Abstract
Highly thermally stable N-aryl,N-alkyl N-heterocyclic carbene (NHC) ruthenium catalysts were designed and synthesized for latent olefin metathesis. These catalysts showed excellent latent behavior toward metathesis reactions, whereby the complexes were inactive at ambient temperature and initiated at elevated temperatures, a challenging property to achieve with second generation catalysts. A sterically hindered N-tert-butyl substituent on the NHC ligand of the ruthenium complex was found to induce latent behavior toward cross-metathesis reactions, and exchange of the chloride ligands for iodide ligands was necessary to attain latent behavior during ring-opening metathesis polymerization (ROMP). Iodide-based catalysts showed no reactivity toward ROMP of norbornene-derived monomers at 25 °C, and upon heating to 85 °C gave complete conversion of monomer to polymer in less than 2 hours. All of the complexes were very stable to air, moisture, and elevated temperatures up to at least 90 °C, and exhibited a long catalyst lifetime in solution at elevated temperatures.
Introduction
Olefin metathesis is widely used as a method of constructing carbon-carbon double bonds.1 Toward this end, highly efficient metathesis catalysts have been designed through improvement of activity,2 stability,3 and selectivity of the catalysts.4 Recently, efforts have been directed toward the development of latent metathesis catalysts.5,6 Latent catalysts are defined as complexes that show little or no activity at a particular (usually ambient) temperature and initiate only upon activation. This activation can be caused by a variety of different stimuli, including heat,7,8 acid,9 light,10-13 and chemical activation.14 Latent metathesis catalysts primarily have applications in polymer chemistry.14 One such application is the advantage of preparing monomer solutions in a mold with a catalyst that is unreactive at ambient temperature, thus allowing for good mixing and even distribution of monomeric solution before initiating polymerization.7 Previous literature reports describe latent ruthenium catalysts whereby the initiators and organic ligand structure were altered to induce latency.15-17 The structure of initiators, such as variations of the Hoveyda-type chelating ligand, has been particularly well-explored and documented.18-20 Another approach toward tuning the latency of a catalyst involves manipulation of the N-heterocyclic carbene (NHC) ligand. This method of inducing latent behavior is attractive in that it enables a straightforward catalyst design and synthesis while maintaining the functional group tolerance and stability of second generation ruthenium catalysts. Reported herein is the investigation of four new ruthenium-based latent catalysts for cross-metathesis and ROMP that were prepared adopting this strategy.
Results and Discussion
Since N-aryl,N-alkyl NHC ruthenium catalysts display good stability, complexes 1 and 2 bearing a sterically hindered N-tert-butyl substituent on the NHC were synthesized and screened for latent behavior during cross-metathesis (CM) and ring-opening metathesis polymerization (ROMP) reactions (Figure 1).21 At ambient temperature, solutions of complexes 1 and 2 in CDCl3 showed no decomposition by NMR spectroscopy over 14 days and were stable in air for over four months. Additionally, complexes 1 and 2 could be heated in chloroform at 60 °C for 5 days without any signs of decomposition, thus indicating good thermal stability. At 90 °C in toluene, catalysts 1 and 2 began showing slight decomposition after 30 hours. This observed catalyst stability was promising for applications in latent metathesis chemistry, where the catalyst must remain active at high temperature for the duration of the reaction.
Figure 1.
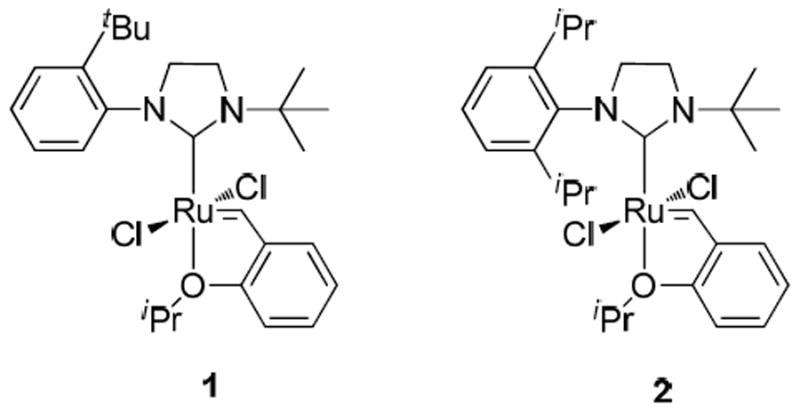
N-aryl,N-alkyl NHC ruthenium catalysts for latent olefin metathesis reactions.
Catalysts 1 and 2 were initially screened and compared for latency for the homodimerization of 1-hexene to 5-decene (Scheme 1). Both catalysts showed less than 5% conversion of 1-hexene after 24 hours at 25 °C while maintaining catalyst structural integrity as confirmed by 1H NMR spectroscopy (Table 1, entries 1 and 2). The reactions were subsequently heated at 85 °C for 24 hours, and the conversions were determined by 1H NMR spectroscopy (Table 1, entries 3 and 4). Both catalysts gave clean conversion of 1-hexene to 5-decene, without detectable side products by 1H NMR spectroscopy. Since catalyst 1 achieved 90% conversion of 1-hexene to 5-decene compared to 41% afforded by catalyst 2, it was considered optimal for further latent cross-metathesis studies. Catalyst 2 was still active with no signs of decomposition after 24 hours at 85 °C. Presumably the better activity of catalyst 1 in relation to catalyst 2 is due to less steric hindrance of the former on the N-aryl ring (mono-tert-butyl versus di-isopropyl). Since both catalysts are latent for cross-metathesis of 1-hexene, the better activity of catalyst 1 is preferential for these reactions. The crystal structures of complexes 1 and 2 show expected geometry (Figure 2).21
Scheme 1.

Latent olefin cross-metathesis of 1-hexene.
Table 1.
Comparison of catalysts for latent homodimerization of 1-hexene.
| Entrya | Catalyst | Temp. (°C) | Time (h) | Conversionb |
|---|---|---|---|---|
| 1 | 1 | 25 | 24 | <5% |
| 2 | 2 | 25 | 24 | <5% |
| 3 | 1 | 85 | 24 | 90% |
| 4 | 2 | 85 | 24 | 41% |
The catalyst loading was 2 mol%. The concentration of 1-hexene in benzene was 0.5M, and the reactions were carried out sealed under a nitrogen atmosphere.
The conversion was determined by 1H NMR spectroscopy. 1-Hexene was cleanly converted to 5-decene.
Figure 2.
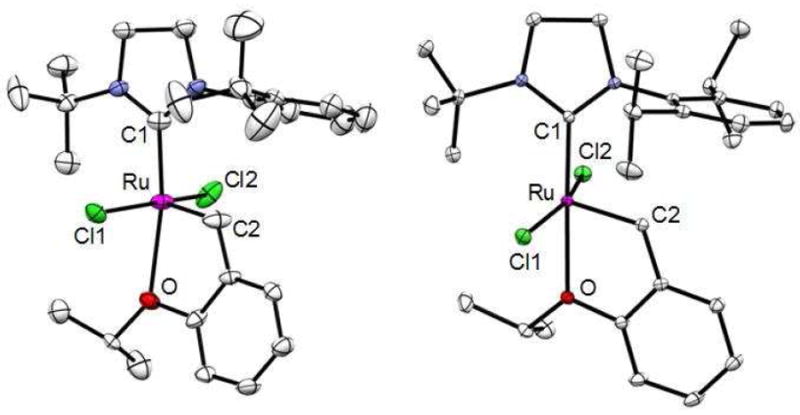
Crystal structures of complexes 1 (left) and 2 (right).
Thermal ellipsoids set at 50% and hydrogen atoms omitted for clarity. Selected bond lengths (Å) and angles (deg.) for 1: C1-Ru, 1.961; C2-Ru, 1.796; O-Ru 2.417; Cl1-Ru, 2.348; Cl2-Ru, 2.376; Cl-Ru-Cl, 7.43; C2-Ru-O, 77.95; and for 2: C1-Ru, 1.982; C2-Ru, 1.837; O-Ru 2.312; Cl1-Ru, 2.369; Cl2-Ru, 2.348; Cl-Ru-Cl, 8.75; C2-Ru-O, 78.50.
Further studies were conducted with catalyst 1 to determine the optimal temperature for carrying out cross-metathesis reactions. In addition to 1-hexene, 1-octene, 5-hexenyl acetate, and 4-penten-1-ol were used as cross-metathesis substrates for homodimerization to assure that the observed results were general to simple alkyl olefins (Table 2). Catalyst 1 proved to be latent for these substrates even at 40 °C, showing no appreciable reactivity until 50 °C. Moderate conversion to product was obtained for all substrates at 60 °C; however, 85 °C was considered optimal for attaining good conversion of the starting material. The reactions gave the desired homocoupled product of these substrates as a mixture of cis and trans isomers.
Table 2.
Temperature optimization and substrate scope of catalyst 1.
 | ||||
|---|---|---|---|---|
| Entrya | Substrate | Temp. (°C) | Time (h) | Conversionb |
| 1 |
|
25 | 24 | <5% |
| 2 | 40 | 24 | 5% | |
| 3 | 50 | 24 | 11% | |
| 4 | 60 | 30 | 35% | |
| 5 | 85 | 24 | 90% | |
|
| ||||
| 6 |
|
25 | 22 | 0% |
| 7 | 40 | 22 | 2% | |
| 8 | 50 | 22 | 13% | |
| 9 | 60 | 30 | 26% | |
| 10 | 85 | 15 | 89% | |
|
| ||||
| 11 |
|
25 | 22 | 0% |
| 12 | 40 | 22 | 0% | |
| 13 | 85 | 22 | 62% | |
|
| ||||
| 14 |
|
25 | 22 | 0% |
| 15 | 40 | 22 | <1% | |
| 16 | 85 | 22 | 76% | |
|
| ||||
| 17 |
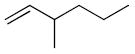
|
25 | 24 | 0% |
| 18 | 85 | 24 | 17% | |
|
| ||||
| 19 |
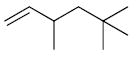
|
25 | 22 | 0% |
| 20 | 85 | 22 | 3% | |
The loading of catalyst 1 was 2 mol%. The concentration of substrate in benzene was 0.5M, and the reactions were carried out sealed under a nitrogen atmosphere.
The conversion was determined by 1H NMR spectroscopy. The reactions went cleanly to the target homodimerization products.
Following temperature optimization, substrate scope was subsequently explored to determine the general applicability of catalyst 1 (Table 2). For simple, longer chain olefins, catalyst 1 efficiently catalyzed conversion of terminal olefin to dimer product (Table 2, entries 5, 10, 13, and 16). Catalyst 1 showed lower reactivity toward more sterically demanding olefins (Table 2, entries 18 and 20), as would be expected given the hindrance of the N-aryl,N-tert-butyl NHC ligand. Functional groups were tolerated well when attached several carbon atoms away from the double bond. However, substrates with functional groups allylic to the olefin, particularly those containing oxygen, underwent significant olefin isomerization upon heating at 85 °C. While catalyst 1 was latent for allyl alcohol, allyl acetate, allyl ethyl ether, and allyl benzene at ambient temperature, subsequent heating of the reactions produced multiple isomerization products in addition to desired product, affording inseparable mixtures of olefinic cross-products. In contrast, catalyst 1 completely isomerized allyloxytrimethylsilane (3) to cis and trans (2-buten-1-yloxy)trimethylsilane (4) after 18 hours at 85 °C, and showed no subsequent cross-metathesis conversion towards the internal olefin product (4) (Scheme 2). Since 1,4-benzoquinone has been reported to prevent olefin isomerization, this additive was tested in the homodimerization of allyl benzene and allyl ethyl ether to see if it would eliminate the observed isomerization and improve selectivity and yield of the desired product.22 However, the addition of 0.1 equivalents of 1,4-benzoquinone resulted in catalyst decomposition. Interestingly, 1 showed no reactivity toward dienes, including 1,3-hexadiene, 1,3-pentadiene, and trans-1-phenyl-1,3-butadiene, even at an elevated temperature of 100 °C, possibly due to the low activity of the ruthenium vinylalkylidene intermediate.23 Longer chain olefins were therefore considered ideal substrates since their conversions to desired homodimerization products were clean.
Scheme 2.

Isomerization of allyloxytrimethylsilane.
Toward the goal of developing a practical latent metathesis catalyst for ROMP applications, catalyst 1 was tested for polymerization of cyclooctadiene (COD) in benzene. In contrast to cross-metathesis reactions, 2 mol% of 1 initiated the ROMP of COD (0.7M in benzene) at ambient temperature, giving 80% conversion to polymer after 35 minutes. Expectedly, higher conversion (90%) of COD to polymer was achieved by 1 at 85°C in 35 minutes. Catalyst 2 was also active for the ROMP of COD (0.7M in benzene) at ambient temperature, affording 39% conversion to polymer in 19 hours. To gain more insight into the reactivity of these catalysts, we screened complex 2 for ROMP of norbornene-derived monomer 5, since 2 would presumably show better latency than 1 due to its increased steric bulk. The increased sterics of 2 was expected to reduce its activity and necessitate higher temperature for initiation. Unfortunately, catalyst 2 polymerized 5 at 25 °C in tetrahydrofuran (THF), giving 56% conversion to polymer in 5 hours. These results showed that 1 and 2 were not effectively latent for ROMP as they were for cross-metathesis. Therefore the complex structure was modified to develop a catalyst that would display latent behavior toward ROMP of norbornene-derived monomers (Figure 3).
Figure 3.
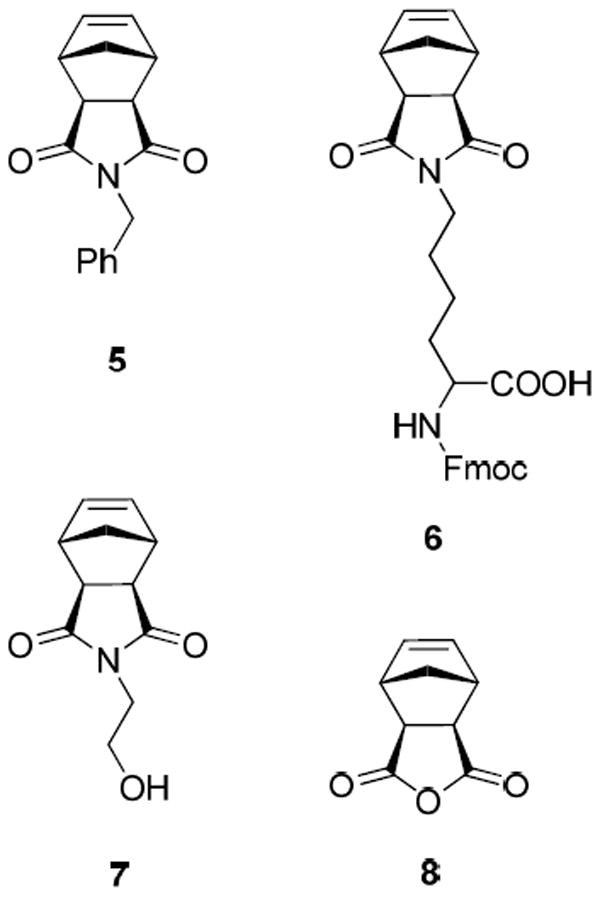
ROMP monomers. Fmoc is fluorenylmethyloxycarbonyl.
In effort to improve the latency of complexes 1 and 2, we converted them to complexes 9 and 10, respectively, by in situ reaction with an excess of sodium iodide according to literature procedure (Scheme 3).24 While 1H NMR chemical shifts of new complexes 9 and 10 are very similar to those of chlorine-based precursors, X-Ray analysis unambiguously established the structure of 10 showing typical spacial arrangement for a second generation ruthenium catalyst (Figure 4).21
Scheme 3.
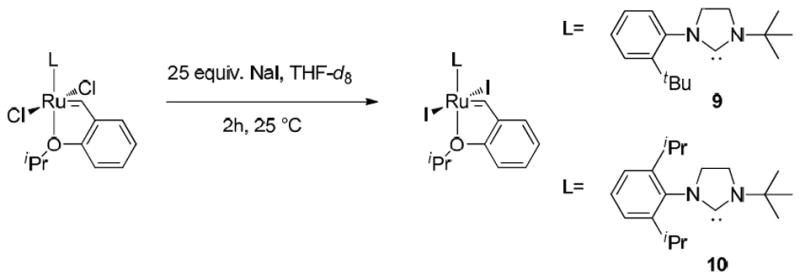
Synthesis of complexes 9 and 10.
Figure 4.
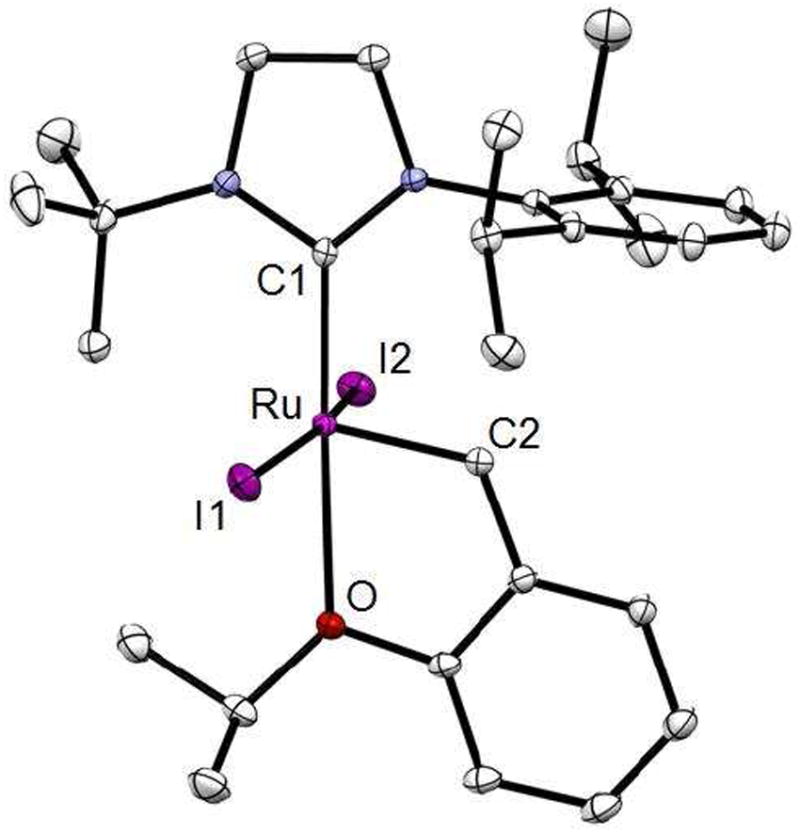
Crystal structure of catalyst 10.
Thermal ellipsoids set at 50% and hydrogen atoms omitted for clarity. Selected bond lengths (Å) and angles (deg.) for 10: C1-Ru, 1.987; C2-Ru, 1.840; O-Ru 2.332; I1-Ru, 2.702; I2-Ru, 2.683; I-Ru-I, 6.66; C2-Ru-O, 78.09.
We anticipated that the effect of changing the chloride ligands to iodide ligands would induce latency for ROMP due to the iodide causing more steric hindrance for the association of the olefin substrate.25 Ruthenium metathesis catalysts with iodide ligands are known to be slower initiators.26 Accordingly, we utilized this property to achieve latent ROMP catalysts.27
Since norbornene-derived polymers have numerous applications, research toward an efficient latent ROMP catalyst was focused on these monomers. Therefore norbornene-derived compounds 5-8, representing a variety of functional groups and different degrees of steric hindrance, were explored as monomers for latent metathesis (Figure 3). Complexes 9 and 10 both proved to be latent for ROMP of 5, affording no conversion of monomer 5 in THF after 4 hours at ambient temperature. Upon heating, >95% conversion to polymer was achieved with both catalysts 9 and 10 in 2 hours. Due to better overall initiation, as well as superior latency after extended time periods, catalyst 10 was used for further latent ROMP studies. Catalyst 10 showed excellent latency at ambient temperature, remaining stable but inactive for at least 24 hours, and subsequently initiating on heating to 85 °C in a sealed reaction vessel to give 99% conversion to polymer 11 (Scheme 4). This observed superior latent behavior of catalysts bearing iodide ligands compared to chloride ligands is consistent with previously reported reactivity trends for catalysts with different halogen ligands.28
Scheme 4.
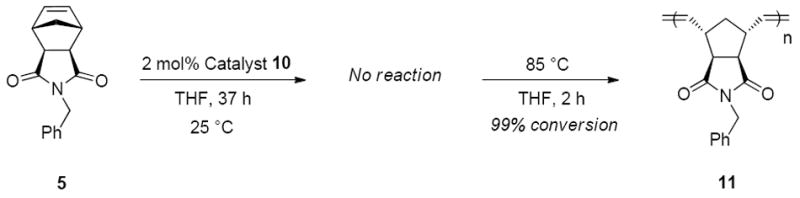
Latent ROMP of 5 with catalyst 10.
The solvent also plays a role in the degree of latency of the catalysts, as THF proved to result in significantly improved latency at 25 °C for ROMP compared to benzene. Specifically, complex 10 showed excellent latency toward the ROMP of COD in THF at 25 °C, giving no polymerization product after 18 hours. However, repeating the same reaction with complex 10 using benzene as the solvent yields 28% conversion of COD to polymer after 30 minutes at 25 °C. THF may increase the latency of the catalysts by functioning as a coordinating solvent, thereby potentially slowing olefin association with ruthenium.
The results of latent ROMP of monomers 5-8 with catalyst 10 are presented in Table 4. Catalyst 10 was latent for the ROMP of all monomers screened, affording no reaction at 25 °C up to 37 hours. Excellent conversion was achieved in 2 hours at 85 °C for each of the monomers (Table 3, entries 2, 4, 6, and 8), and the corresponding polymers were isolated in good yield. The polydispersity index (PDI) was moderately low for the polymerization of 5 and 6 (Table 3, entries 2 and 4), indicating good catalyst initiation and propagation. The PDI for the polymerization of 7 was significantly broader and for 8 was moderately broader (Table 3, entries 6 and 8, respectively), suggesting poorer catalyst initiation for these monomers.
Table 3.
Latent ROMP of norbornene-derived monomers with catalyst 10.
| Entrya | Monomer | Temp. (°C) | Time (h) | Conversionb | Yieldc | Mn (g/mol)d | PDId |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 25 | 24 | 0% | — | — | — |
| 2 | 5 | 85 | 2 | 99% | 81% | 24,300 | 1.16 |
| 3 | 6 | 25 | 37 | 0% | — | — | — |
| 4 | 6 | 85 | 2 | 95% | 94% | 36,900 | 1.27 |
| 5 | 7 | 25 | 24 | 0% | — | — | — |
| 6 | 7 | 85 | 2 | 99% | 48% | 2,000 | 3.25 |
| 7 | 8 | 25 | 37 | 0% | — | — | — |
| 8 | 8 | 85 | 2 | 78% | 68% | 3,800 | 1.79 |
The loading of catalyst 10 was 2 mol%. The substrate concentrations were 0.5M in THF, and the reactions were carried out sealed under a nitrogen atmosphere.
The conversion was determined by 1H NMR spectroscopy.
Isolated polymer yield.
The molecular weight and PDI were determined by GPC.
Conclusions
We have developed N-aryl,N-alkyl NHC ruthenium catalysts showing excellent latent behavior toward cross-metathesis and ROMP reactions, providing fine thermal control for initiation. These complexes demonstrate remarkable thermal stability over extended periods of time, enabling metathesis reactions to be successfully carried out at high temperatures. Exchanging out the chloride ligands for iodide ligands is important for producing complexes that are latent for ROMP. Catalyst studies showed that elevated temperatures are required for metathesis activity, and upon reacting at these temperatures, the catalysts afford good conversion of substrate to product. These N-aryl,N-tert-butyl ruthenium complexes are attractive for applications in latent chemistry due to their properties and behavior, and their thermal stability lends them to be promising metathesis catalysts where elevated temperatures are required.
Supplementary Material
Acknowledgments
This research was supported by the National Science Foundation through a Graduate Fellowship to Renee Thomas and by the Department of Defense through a Graduate Fellowship to Benjamin Keitz. Alexey Fedorov thanks the Swiss National Science Foundation for a post-doctoral fellowship. We thank Dr. Rosemary Conrad for the synthesis of monomer 6. The authors thank the NSF and NIH for providing funding and Materia for gifts of catalyst precursors.
Footnotes
Supporting Information Available. Catalyst syntheses and experimental procedures. X-ray crystallographic data. Supporting information is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Trnka TM, Grubbs RH. Acc Chem Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]; (b) Hoveyda AH, Zhugralin AR. Nature. 2007;450:243–251. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]; (c) Chatterjee AK, Morgan JP, Scholl M, Grubbs RH. J Am Chem Soc. 2000;122:3783–3784. [Google Scholar]; (d) Fu GC, Nguyen ST, Grubbs RH. J Am Chem Soc. 1993;115:9856–9857. [Google Scholar]
- 2.Ritter T, Hejl A, Wenzel AG, Funk TW, Grubbs RH. Organometallics. 2006;25:5740–5745. [Google Scholar]
- 3.Kuhn KM, Bourg JB, Chung CK, Virgil SC, Grubbs RH. J Am Chem Soc. 2009;131:5313–5320. doi: 10.1021/ja900067c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Endo K, Grubbs RH. J Am Chem Soc. 2011;133:8525–8527. doi: 10.1021/ja202818v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 5.Monsaert S, Lozano Vila A, Drozdzak R, Van Der Voort P, Verpoort F. Chem Soc Rev. 2009;38:3360–3372. doi: 10.1039/b902345n. [DOI] [PubMed] [Google Scholar]
- 6.Szadkowska A, Grela K. Current Organic Chemistry. 2008;12:1631–1647. [Google Scholar]
- 7.Hejl A, Day MW, Grubbs RH. Organometallics. 2006;25:6149–6154. [Google Scholar]
- 8.Ung T, Hejl A, Grubbs RH. Organometallics. 2004;23:5399–5401. [Google Scholar]
- 9.Samec JS, Keitz BK, Grubbs RH. J Organomet Chem. 2010;695:1831–1837. [Google Scholar]
- 10.Wang D, Wurst K, Knolle W, Decker U, Prager L, Naumov S, Buchmeiser MR. Angew Chem Int Ed. 2008;47:3267–3270. doi: 10.1002/anie.200705220. [DOI] [PubMed] [Google Scholar]
- 11.Ginzburg Y, Anaby A, Vidavsky Y, Diesendruck CE, Ben-Asuly A, Goldberg I, Lemcoff NG. Organometallics. 2011;30:3430–3437. [Google Scholar]
- 12.Wang D, Wurst K, Buchmeiser MR. Chem Eur J. 2010;16:12928–12934. doi: 10.1002/chem.201001999. [DOI] [PubMed] [Google Scholar]
- 13.Kost T, Sigalov M, Goldberg I, Ben-Asuly A, Lemcoff NG. J Organomet Chem. 2008;693:2200–2203. [Google Scholar]
- 14.(a) Monsaert S, Ledoux N, Drozdzak R, Verpoort F. J Polym Sci Part A: Polym Chem. 2010;48:302–310. [Google Scholar]; (b) Zirngast M, Pump E, Leitgeb A, Albering JH, Slugovc C. Chem Comm. 2011;47:2261–2263. doi: 10.1039/c0cc04897f. [DOI] [PubMed] [Google Scholar]
- 15.Lexer C, Burtscher D, Perner B, Tzur E, Lemcoff GN, Slugovc C. J Organomet Chem. 2011;696:2466–2470. [Google Scholar]
- 16.Hudson DM, Valerte EJ, Schachner J, Limbach M, Müller K, Schanz HJ. ChemCatChem. 2011;3:297–301. [Google Scholar]
- 17.Diesendruck CE, Vidavsky Y, Ben-Asuly A, Lemcoff NG. Journal of Polymer Science: Part A: Polymer Chemistry. 2009;47:4209–4213. [Google Scholar]
- 18.Ben-Asuly A, Tzur E, Diesendruck CE, Sigalov M, Goldberg I, Lemcoff NG. Organometallics. 2008;27:811–813. [Google Scholar]
- 19.Kabro A, Roisnel T, Fischmeister C, Bruneau C. Chem Eur J. 2010;16:12255–12261. doi: 10.1002/chem.201001659. [DOI] [PubMed] [Google Scholar]
- 20.Szadkowska A, Gstrein X, Burtscher D, Jarzembska K, Wozniak K, Slugovc C, Grela K. Organometallics. 2010;29:117–124. [Google Scholar]
- 21.Thomas RM, Keitz BK, Champagne TM, Grubbs RH. J Am Chem Soc. 2011;133:7490–7496. doi: 10.1021/ja200246e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong SH, Sanders DP, Lee CW, Grubbs RH. J Am Chem Soc. 2005;127:17160–17161. doi: 10.1021/ja052939w. [DOI] [PubMed] [Google Scholar]
- 23.(a) Nguyen ST, Johnson LK, Grubbs RH. J Am Chem Soc. 1992;114:3974–3975. [Google Scholar]; (b) Schwab P, France MB, Ziller JW, Grubbs RH. Angew Chem Int Ed. 1995;34:2039–2041. [Google Scholar]
- 24.Funk TW, Berlin JM, Grubbs RH. J Am Chem Soc. 2006;128:1840–1846. doi: 10.1021/ja055994d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hejl A. Ph D Dissertation, California Institute of Technology. 2007. [Google Scholar]
- 26.Sanford MS, Love JA, Grubbs RH. J Am Chem Soc. 2001;123:6543–6554. doi: 10.1021/ja010624k. [DOI] [PubMed] [Google Scholar]
- 27.Catalyst 10 was investigated for latent RCM of diethyl diallylmalonate; however, the complex never initiated even at 85 °C for this substrate. Catalyst 2, with chloride ligands in place of iodide ligands, showed low activity for RCM of diethyl diallylmalonate at 25 °C. These complexes were therefore concluded to not be optimal for latent RCM.
- 28.Wappel J, Urbina-Blanco CA, Abbas M, Albering JH, Saf R, Nolan SP, Slugovc C. Beilstein J Org Chem. 2010;6:1091–1098. doi: 10.3762/bjoc.6.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


