The method for AAV-luciferase transfer to lacrimal gland of mice is reported. AAV 9 and AAV 5 serotypes were more efficient, as confirmed by in vivo luminescence and immunohistochemistry. Despite inducing neutralizing antibodies, there was no induced inflammatory response or tear dysfunction.
Abstract
Purpose.
The lacrimal gland (LG) delivers defensive and metabolic factors to the ocular surface. These functions may be disrupted in several diseases, and for most of them there is no cure. The aim of this study is to investigate conditions and limitations for using adeno-associated virus (AAV) vectors as gene transfer agents to LG.
Methods.
Eight-week-old Balb/c mice were used to investigate route, gene expression, and time course of AAV gene vector transfer to LG. AAV vectors encoding firefly luciferase were administered to the LG and luciferase expression was evaluated in vivo by immunohistochemistry. Ocular surface and neutralizing antibodies were also evaluated.
Results.
The present work revealed that AAV vectors are able to delivery DNA to the LGs of mice. Direct injection had the highest level of transduction, and topical ocular drops the lowest. Overall, the AAV strain with highest transduction activity as measured by both luminescence and immunohistochemistry was AAV9, followed by AAV 5w8 and AAV5. Transduction was not different between sexes, could be detected as soon as 24 hours after injection, and lasted for at least 30 days (study termination). No tissue damage was observed when compared with controls. All vectors with detectable LG transduction induced neutralizing antibodies.
Conclusions.
LG gene delivery by AAV vectors appears to be both safe and well tolerated. The choice of vector influences both the overall transduction activity, as well as the spread of vector to other organs. This work supports the use of AAV-mediated gene therapy for dry eye.
The lacrimal gland (LG) is part of the immune secretory system responsible for maintaining moisture, protection, and secretion of metabolic elements necessary for clear vision and maintenance of the ocular surface.1,2 Sjögren′s Syndrome (SS) and many other diseases cause dryness of the ocular surface as well as other mucosal and skin tissues.3 The tears are complex secretions that wet and protect the cornea and the conjunctiva and are controlled by neural, immune, and endocrine systems inputs. Disruption in one or more of these systems are known to cause LG dysfunction; they induce clinical signs and symptoms of dryness in several organs, and most have no cure.4–6
Considering that LG dysfunction is presently incurable, has several different causes, and the diagnostic tools have low sensitivity and specificity, better understanding of the viral vector gene transfer to this organ may support the development of tools for future diagnostic and therapeutic strategies.
In support of that assumption, previous work has demonstrated gene transfer to the LG using adenoviral vectors (AdV) encoding TNF-α inhibitor, or adeno-associated viral vector (AAV) encoding IL-10 in rabbit LG.7,8 In addition, overexpression of protein kinase C delivered by adenoviral gene transfer to the acinar cells of the rat LG increased basal- and adrenergic-stimulated protein secretion.9 Furthermore, ocular surface treatment with vectors has been recently reported by delivery of alkaline phosphatase to mouse and human corneal stroma by AAV-6, 8, and 9 vectors, between 14 and 30 days, without signs of inflammation.10
The aims of this study are to investigate the safety and efficiency of AAV as a gene transfer vector for potential lacrimal gland therapy, and to establish the best route, AAV serotype, dose, and kinetics in mice LG in preparation for future studies with dry eye disease and SS.
Materials and Methods
Eight-week-old male and female mice were obtained from The Jackson Laboratories (Bar Harbor, ME). All experimental studies were performed according to the “Principles of laboratory animal care” (NIH publication no. 85-23) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the Animal Care and Use Committee and NIH Biosafety Committee.
Animals had free access to standard rodent chow and water. Anesthesia consisted of a mix of ketamine (KetaVed Vedco Inc., St. Joseph, MO) (100 mg/mL), 1 mL/kg of body weight; and xylazine (Lloyd Inc., Shenandoah, IA) (20 mg/mL), 0.7 mL/kg body weight; was administered intramuscularly before surgical procedure, and later before tissue collection. In vivo luminescence expression and tear film measurements were conducted under mild anesthesia with 3% isoflurane (Florane; Baxter, Deerfield, IL), together with O2 in a vaporizer (Impac 6, VetEquip Inc., Pleasanton, CA). All procedures and manipulation were conducted just after confirming abolition of the caudal and corneal reflex.
Vector Preparation
Generation of recombinant AAV vectors was performed as previously described.11 Briefly, 293T cells were cotransfected by calcium phosphate precipitation with the following trans plasmids: pMMTV2.1 (provides the AAV serotype 2 Rep); the AAV Cap genes corresponding to the strains studied here; pAd12 (provides adenoviral helper genes); and a vector plasmid, containing luciferase cDNA with a cytomegalovirus (CMV) promoter flanked by the AAV 2, inverted terminal repeats. The cells were harvested 48 hours posttransfection and a crude viral lysate was obtained after three freeze-thaw cycles. The lysate was treated with benzonase, adjusted to a refractive index of 1.372 by addition of CsCl, and centrifuged at 38,000g for 65 hours at 20°C (Sorvall Discovery 100 SE, Kendro Laboratory Products, Newtown, CT). Equilibrium density gradients were fractionated, and fractions with a refractive index of 1.369 to 1.375 were collected and stored at 4°C before assay for transduction activity. Immediately before use, vector fractions were dialyzed against 0.9% NaCl. The number of AAV genomes (particles) was estimated using a qPCR assay, amplifying a CMV promoter region of the vector genome using an automated sequence detector (ABI Prism 7700 Sequence Detector; Applied Biosystems, Carlsbad, CA). Vector titers and concentrations were adjusted based on the qPCR assay to achieve values of 5 × 1012 particles/mL.
Animal Procedure and Evaluation
Before gene vector studies, trypan blue (TB) (Lonza, Walkensville, MD) at a dilution of 0.04% staining assays were conducted to investigate the reproducibility of drug delivery by different approaches and solution volumes. The mouse extra orbital LG was injected with TB via each one of the following four methods in anesthetized mice: (a) injection of 50 μL into the LG, through the skin, into the subdermal space (at a point of the head, 3 mm equidistant between the eye and ear) with insulin syringes and 28-gauge needle (Becton Dickinson, Franklin Lakes, NJ); (b) retrograde lacrimal ductal delivery of 10 to 50 μL to the LG, using a 30-gauge injection needle or propylethylene micro tubes (Becton Dickinson), starting in the temporal canthus of the lid opening, using a surgical microscope; (c) injection of 2 μL into the LG with a 10-μL microsyringe (NanoFil; World Precision Instruments, Sarasota, FL) and a 34-gauge blunt needle (NanoFil; World Precision Instruments), after removal of fur from the cheek, followed by cleaning with betadine and alcohol and a vertical small scissor cut (5 mm in length), followed by incision closure with cianoacrylate glue, application of 0.5% proparacaine hydrochloride (Alcon Laboratories Inc., Fort Worth, TX) and triple antibiotic ointment (Bausch & Lomb, Tampa, FL), as previously described12; or (d) eye drop solution over the experimental eye, after gentle luxation while animals were anesthesized with inhalant isoflurane for 2 minutes.
For vector instillation, the methods described above as “c” and “d” were used, and 3 to 5 mice per group received 2 μL, or otherwise specified in the text, directly injected in the exposed LG, or instilled over the cornea. The gene vector transduction was monitored by luminescence evaluated in vivo with an imaging system (Xenogen IVIS; Caliper Life Sciences, Hopkinton, MA). After isoflurane inhalation anesthesia, the animals were injected intraperitoneally (IP) with 100 μL 40 mg/mL luciferin (D Luciferin Fire Fly; Gold Biotechnology, St. Louis, MO). The captured images were analyzed with image analysis software (Igor Pro 5.0; WaveMetrics, Inc., Portland, OR) and stored on a PC (Apple Power PC G4, with Mac OS 10.4.11 system software; Apple Inc., Cupertino, CA). Luminescence value was expressed per area over exposure time.
Body and Eye Examination
At Day 30 after vector delivery, the mice were anesthetized with isoflurane (n = 2 to 3 per group on serotype comparison and n = 5 per group on sex comparison) and weighed. The ocular surface was evaluated by direct observation and a microscope (Leica MZ 75; Leica Microsystems Inc., Bannockburn, IL) to evaluate eye redness, surface smoothness, and/or corneal opacity. The tear secretion was measured by modified Schirmer test at the site of injection (right side) or from naïve controls, which consist of placing a phenol red thread (Zone-Quick; Mericon America, Inc., San Mateo, CA), in the conjunctival fornix of the eyes for 20 seconds.
Tissue Collection and Analysis
After the above-mentioned data were collected, mice were euthanized with an excess of anesthesia followed by CO2 inhalation in a CO2 chamber. LG and eye globes tissue from AAV-injected and naïve mice were harvested for morphologic and immunohistochemical evaluation.
Samples were embedded in 4% paraformaldehyde overnight, washed three times in tap water and fixed in 70% ethanol. After paraffin blocking, 5-μm thick sections were obtained; for each sample one section was stained with H&E and immunohistochemistry staining were also performed. Slides were observed and images captured by microscope (Leica Leitz DM IRB, Leica Microsystems GmbH, Wetzlar, Germany) connected to a PC (Optiplex 960 Desktop Personal Computer; Dell, Round Rock, TX) by a digital camera (Q color 5; Olympus, Center Valley, PA) using optical imaging software (Meta-Morph MAG Biosystems Software version 7.5.6; MDS Analytical Technologies, Downingtown, PA).
Immunohistochemistry for Luciferase
To confirm luciferase gene transduction and to identify the location of the vectors, immunoperoxidase assay was performed with an immunohistochemistry kit (Invitrogen Co., Carlsbad, CA). In brief, deparafinization and rehydration of tissue was performed with xylene and progressively lower concentrations of ethanol. Endogenous peroxidase was quenched with 3% H2O2 and nonspecific binding blocked with blocking solution provided in the kit, before overnight incubation with primary antibody anti-luciferase rabbit IgG (10.8 μg/mL) (Sigma-Aldrich, St. Louis, MO). These procedures were followed by secondary antibody, streptavidin-biotin-peroxidase complex incubations, and DAB staining. Finally, slides were counterstained with Ehrlich's hematoxilin (Electronic Microscopy Sciences, Hatfield, PA), mounted (Permount; Fisher, Fair Lawn, NJ) and coverslipped.
AAV Neutralizing Antibody Assay
Cos-7 cells were seeded at 7 × 103 cells per well of a 96-well plate overnight. The next day, 50 μL of serum (previously diluted, twofold from 1:25 to 1:1600 in 1% horse serum DMEM) from mice injected in the LG with one of the following viruses: AAV2, 4, 5, x5, 5 w8, 9, 12, and BAAV containing luciferase gene was pre-incubated with an equal volume of their respective virus particles for 1 hour at room temperature. Serum/vector mixture was added in triplicate to Cos-7 cells and incubated for 1 hour at 37°C. Serum from naïve animals was used as a negative control. Afterward, the solution was removed and the cells incubated for 3 days with DMEM containing 1% fetal calf serum, 1% antibiotic/antimycotic solution and 1% l-glutamine (Gibco; Invitrogen Co.). Measurement of luciferase transduction was performed by adding 20 μL of firefly luciferin per well (Bright-Glo; Promega, Madison, WI), and relative light units detected in each well by a luminometer (Optocomp I; MGM Instruments Inc., Hamden, CT). Results were expressed as the neutralization titer that inhibited 50% of transduction with AAV vector, compared with serum from naïve animals. Therefore the lower the titer, the fewer antibodies are present. All samples were assayed in triplicate.
Statistical Analysis
Data, unless otherwise indicated, were expressed as mean ± SD. Comparisons were made using the Kruskal-Wallis test for continuous data comparison among several groups and the Mann-Whitney U test for continuous data comparison between males and females (Graphpad 5.0 software; Prism, San Diego, CA). The level of significance used was P < 0.05.
Results
Delivery Route for LG Medication
Mice LG duct cannulation as has been reported for the salivary gland followed by trypan blue (TB) staining failed to stain the LG (data not shown). Puncture injection through the skin left TB in the subcutaneous space. The most successful route of application of recombinant (r)AAV-Luc was by injection of the LG after surgical opening of skin and LG exposure. This method allowed good distribution of TB in the target tissue. In addition, topical ocular application was conducted with the findings described below.
In Vivo Imaging Detects Serotype-Dependent Transgene Expression Assay and Biodistribution
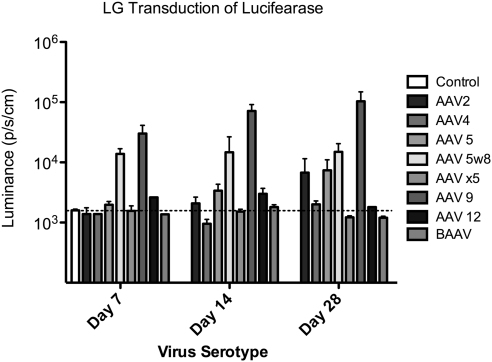
Twenty-four hours, and 7, 14, and 30 days after injection, expression of luciferase was measured by luminescence imaging. AAV9, followed by AAV 5w8 and AAV5 demonstrated the fastest and most intense transduction. AAV9 and AAV2 showed a continuous increase in luciferase expression over the 30-day period of study and at Day 30 AAV2 showed levels of expression similar to AAV 5. In contrast, AAV 5w8 showed stable expression, initially starting at Day 7 and continuing until the end of the study (30 days) (Figs. 1, 2A).
Figure 1.
In vivo luminescence measurement in mice injected with AAV 109 viral particles into the right LG (horizontal line: the base line obtained from control naïve mouse); P > 0.05. For comparison of luminescence progression of AAV vectors at different times (Day 7, 14, and 28) or among different serotypes at the same day (n = 3 per group), however, only one mouse that received AAV4, AAV12, and BAAV, and two that received AAVx5 were measured at Day 7, and only two that received AAV4, AAV5w8, AAV9, and AAV12 were measured at Day 28.
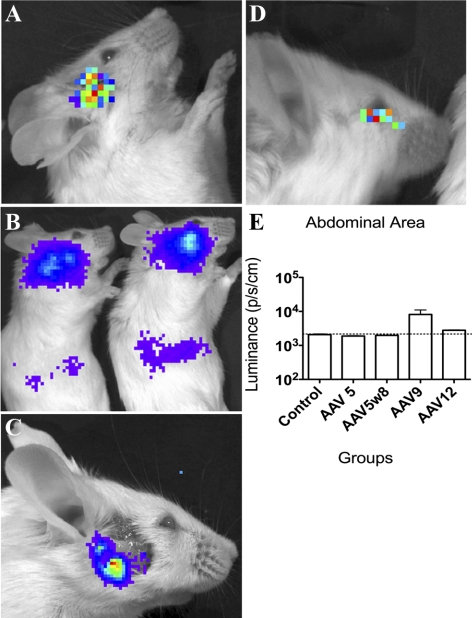
Figure 2.
Imaging of luminescence (A) at the site of injection of AAV2 in Balb/c mouse after 14 days (LG) with microsyringe; (B) in the abdominal area of mouse injected with AAV9 after 14 days; (C) facial area with persistent luminescence in mouse injected with AAV5 after LG removal at Day 30; (D) corneal luminescence 24 hours after topical AAV9 application (2 μL of 5 × 1012 particles per mL); and (E) comparative measurement of AAV-Luc transduction in vivo in the abdominal area 30 days after injection in LG.
In addition to expression at the site of injection, luminescence imaging detected AAV9 and AAV12 transduction at distal sites, more specifically in the abdominal area, right subcostal region for AAV9 (Fig. 2B) and inferior central area of the abdomen for AAV12 (data not shown). Quantification of luminescence in the abdominal area revealed high levels of AAV9 expression (Fig. 2E). In contrast AAV5 and AAV 5w8 transduction appeared to stay more localized to the LG, however some expression was still detected after removal of the gland suggesting some spreading to the adjacent tissues (Fig. 2C). More extensive spreading was detected if the AAV 5 vector dose was increased fivefold, including in the abdomen (i.e., from 1 × 1010 to 5 × 1010 particles) (data not shown).
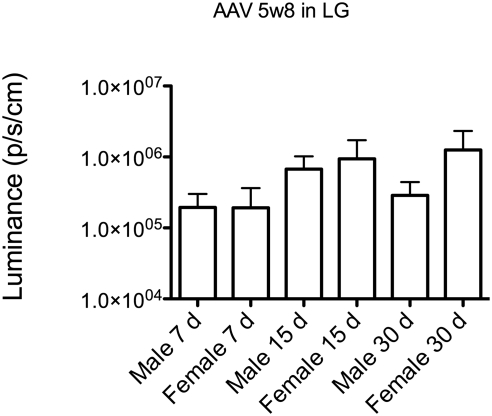
Previous studies revealed striking sex-related differences in the LG.13 Moreover, gene transfer studies with AAV2 into mouse submandibular salivary glands have suggested that transduction activity can be significantly (260-fold) lower in female mice.14 However in LG no sex bias in transduction activity was detected at 7, 14, and 30 days using AAV 5w8 vector (Fig. 3).
Figure 3.
Comparison of in vivo luminescence between males and females injected with AAV5w8 in the LG (P = 0.0967, Kruskal-Wallis test).
To test for transduction after topical application of AAV vectors, 2 μL of AAV2, BAAV, or AAV 9 at 5 × 1012 particles per mL were applied to the ocular surface and then mice were prevented from blinking or moving for 2 minutes. Mild luminescence was observed in the first 24 hours, with AAV9 only (Fig. 2D). However, this corneal or regional luminescence was not seen in subsequent evaluations at Day 5 and Day 30 for all virus serotypes (data not shown).
Serotype-Dependent Immunohistochemical Staining of Lacrimal Ductal and Acinar Cells
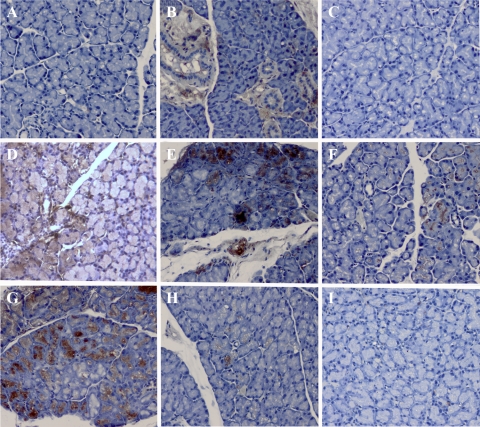
Immunoperoxidase staining was used to confirm AAV transduction and to locate positive cells within the gland. AAV4 and BAAV showed no staining in the LG, confirming the lack of transduction seen with luciferase imaging. AAV9, AAV5w8, and AAV5 revealed intense staining in acinar cells. AAVx5 did not show in vivo luminescence above what was seen with the naïve controls. However imunohistochemistry detected some positive acinar cells. On the other hand, AAV2 primarily transduced ductal epithelial cells whereas AAV12 showed mild but mixed transduction in both acinar and ductal cells (Fig. 4A–I).
Figure 4.
Immunohistochemistry images of LG transduced with luciferase: (A) naïve control, (B) AAV 2, (C) AAV 4, (D) AAV 5, (E) AAV5w8, (F) AAVx5, (G) AAV9, (H) AAV 12, and (I) BAAV. The mice received LG injection of 2 μL of AAV serotypes (5 × 1012 particles per mL) encoding the luciferase gene. Slides were counterstained with hematoxylin. Magnification, ×200.
Neutralizing Antibody Formation after Lacrimal Gland Transduction
To investigate whether delivery of AAV vectors to the LG induced an immune response, neutralizing antibody assays were performed. The assay showed that animals injected with AAV5, AAV5w8, AAV9, AAV12, and BAAV produce a significant amount of neutralizing antibodies compared with serum from mice injected with AAV2, AAV4, AAVx5, and BAAV which generated a lower neutralizing response (Table 1).
Table 1.
Neutralization Titers that Inhibited 50% of Transduction, with AAV Vector Compared with Media with Serum of Naïve Controls
| Neutralization Titers | |
|---|---|
| rAAV 2 | ≤1:25 |
| rAAV 4 | ≤1:25 |
| rAAV 5 | 1:200 |
| rAAV 5w8 | 1:200 |
| rAAV x5 | ≤1:25 |
| rAAV 9 | 1:200 |
| rAAV 12 | 1:200 |
| rBAAV | ≤1:25 |
AAV Gene Transfer to the Lacrimal Gland Is Well Tolerated
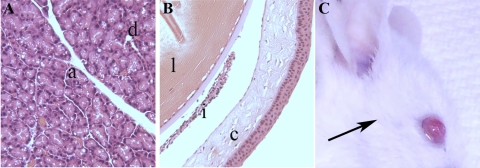
To test whether AAV either injected into the LG or applied topically, would disrupt the homeostasis between the LG and ocular surface, the eyes of mice were examined directly and by histologic analysis. The direct observation of the eyes did not show signs of inflammation, neovascularization, epithelial defect, corneal edema or opacity, regardless of whether AAV suspension was injected into their LG or topically applied to the ocular surface (Fig. 5C). Tear secretion measurement did not show differences between transduced and naïve animals. Similarities were also seen in the body and LG weight of these animals compared with controls (Table 2). Slides stained with H&E revealed that neither LG nor ocular surface structures were altered by the presence of AAV vectors of all serotypes. There was no inflammatory infiltrate and the architecture of acinar or ductal epithelial cells did not change. In addition, the corneal epithelium was intact, with all epithelial layers preserved and no inflammatory cells present in the corneal stroma or conjunctival tissue (Figs. 5A, 5B).
Figure 5.
Representative histologic images of (A) mouse LG injected with AAV 5, showing preserved acini (a) and ductal (d) structures and (B) the eye globe of the same side of injection, where cornea (c), iris (i), and lens (l) are also preserved after 30 days. The slides were stained with H&E; magnification, ×100. (C) Aspect of the eye of mouse, after 30 days of being injected with AAV 9 (surgical incision area, arrow).
Table 2.
Comparison among Body Weight, LG Weight, and Tear Secretion Measurement with Phenol Red Thread in Naïve Controls and Mice Injected with AAV in the LG
| Body Weight (g) | LG Weight (mg) | Phenol Red Thread (mm) | |
|---|---|---|---|
| rAAV 2 (n = 3) | 29.7 ± 0.6 | 17.0 ± 2.4 | 5.5 ± 1.1 |
| rAAV 4 (n = 2) | 29.5 ± 1.3 | 16.3 ± 3.6 | 6.0 ± 1.8 |
| rAAV 5 (n = 3) | 28.8 ± 1.3 | N/M | 5 ± 0.5 |
| rAAV 5w8 (n = 2) | 29.3 ± 1.7 | N/M | 5.8 ± 0.5 |
| rAAV x5 (n = 3) | 28.1 ± 2.7 | 19.6 ± 2.5 | 6.5 ± 1.8 |
| rAAV 9 (n = 2) | 28.1 ± 0.1 | N/M | 6.0 ± 1.6 |
| rAAV 12 (n = 2) | 28.9 ± 0.6 | N/M | 6.0 ± 0.8 |
| rBAAV (n = 3) | 30.8 ± 0.4 | 24.8 ± 2.1 | 6.5 ± 0.6 |
| Control (n = 3) | 29.8 ± 1.8 | 21.5 ± 2.1 | 6.3 ± 1.6 |
Data are expressed as mean ± SD. N/M, not measured.
Discussion
The present work describes the activity, biodistribution, and host response to eight serotypes of adeno-associated viral vectors on injection into the LG gland of mice. Current treatment for dry eye symptoms related to Sjogren's syndrome or other causes is typically delivered as a topical therapy, yet recent studies have revealed that the actual therapeutic benefit of this method of treatment is modest according to clinical and patient perspectives.15–17 Viral gene therapy to induce production of proteins locally in the LG represents an alternative approach. This finding is in agreement with previous studies on LG gene therapy in rabbits, where AAV2 encoding IL-10 gene under the control of the tetracycline-inducible tetON system, modulated the inflammatory infiltrate of the LG and improve the ocular surface for up to 4 months.8 In large mammals and humans, LG retroductal instillation could be a better approach to improve efficiency and reduce spread to other organs as has been developed for gene transfer to the salivary gland.18 However, the most robust method to deliver genes to the mice LG was by direct injection with guidance of a dissecting microscope, because the volume of solution injected combined with the size of their LG duct do not allow reproducible retroductal injection with present techniques. Considering that mice are the major animal model available to study dry eye and Sjogren's syndrome, direct injection with the LG exposure method should be selected for future studies in those models. Moreover, this method allows direct cell contact among vectors and acinar cells and access to the basolateral surface of those cells, which may have relevance in transduction of some AAV serotypes, as the polarization of the cells results in expression of different proteins on the apical or basolateral surfaces, which would work as receptor for AAV vectors.
The attempt to use topically applied AAV failed to result in stable transduction of the cornea epithelia, which is challenging, because major diseases in the ocular surface are located in this layer.10,19 The possible reason for this could be the rapid turnover of the post-mitotic epithelial cells as well as the incapacity of these viruses to incorporate in the host cell genome.20,21
Due to the extensive genetic manipulation of mice, many models of ocular disease have been established.22 However, due to their limited lacrimal secretory activity (0.8 μL in a normal strain) it can be difficult to measure recombinant protein production. Although not tested in this study, proteins should be able to be secreted into the tears from the transduced LG tissue. In two previous studies using rabbit models, which have a larger tear volume, transduction of LG resulted in detection of the recombinant protein (i.e., TNF-α soluble receptor and IL-10) in the tears.8,23
The efficiency of transduction is determined by several factors including mechanical barriers, innate and immune defense, concentration of virus suspension, cell affinity to the different viral capsids, and the capacity of the cell to express the encoded gene. Among the 100 AAV isolates reported, less than 10 were tested in exocrine gland or ocular tissue. As observed here, by in vivo luminescence assays, the transduction of LG was more efficient with AAV9 encoding luciferase. A similar effect was obtained in the corneal stroma of mice, when AAV 9 was compared with rAAV8 and 6 encoding alkaline phosphatase.10
Another relevant aspect related to the development of a vector for gene transfer is the host immune response to the vector. The mice in our study maintained body weight and had no inflammation or impaired function of the LG, or change in ocular surface with all AAV tested, which suggests that this is a safe strategy for potential use in LG disease treatment. In contrast, one aspect that needs further consideration is the spread of AAV observed with high doses of AAV5, as well as lower doses of AAV9 and AAV12 and their tropism for organs in the abdominal area. It was observed before that adenovirus administered to rat salivary gland has dose-dependent capacity to spread to the abdominal area, heart, and central nervous system.24 In addition, after retroductal injection into salivary gland of mice, AAV2, revealed higher capacity to spread compared with AAV4 and 5.25
Ideally, a therapeutic viral vector should be able to be readministered if necessary (e.g., expression decrease over time). However, the present work indicates that in LGs, AAV vectors that lead to higher transduction tend to induce higher levels of neutralization antibodies and are therefore likely to have reduced performance in repeat administrations.
Considering the present findings, this work suggests that AAV5, or its related mutant AAV5 w8 may be better suited for LG gene therapy. However, other serotypes also demonstrate transduction activity in the lacrimal gland. Previous studies observed transient improvement in function and inflammation in a rabbit model of autoimmunity with TNF-alpha inhibitor or IL-10 expressed from adenovirus vector and longer benefits when IL-10 was expressed from AAV2 vector.7,8,26
In conclusion, the present work demonstrates for the first time the feasibility of gene transfer to the LG in mice and the systemic repercussions, to the vector as well as spread to other organs. Use of these vectors should enhance our understanding of lacrimal gland biology and the development of new therapies for the treatment of ocular dryness and lacrimal gland dysfunction.
Acknowledgments
The authors thank William Swaim, Hongen Yin, Ana Paola Cotrim, Milton Papa, and Ilias Alevizos from NIDCR/NIH and Driss Zoukhri from Tufts University School of Dental Medicine for assistance with this work.
Footnotes
Supported by an NIH intramural grant (JAC), and grants from the following Brazilian governmental institutions (EMR): Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clinicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA).
Disclosure: E.M. Rocha, None; G. Di Pasquale, None; P. Perez Riveros, None; K. Quinn, None; B. Handelman, None; J.A. Chiorini, None
References
- 1. Montés-Micó R, Cerviño A, Ferrer-Blasco T, García-Lázaro S, Madrid-Costa D. The tear film and the optical quality of the eye. Ocul Surf. 2010;8:185–192 [DOI] [PubMed] [Google Scholar]
- 2. Scherz W, Dohlman CH. Is the lacrimal gland dispensable? Keratoconjunctivitis sicca after lacrimal gland removal. Arch Ophthalmol. 1975;93:281–283 [DOI] [PubMed] [Google Scholar]
- 3. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:93–107 [DOI] [PubMed] [Google Scholar]
- 4. Sullivan DA, Wickham LA, Rocha EM, et al. Androgens and dry eye in Sjogren's syndrome. Ann N Y Acad Sci. 1999;876:312–324 [DOI] [PubMed] [Google Scholar]
- 5. Garcia-Carrasco M, Fuentes-Alexandro S, Escarcega RO, Salgado G, Riebeling C, Cervera R. Pathophysiology of Sjogren's syndrome. Arch Med Res. 2006;37:921–932 [DOI] [PubMed] [Google Scholar]
- 6. Tzioufas AG, Tsonis J, Moutsopoulos HM. Neuroendocrine dysfunction in Sjogren's syndrome. Neuroimmunomodulation. 2008;15:37–45 [DOI] [PubMed] [Google Scholar]
- 7. Trousdale MD, Zhu Z, Stevenson D, Schechter JE, Ritter T, Mircheff AK. Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with induced autoimmune dacryoadenitis. J Autoimmune Dis. 2005;2:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas PB, Samant DM, Selvam S, et al. Adeno-associated virus-mediated IL-10 gene transfer suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2010;51:5137–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodges RR, Raddassi I, Zoukhri D, Toker A, Kazlauskas A, Dartt DA. Effect of overexpression of constitutively active PKCalpha on rat lacrimal gland protein secretion. Invest Ophthalmol Vis Sci. 2004;45:3974–3981 [DOI] [PubMed] [Google Scholar]
- 10. Sharma A, Tovey J, Ghosh A, Mohan R. AAV serotype influences gene transfer in corneal stroma in vivo. Exp Eye Res. 2010;91:440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaludov N, Handelman B, Chiorini JA. Scalable purification of adeno-associated virus type 2, 4, or 5 using ion-exchange chromatography. Hum Gene Ther. 2002;13:1235–1243 [DOI] [PubMed] [Google Scholar]
- 12. Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res 2007;84:894–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullivan DA, Wickham LA, Rocha EM, Kelleher RS, da Silveira LA, Toda I. Influence of gender, sex steroid hormones, and the hypothalamic-pituitary axis on the structure and function of the lacrimal gland. Adv Exp Med Biol. 1998;438:11–42 [DOI] [PubMed] [Google Scholar]
- 14. Voutetakis A, Zheng C, Mineshiba F, et al. Adeno-associated virus serotype 2-mediated gene transfer to the parotid glands of nonhuman primates. Hum Gene Ther. 2007;18:142–150 [DOI] [PubMed] [Google Scholar]
- 15. Doughty MJ, Glavin S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review. Ophthalmic Physiol Opt. 2009;29:573–583 [DOI] [PubMed] [Google Scholar]
- 16. Ramos-Casals M, Tzioufas A, Stone J, Sisó A, Bosch X. Treatment of primary Sjögren syndrome: a systematic review. JAMA. 2010;304:452–460 [DOI] [PubMed] [Google Scholar]
- 17. Asbell PA, Spiegel S. Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey. Eye Contact Lens. 2010;36:33–38 [DOI] [PubMed] [Google Scholar]
- 18. Baum BJ, Zheng C, Alevizos I, et al. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncol. 2010;46:4–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohan RR, Schultz GS, Hong JW, Wilson SE. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp Eye Res. 2003;76:373–383 [DOI] [PubMed] [Google Scholar]
- 20. Gloor BP, Rokos L, Kaldarar-Pedotti S. Cell cycle time and life-span of cells in the mouse eye. Measurements during the postfetal period using repeated 3H-thymidine injections. Dev Ophthalmol. 1985;12:70–129 [PubMed] [Google Scholar]
- 21. Goncalves MA. Adeno-associated virus: from defective virus to effective vector. Virol J. 2005;2:43–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavoie TN, Lee BH, Nguyen CQ. Current concepts: mouse models of Sjögren's syndrome. J Biomed Biotechnol. 2011;2011:549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Z, Stevenson D, Schechter JE, et al. Tumor necrosis factor inhibitor gene expression suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:343–351 [DOI] [PubMed] [Google Scholar]
- 24. O'Connell BC, Zheng C, Jacobson-Kram D, Baum BJ. Distribution and toxicity resulting from adenoviral vector administration to a single salivary gland in adult rats. J Oral Pathol Med. 2003;32:414–421 [DOI] [PubMed] [Google Scholar]
- 25. Katano H, Kok MR, Cotrim AP, et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther. 2006;13:594–601 [DOI] [PubMed] [Google Scholar]
- 26. Zhu Z, Stevenson D, Schechter JE, et al. Prophylactic effect of IL-10 gene transfer on induced autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2004;45:1375–1381 [DOI] [PubMed] [Google Scholar]