every year starting the year after surgery. During OGTT, plasma insulin and glucose measurements at 30 and 60 min of the OGTT are not informative and can be omitted, as they have shown little impact for the detection of impaired glucose tolerance. IVGTT is not recommended during the follow-up, as it does not provide any pertinent supplemental information. HbA1c is of less interest for screening but becomes important when the patients reach the state of diabetes for the decision to start insulin therapy.
In conclusion, hypoglycemia due to focal forms of congenital hyperinsulinism is cured after partial elective pancreatectomy, but the dramatic outcome of the diffuse forms illustrates the urgent need for progress in medical treatment to reduce the surgical indications and prevent these patients from a high risk of insulin-dependent diabetes in childhood and adolescence.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
J.B. analyzed data and wrote the paper. M.C. collected and analyzed data. J.-B.A. collected data. K.L. performed the biochemical analysis and reviewed the RESEARCH DESIGN AND METHODS section. G.V. helped analyze IVGTT and OGTT results and reviewed the RESEARCH DESIGN AND METHODS and the RESULTS sections. V.V. and J.R. analyzed the biopsies. F.B. performed the radiological examination and reviewed the RESEARCH DESIGN AND METHODS section. C.N.-F. performed the surgery and collected data during the procedure. J.-M.S. collected data. J.-J.R. and P.d.L. collected and analyzed data and wrote the manuscript. J.-J.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Bellane-Chantelot (Unité fonctionnelle de génétique des maladies métaboliques et des neutropénies congénitale, groupe hospitalier Pitié-Salpétrière, Paris, France), who performed the genetic analysis. The authors are very grateful to Alan Delamater (Department of Pediatrics, University of Miami, Miami, FL), who corrected their English.
References
- 1.Aynsley-Green A, Hussain K, Hall J, et al. Practical management of hyperinsulinism in infancy. Arch Dis Child Fetal Neonatal Ed 2000;82:F98–F107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menni F, de Lonlay P, Sevin C, et al. Neurologic outcomes of 90 neonates and infants with persistent hyperinsulinemic hypoglycemia. Pediatrics 2001;107:476–479 [DOI] [PubMed] [Google Scholar]
- 3.Hussain K, Cosgrove KE. From congenital hyperinsulinism to diabetes mellitus: the role of pancreatic beta-cell KATP channels. Pediatr Diabetes 2005;6:103–113 [DOI] [PubMed] [Google Scholar]
- 4.Lovvorn HN 3rd, Nance ML, Ferry RJ Jr, Stolte L, Baker L, O'Neill JA Jr, Schnaufer L, Stanley CA, Adzick NS. Congenital hyperinsulinism and the surgeon: lessons learned over 35 years. J Pediatr Surg 1999;34:786–792 [DOI] [PubMed]
- 5.Shilyansky J, Fisher S, Cutz E, Perlman K, Filler RM. Is 95% pancreatectomy the procedure of choice for treatment of persistent hyperinsulinemic hypoglycemia of the neonate? J Pediatr Surg 1997;32:342–346 [DOI] [PubMed] [Google Scholar]
- 6.Thomas CG, Jr, Cuenca RE, Azizkhan RG, Underwood LE, Carney CN. Changing concepts of islet cell dysplasia in neonatal and infantile hyperinsulinism. World J Surg 1988;12:598–609 [DOI] [PubMed] [Google Scholar]
- 7.Thomas CG, Jr, Underwood LE, Carney CN, Dolcourt JL, Whitt JJ. Neonatal and infantile hypoglycemia due to insulin excess: new aspects of diagnosis and surgical management. Ann Surg 1977;185:505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sempoux C, Guiot Y, Dahan K, et al. The focal form of persistent hyperinsulinemic hypoglycemia of infancy: morphological and molecular studies show structural and functional differences with insulinoma. Diabetes 2003;52:784–794 [DOI] [PubMed] [Google Scholar]
- 9.Jack MM, Walker RM, Thomsett MJ, Cotterill AM, Bell JR. Histologic findings in persistent hyperinsulinemic hypoglycemia of infancy: Australian experience. Pediatr Dev Pathol 2000;3:532–547 [DOI] [PubMed] [Google Scholar]
- 10.Fékété CN, de Lonlay P, Jaubert F, Rahier J, Brunelle F, Saudubray JM. The surgical management of congenital hyperinsulinemic hypoglycemia in infancy. J Pediatr Surg 2004;39:267–269 [DOI] [PubMed] [Google Scholar]
- 11.Brunelle F, Negre V, Barth MO, et al. Pancreatic venous samplings in infants and children with primary hyperinsulinism. Pediatr Radiol 1989;19:100–103 [DOI] [PubMed] [Google Scholar]
- 12.Doppman JL, Miller DL, Chang R, Shawker TH, Gorden P, Norton JA. Insulinomas: localization with selective intraarterial injection of calcium. Radiology 1991;178:237–241 [DOI] [PubMed] [Google Scholar]
- 13.de Lonlay P, Simon-Carre A, Ribeiro MJ, et al. Congenital hyperinsulinism: pancreatic [18F]fluoro-L-dihydroxyphenylalanine (DOPA) positron emission tomography and immunohistochemistry study of DOPA decarboxylase and insulin secretion. J Clin Endocrinol Metab 2006;91:933–940 [DOI] [PubMed] [Google Scholar]
- 14.Cade A, Walters M, Puntis JW, Arthur RJ, Stringer MD. Pancreatic exocrine and endocrine function after pancreatectomy for persistent hyperinsulinaemic hypoglycaemia of infancy. Arch Dis Child 1998;79:435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lonlay-Debeney P, Poggi-Travert F, Fournet JC, et al. Clinical features of 52 neonates with hyperinsulinism. N Engl J Med 1999;340:1169–1175 [DOI] [PubMed] [Google Scholar]
- 16.Jack MM, Greer RM, Thomsett MJ, et al. The outcome in Australian children with hyperinsulinism of infancy: early extensive surgery in severe cases lowers risk of diabetes. Clin Endocrinol (Oxf) 2003;58:355–364 [DOI] [PubMed] [Google Scholar]
- 17.Rother KI, Matsumoto JM, Rasmussen NH, Schwenk WF. Subtotal pancreatectomy for hypoglycemia due to congenital hyperinsulinism: long-term follow-up of neurodevelopmental and pancreatic function. Pediatr Diabetes 2001;2:115–122 [DOI] [PubMed] [Google Scholar]
- 18.Rahier J, Fält K, Müntefering H, Becker K, Gepts W, Falkmer S. The basic structural lesion of persistent neonatal hypoglycaemia with hyperinsulinism: deficiency of pancreatic D cells or hyperactivity of B cells? Diabetologia 1984;26:282–289 [DOI] [PubMed] [Google Scholar]
- 19.Rahier J, Sempoux C, Fournet JC, et al. Partial or near-total pancreatectomy for persistent neonatal hyperinsulinaemic hypoglycaemia: the pathologist’s role. Histopathology 1998;32:15–19 [DOI] [PubMed] [Google Scholar]
- 20.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301 [DOI] [PubMed] [Google Scholar]
- 21.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 23.Giurgea I, Sempoux C, Bellanné-Chantelot C, et al. The Knudson’s two-hit model and timing of somatic mutation may account for the phenotypic diversity of focal congenital hyperinsulinism. J Clin Endocrinol Metab 2006;91:4118–4123 [DOI] [PubMed] [Google Scholar]
- 24.Leibowitz G, Glaser B, Higazi AA, Salameh M, Cerasi E, Landau H. Hyperinsulinemic hypoglycemia of infancy (nesidioblastosis) in clinical remission: high incidence of diabetes mellitus and persistent beta-cell dysfunction at long-term follow-up. J Clin Endocrinol Metab 1995;80:386–392 [DOI] [PubMed] [Google Scholar]
- 25.Mazor-Aronovitch K, Landau H, Gillis D. Surgical versus non-surgical treatment of congenital hyperinsulinism. Pediatr Endocrinol Rev 2009;6:424–430 [PubMed] [Google Scholar]