Abstract
Current strategies to deliver therapeutic molecules to specific cell and tissue types rely on conjugation of antibodies and other targeting ligands directly to the therapeutic molecule itself or its carrier. This work describes a novel strategy to deliver therapeutic molecules into macrophages that takes advantage of the native hemoglobin (Hb) scavenging activity of plasma haptoglobin (Hp) and the subsequent uptake of the Hb-Hp complex into macrophages via CD163 receptor mediated endocytosis. The drug delivery system described in this work consists of hemoglobin decorated liposomes that can encapsulate any therapeutic molecule of interest, in this case the model fluorescent dye calcein was used in this study. The results of this study clearly demonstrate that this delivery system is specific towards macrophages and demonstrates the feasibility of using this approach in targeted drug delivery.
Keywords: Hemoglobin, liposome, Hb-liposome, targeted drug delivery, macrophage, CD-163 receptor
Introduction
Liposome-based drug delivery systems are generally preferred to the free drug form because of their longer circulatory half-life, sustained drug release rate, compatibility with both hydrophilic and hydrophobic drugs, and cell/tissue selectivity when they are surface modified with appropriate targeting ligands (Peer et al. 2007; Samad et al. 2007). Various types of therapeutic molecules have been successfully encapsulated and delivered via liposomes, including DNA and RNA (Perrie et al. 2004; Shen 2008), peptides and proteins (Vangala et al. 2007) as well as small drug molecules (Danoff et al. 2007).
Typical strategies that permit targeting of liposomes to a specific type of cell or tissue include conjugating antibodies (Torchilin 2008), aptamers (Cao et al. 2009), glycoproteins (Soni et al. 2005), and peptides (Wang et al. 2009) to the surface of the liposome. Among these strategies, antibodies and aptamers are highly selective towards their target.
In this work, we describe a novel system that can be used to specifically targeted delivery of liposomes carrying a therapeutic cargo towards macrophages. This approach takes advantage of the native hemoglobin (Hb) scavenging machinery of the body (Kristiansen et al. 2001). In the body, acellular Hb generated from lysed red blood cells (RBCs) binds to plasma haptoglobin (Hp) in a 1:1 molar ratio and is subsequently scavenged by the macrophages via the CD163 scavenging receptor. This is the major route by which Hb is cleared from the blood. If the available Hp in the blood becomes saturated with Hb, the unbound Hb is then eliminated from the body via the kidneys (Bunn et al. 1969).
Two groups have used this Hb scavenging mechanism to deliver small drug molecules to monocytes and macrophages, by conjugating these molecules to the surface of Hb. In 2006, Brookes et al. conjugated ribavirin to the surface of Hb in order to treat hepatitis (Brookes et al. 2006). This work was extended to cancer treatment by Palmer's group, who showed that it is possible to conjugate an anti-cancer drug to the surface of hemoglobin in order to kill monocytic cancer cells (Zhang and Palmer 2011).
However, conjugation of small drug molecules to the surface of Hb only results in the conjugation of a limited number of drug molecules, and low drug delivery efficiency which is caused by the absence of multivalency with respect to the targeting ligand (i.e. Hb) (Pastan et al. 2006). In this work, Hb is conjugated to the surface of liposomes in order to serve as a ligand to specifically target uptake by macrophages. This approach dually serves to facilitate encapsulation of large amounts of therapeutic molecules per liposome along with conferring a high degree of multivalency due to the multiple copies of Hb displayed on the liposome surface.
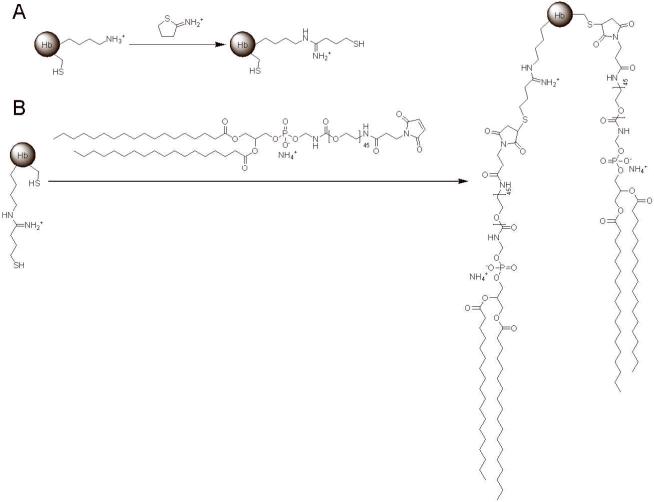
To attach Hb to the surface of liposomes, Hb is first thiolated using the reagent 2-iminothiolane. The thiolated Hb can then react with any free maleimide groups via any free thiol groups on the surface of Hb. In this work, the maleimide group is conjugated to one end of the polyethylene glycol-2000 linker (PEG(2000)), while the other end of the PEG(2000) linker is conjugated to the lipid distearoylphosphatidylethanolamine (DSPE). Therefore, liposomes generated with maleimide functionalized DSPE possess maleimide groups incorporated both on the inside and outer leaflet of the liposome membrane. Therefore, mixing maleimide functionalized liposomes and thiolated Hb will covalently link Hb to the outer leaflet of the liposome membrane. Several of these chemical routes (i.e. thiolation of Hb and Hb conjugation) are well established in the literature (Manjula et al. 2003; Vandegriff et al. 2003).
The pathway by which Hb modified liposomes specifically target macrophages is hypothesized to occur as follows: 1) Hb conjugated liposomes (liposome-Hb) bind to free Hp in the blood, and forms stable liposome-Hb-Hp complexes; 2) The liposome-Hb-Hp complex is then recognized by the CD163 receptor present on the surface of macrophages; 3) The liposome-Hb-Hp-CD163 complex then mediates its internalization into macrophages. At this point, any encapsulated therapeutic molecule can be released into the cytosol of macrophages to treat macrophage-specific diseases, such as arthritis (Kinne et al. 2000), diabetes (Odegaard et al. 2007), hepatitis (Brookes et al. 2006) and monocytic leukemia (Honma 2001).
Materials and Methods
Materials
Human hemoglobin (Hb) was purified via tangential flow filtration as described in the literature (Elmer et al. 2010; Palmer et al. 2009). Human Hp was purchased from Abcam, Inc. (Cambridge, MA). 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (DSPE-PEG(2000) Maleimide) (i.e. maleimide-DSPE) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). All other chemicals including calcein, cholesterol, Triton X-100, chloroform and 2-iminothiolane were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of Liposomes Conjugated with Hb
7 μmol of DSPC, 3 μmol of cholesterol and 1 μmol of maleimide-DSPE were dissolved in chloroform separately and mixed together. The lipid mixture was initially air dried and then vacuumed dried for 1 h to form dry lipid films. Then the lipid film (11 μmol) was rehydrated in 1 mL of 50 mM calcein in low salt phosphate buffered saline (PBS) (37 mM sodium chloride, 2.7 mM potassium chloride, 8.1 mM sodium phosphate dibasic, and 1.76 mM potassium phosphate monobasic, pH 7.4) and extruded through a 0.1 μm membrane filter (Whaterman Ltd, England) at 65°C to form liposomes functionalized with maleimide (maleimide-liposomes). Maleimide-liposomes were purified on a Sephacryl S-200 gel filtration column (GE Healthcare, Waukesha, WI) equilibrated with PBS (137 mM sodium chloride, 2.7 mM potassium chloride, 8.1 mM sodium phosphate dibasic, and 1.76 mM potassium phosphate monobasic, pH 7.4) using an AKTA FPLC system (Pharmacia, Sweden).
Thiolated Hb was previously prepared by reacting 1 equivalent of human Hb with 4 equivalents of 2-iminothiolane in degassed PBS (pH 7.4) at room temperature for 2 h, and subsequently purified using a 10 kDa cutoff centrifugal filter unit (Millipore, Billerica, MA).
11 μmol of maleimide-liposomes was then reacted with either 0.25 μmol of thiolated Hb or β-mercaptoethanol in PBS (pH 7.4) in a 300 rpm shaker at 37°C for 1 h, in order to produce Hb conjugated liposomes (Hb-liposomes) or control liposomes with blocked maleimide groups (control-liposomes). Then both types of liposome preparations were purified on a Sephacryl S-200 gel filtration column, with PBS (pH 7.4) as running buffer. Three batches of Hb-liposomes and control-liposomes were made separately.
Calcein Concentration
Calcein concentration was determined on a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE) at 493 nm, and calculated based on the extinction coefficient of 18,258 M-1 cm-1.
Hb Concentration
Hb concentration was determined by the Bradford protein assay (Bio-Rad, Philadelphia, PA) using bovine serum albumin (BSA) standards (Thermo Scientific).
UV-Visible Spectra
UV-Visible spectra were recorded on a Nanodrop ND-1000 spectrophotometer. Hb-liposomes and control-liposomes were diluted with PBS (137 mM sodium chloride, 2.7 mM potassium chloride, 8.1 mM sodium phosphate dibasic, and 1.76 mM potassium phosphate monobasic, pH 7.4) to a concentration of 0.8 mM calcein. Hb was diluted with the same buffer to a concentration of 1 mg/mL.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Hb-liposomes containing 10 μg of Hb, equal amounts of control-liposomes, and 10 μg of Hb were separately boiled with Laemmli sample buffer (Bio-Rad), and loaded onto a 12% SDS-PAGE gel, and the resulting gel was imaged on a Gel Doc XR imaging system (Bio-Rad). Subsequent densitometric analysis was carried out by Quantity One 4.6.3 software (Bio-Rad).
Dynamic Light Scattering (DLS)
50 μL of 5 mM sample was transferred into a 45 μL low-volume quartz batch cuvette (Malvern, United Kingdom), and the liposome size distribution was recorded on a Malvern Zetasizer Nano ZS (Malvern,) at 37°C. Statistics were calculated based on the results from three different batches of the two types of liposomes.
Calcein Release
Each sample was diluted with 30% HyClone fetal bovine serum (FBS) (Thermo Scientific) in PBS (pH 7.4) to 100 μM lipid, and incubated in a 300 rpm shaker at 37°C. At each time point (t, t=0, 2, 4, 8, 24, 48, 72, 96 h), the calcein emission at 518 nm (Ft) was recorded on a PerkinElmer LS-50B fluorimeter (PerkinElmer, Waltham, MA), using an excitation wavelength at 493 nm and an emission wavelength at 515 nm. At the end of 96 h, each sample was lysed by incubating it with10 μL of 20% Triton X-100 for 30 min at 65°C in order to release all the encapsulated calcein, and the fluorescence emission was recorded as Ftotal. The percentage of calcein released at each time point was then calculated by using the following equation (Ft-F0)/(Ftotal-F0).
Surface Plasmon Resonance (SPR)
SPR was conducted at a flow rate of 10 μL/min of HBS-EP running buffer (pH 7.4) (Biacore, Sweden) on a Biacore 3000 system (Biacore) at 37°C. Before experiments were conducted, Hp was diluted to 30 mg/mL and immobilized on a CM5 sensor chip (Biacore) using an amine coupling kit (Biacore) according to the manufacturer's instructions. During experiments, control-liposomes at the 5 indicated concentrations were loaded into 5 separate Hp channels for 7 min, in order to test any association between control-liposomes and Hp, and then washed by running buffer to facilitate dissociation for 2 min. Because these 5 Hp channels showed no association with control-liposomes at all, the same channels were used for Hb-liposome association and dissociation experiments. Hb-liposomes at the 5 concentrations indicated were loaded into these 5 channels to bind Hp for 7 min, and washed to detect any dissociation for 8 min. Data was collected using Biacore 3000 Control Software and processed in BIAevaluation Software (Biacore).
Cell Experiments
THP-1 (ATCC, Manassas, VA) and U251 (ATCC) cells were maintained in GIBCO RPMI 1640 (Invitrogen) supplemented with 10% FBS and 1% GIBCO penicillin streptomycin (Invitrogen), at a concentration of 0.2-1 million cells/mL. Before experiments were conducted, THP-1 cells were transformed into macrophages by incubating them with 100 nM of phorbol-12-myristate-13-acetate (Sigma-Aldrich) for 48 h, and then induced to a high CD163 expressing level by incubating them with 0.1 mg/mL dexamethasone (Sigma-Aldrich) for another 24 h. The cells were then treated with the indicated liposomes for 24 h, fixed with 4% formaldehyde (Thermo Scientific) in PBS, and mounted on ProLong antifade reagent with DAPI (Invitrogen). Images were taken 18 h later using an Olympus IX71 inverted fluorescent microscope (Olympus,Center Valley, PA), and processed using ImageJ software (NIH, Bethesda, MD).
Results and Discussion
Surface modification of liposome with Hb
In this work, we seek to design a lipid liposome-based therapeutic delivery system that is targeted towards macrophages. Since calcein is an easy molecule to detect, calcein was used in this study as the model drug that is encapsulated in all liposome preparations in order to evaluate the feasibility of this approach.
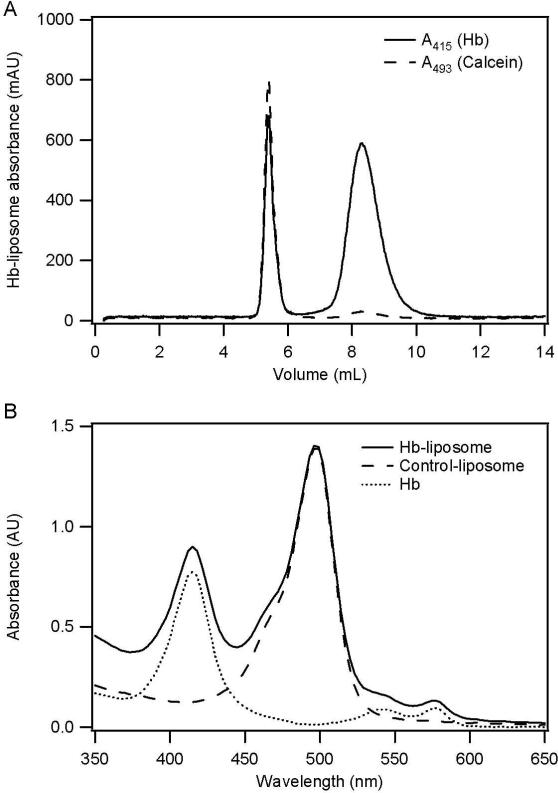
Calcein incorporated maleimide-liposomes were prepared by rehydrating the dry lipid film composed of DSPC, cholesterol, and maleimide-DSPE (7:3:1 molar ratio), with calcein solution, followed by extrusion of this suspension through a 0.1 μm membrane filter. In order to conjugate Hb to the maleimide-liposomes, thiolated Hb was produced by reacting Hb with 2-iminothiolane (Figure 1A). The thiolated Hb was then attached to the outer surface of the maleimide-liposomes via a maleimide-thiol coupling reaction (Figure 1B). Relatively large Hb-liposomes were then separated from smaller sized Hb molecules by size exclusion chromatography (SEC) (Figure 2A). As shown in Figure 2A, calcein incorporated liposomes eluted at 5.5 mL, while a fraction of Hb appears to also co-elute with the liposomes at 5.5 mL, indicating that this fraction of Hb is attached to the outer surface of liposomes and therefore forms Hb-liposomes. Conversely, unattached Hb eluted at 8.5 mL. Because Hb also exhibits some absorption at 493 nm, which is about one tenth of its absorption at 415 nm (Soret band), the small A493 peak at 8.5 mL is caused by acellular Hb, and not the Hb-liposomes encapsulating calcein. Therefore, Hb-liposomes were separated from free Hb, and this peak was collected for further study.
Figure 1.
Chemical routes for conjugating DSPE-PEG(2000)-Maleimide to Hb. A, Thiolated Hb was synthesized by reacting the side chain of Hb surface lysine residues with 2-iminothiolane, in order to add free thiol groups to the surface of Hb. B, DSPE-PEG(2000)-Maleimide was conjugated to native cysteine residues on the surface of Hb or thiolated lysine residues on the surface of Hb through maleimide-thiol coupling chemistry.
Figure 2.
SEC and UV-Visible spectrum of Hb-liposomes. A, SEC purification of Hb-liposomes and unreacted Hb, showing that a fraction of Hb co-eluted with the liposomes. The solid line, A415 is due to the Soret band of Hb; while the dashed line, A493 is due to the encapsulated calcein inside the Hb-liposomes. B, UV-Visible spectrum of Hb-liposomes, control-liposomes, and free Hb. The spectrum of Hb-liposomes is clearly a summation of the spectra from control-liposomes and free Hb. The solid line represents Hb-liposomes, while the dashed line represents control-liposome and the dotted line represents free Hb.
Figure 2B shows the UV-Visible spectrum of the purified Hb-liposomes, recorded in PBS (pH 7.4). Besides the large peak around 493 nm which is primarily due to the calcein incorporated inside the liposomes, Hb-liposomes possess three other peaks, which are known as Hb Soret-band (415 nm) and Hb Q-bands (542 nm and 577 nm). Therefore, the UV-Visible absorption spectrum of Hb-liposomes is composed of the absorption from calcein encapsulated inside the liposomes and Hb that is covalently attached to the surface of the liposomes, thereby indicating the existence of Hb-liposomes in solution. Since Hb is attached to the outer surface of the liposomes via a PEG(2000) linker, and it is known that PEGylation of Hb influences the stability of the Hb tetramer and Hb autoxidation which leads to methemoglobin formation (Hu et al. 2008; Vandegriff et al. 2006), the absorption of methemoglobin at 630 nm is also shown in Figure 2B. The increase in the absorption at 630 nm is unobservable for Hb-liposomes compared to Hb, indicating negligible methemoglobin formation during the preparation of Hb-liposomes.
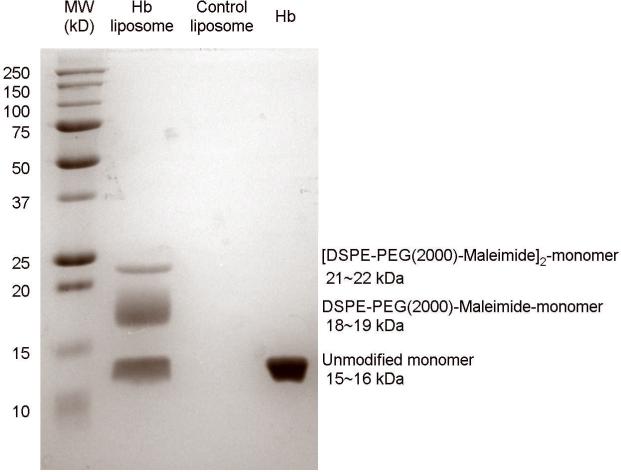
To prove that the thiolated Hb is chemically coupled to the maleimide group on the liposome surface, SDS-PAGE results are shown in Figure 3. After undergoing denaturing electrophoresis conditions, Hb-liposomes exhibit three bands, which are due to unreacted α and β subunits (46% of loaded protein), α and β subunits covalently coupled with one DSPE-PEG(2000)-Maleimide molecule (42% of loaded protein), and α and β subunits coupled with two DSPE-PEG(2000)-Maleimide molecules (12% of loaded protein), respectively. From the SDS-PAGE it is evident that 54% of the α and β subunits were conjugated to the maleimide lipid. The presence of unmodified α and β subunits could be explained by the fact that they were incorporated into Hb tetramers, in which other subunits in the tetramer reacted with the maleimide-liposomes. Therefore, the SDS-PAGE results show that Hb tetramers are covalently attached to the liposomes.
Figure 3.
SDS-PAGE of Hb-liposomes, control-liposomes and Hb. Hb-liposomes exhibit three bands corresponding to unmodified individual α (15.2 kDa) and β (15.9 kDa) subunits (46% of loaded protein), α and β subunits coupled with one DSPE-PEG(2000)-Maleimide molecule (18.2 kDa and 18.9 kDa, respectively, 42% of loaded protein), and α and β monomers coupled with two DSPE-PEG(2000)-Maleimide molecules (21.2 kDa and 21.9 kDa, respectively, 12% of loaded protein).
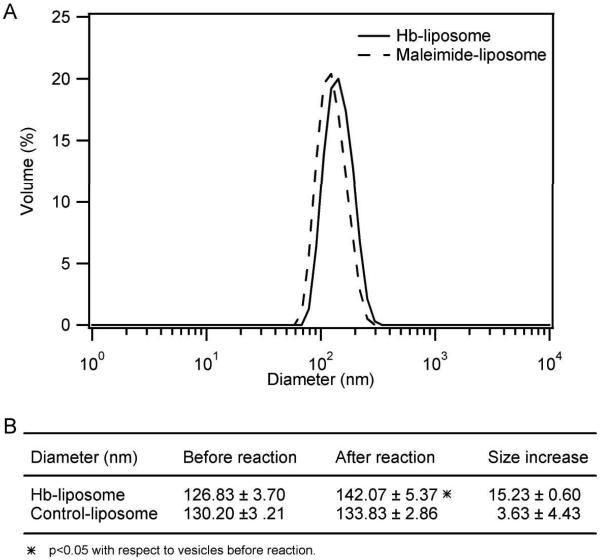
Hb modification also increases the size of the liposomes, as indicated by the DLS results. As shown in Figure 4A, the size distribution of maleimide-liposomes is shifted towards large values after reaction with Hb, but it is not shifted after incubation with β-mercaptoethanol in order to make control-liposomes (data not shown). Figure 4B shows that the increase in liposome diameter is significant for Hb-liposomes, but not significant for control-liposomes. Interestingly, Hb modification increases the liposome diameter by 15.23±0.60 nm, which happens to be twice the diameter of tetrameric Hb (7 nm) (GhoshMoulick et al. 2007). Therefore, this indicates that the increase in diameter of Hb-liposomes is caused by adding a layer of Hb tetramers to the surface of the liposomes.
Figure 4.
DLS of Hb-liposomes and control-liposomes. A, DLS size distribution of the maleimide-liposomes before (dashed line) and after (solid line) reaction with Hb; B, DLS diameter results of Hb-liposomes, control-liposomes, and size increase caused by covalent modification of the liposome surface with Hb. The statistics are based on results from three different batches of liposomes.
Drug release
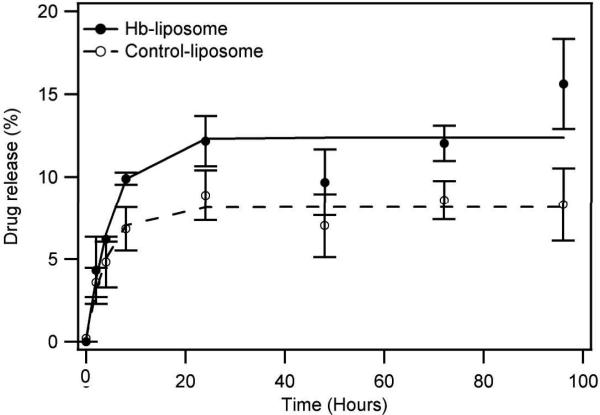
To test the stability of Hb-liposomes in a plasma-like environment, calcein release experiments from Hb-liposomes was conducted in 30% FBS/PBS in a 300 rpm shaker at 37°C (Figure 5). The results show both Hb-liposomes and control-liposomes quickly release encapsulated calcein within the first 24 h, but become stable after that time period. At the end of the experiment, Hb-liposomes released approximately 15% of the incorporated calcein, slightly higher than the control-liposomes which released less than 10% of the calcein. Both leakage rates are acceptable in medicine, considering that most long circulating liposomes are cleared during the first 24 h of circulation (Takeuchi et al. 2001). Therefore, Hb-liposomes are stable enough to deliver more than 85% of the incorporated drug into the target cell, even after four days of incubation in a plasma-like setting.
Figure 5.
Calcein release from Hb-liposomes and control liposomes. Both Hb-liposomes and control-liposomes possess low rates of drug release. The solid line represents Hb-liposomes, while the dashed line represents control-liposomes.
Hb-liposomes bind Hp and form Hb-Hp modified liposomes
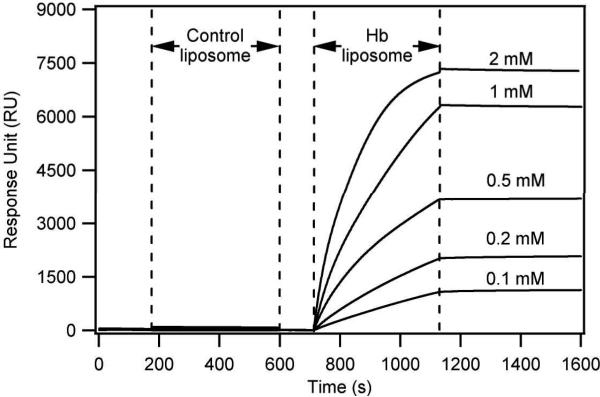
A special characteristic of Hb is its ability to bind Hp, and form stable Hb-Hp complexes. The Hb-Hp complex will be recognized by the CD163 receptor on the surface of macrophages, and this recognition will mediate Hb-Hp receptor mediated uptake by the macrophages. Therefore, in order for Hb-modified liposomes to deliver encapsulated therapeutics into target cells, it is important that these Hb-liposomes be able to tightly bind Hp. To test whether the interaction between Hb-liposomes and Hp exists, and whether this interaction is stable, SPR analysis was conducted (Figure 6). As shown in Figure 6, the signal indicating that Hb-liposomes bind to the immobilized Hp on the chip surface increases during the association stage, and plateaus during the dissociation stage, indicating that the Hb-liposomes bind to the immobilized Hp and form stable Hb-Hp modified liposomes. In the same Hp fixed channels, control liposomes without Hb conjugated on their surface only bind Hp at negligible levels during the association stage, and quickly detach Hp during the dissociation stage, indicating that the interaction between control-liposomes and Hp is unspecific in nature.
Figure 6.
SPR of Hb-liposomes and control-liposomes.
Hb-Hp modified liposomes as targeted delivery for macrophages
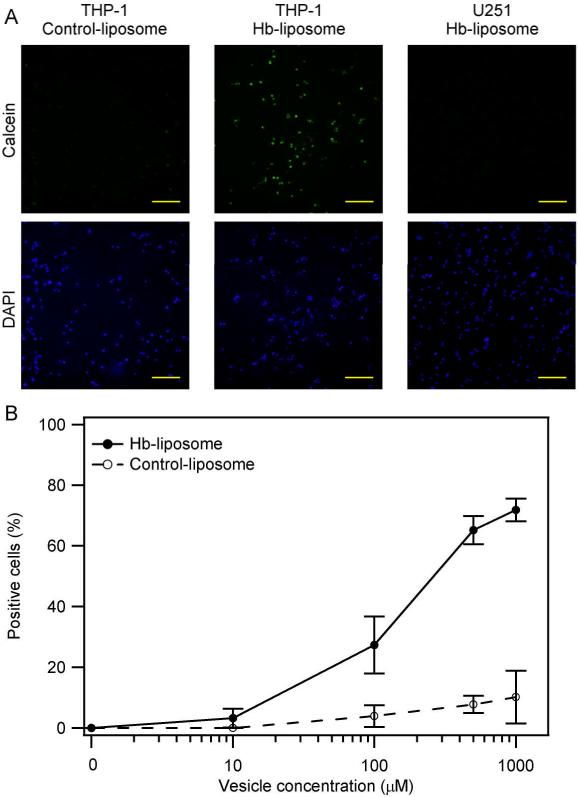
It is known that the Hb-Hp complex will undergo endocytosis via the Hb-Hp receptor CD163 that is present on the surface of macrophages. However, it is not clear whether Hb-Hp complexes conjugated to liposomes will also undergo CD163 receptor-mediated endocytosis. Figure 7A shows that less than 5% of the macrophages expressing the CD163 receptor on the cell surface internalized control liposomes without Hb modification, as a result of unspecific endocytosis; while approximately 70% of the macrophages internalized Hb-liposomes. This result indicates that Hb-liposomes together with Hp are specifically taken up by macrophages via CD163-dependent endocytosis. On the other hand, human glioma cells (U251) treated with the same amount of Hb-liposomes and Hp did not take up the Hb-liposomes. This is result is expected, since U251 cells do not express the CD163 receptor on their surface. Figure 7B shows that the uptake of Hb-liposomes by macrophages is dose-dependent.
Figure 7.
Cell uptake experiments. Hb-liposomes are specifically internalized by macrophages, and not non-macrophage cells, while control-liposomes are unspecifically taken up by the cells. A, Fluorescent images of the indicated cell line treated with 1 mM of the indicated liposomes. The green signal represents calcein, while the blue signal represents cell nuclei. Bars represent 100 μm. B, Cell count results from the fluorescent images. The solid line represents Hb-liposomes, while the dashed line represents control-liposomes.
Conclusions
In this work, a new strategy was developed to targeted delivery of liposomes carrying a therapeutic payload to macrophages. Liposomes encapsulating therapeutic molecules are conjugated with Hb, which, together with Hp, mediate CD163-dependent endocytosis by the macrophages. These results show that, Hb-liposomes are stable even after 4 days of shaking in a plasma-like environment. And after exposure to 1 mM liposomes, approximately 70% of macrophages internalize drug encapsulated Hb-liposomes, supporting the potential use of Hb-liposomes as a drug delivery system targeting macrophages.
Acknowledgements
This work was supported by National Institutes of Health grants R01HL078840 and R01DK070862 to AFP. The authors would like to thank David R. Harris for purifying the human hemoglobin used in these studies.
References
- Brookes S, Biessels P, Ng NF, Woods C, Bell DN, Adamson G. Synthesis and characterization of a hemoglobin-ribavirin conjugate for targeted drug delivery. Bioconjug Chem. 2006;17(2):530–7. doi: 10.1021/bc0503317. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Esham WT, Bull RW. The renal handling of hemoglobin. I. Glomerular filtration. J Exp Med. 1969;129(5):909–23. doi: 10.1084/jem.129.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Tong R, Mishra A, Xu W, Wong GC, Cheng J, Lu Y. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew Chem Int Ed Engl. 2009;48(35):6494–8. doi: 10.1002/anie.200901452. [DOI] [PubMed] [Google Scholar]
- Danoff EJ, Wang X, Tung SH, Sinkov NA, Kemme AM, Raghavan SR, English DS. Surfactant vesicles for high-efficiency capture and separation of charged organic solutes. Langmuir. 2007;23(17):8965–71. doi: 10.1021/la070215n. [DOI] [PubMed] [Google Scholar]
- Elmer J, Buehler PW, Jia YP, Wood F, Harris DR, Alayash AI, Palmer AF. Functional Comparison of Hemoglobin Purified by Different Methods and Their Biophysical Implications. Biotechnology and Bioengineering. 2010;106(1):76–85. doi: 10.1002/bit.22659. [DOI] [PubMed] [Google Scholar]
- GhoshMoulick R, Bhattacharya J, Roy S, Basak S, Dasgupta AK. Compensatory secondary structure alterations in protein glycation. Biochim Biophys Acta. 2007;1774(2):233–42. doi: 10.1016/j.bbapap.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Honma Y. A novel therapeutic strategy against monocytic leukemia with deoxyadenosine analogs and adenosine deaminase inhibitors. Leuk Lymphoma. 2001;42(5):953–62. doi: 10.3109/10428190109097714. [DOI] [PubMed] [Google Scholar]
- Hu T, Li D, Manjula BN, Acharya SA. Autoxidation of the site-specifically PEGylated hemoglobins: role of the PEG chains and the sites of PEGylation in the autoxidation. Biochemistry. 2008;47(41):10981–90. doi: 10.1021/bi800906z. [DOI] [PubMed] [Google Scholar]
- Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2(3):189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Manjula BN, Tsai A, Upadhya R, Perumalsamy K, Smith PK, Malavalli A, Vandegriff K, Winslow RM, Intaglietta M, Prabhakaran M. Site-specific PEGylation of hemoglobin at Cys-93(beta): correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjug Chem. 2003;14(2):464–72. doi: 10.1021/bc0200733. others. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. doi: 10.1038/nature05894. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AF, Sun G, Harris DR. Tangential flow filtration of hemoglobin. Biotechnol Prog. 2009;25(1):189–99. doi: 10.1002/btpr.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6(7):559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- Perrie Y, Barralet JE, McNeil S, Vangala A. Surfactant vesicle-mediated delivery of DNA vaccines via the subcutaneous route. Int J Pharm. 2004;284(1-2):31–41. doi: 10.1016/j.ijpharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4(4):297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- Shen Y. Advances in the development of siRNA-based therapeutics for cancer. IDrugs. 2008;11(8):572–8. [PubMed] [Google Scholar]
- Soni V, Kohli DV, Jain SK. Transferrin coupled liposomes as drug delivery carriers for brain targeting of 5-florouracil. J Drug Target. 2005;13(4):245–50. doi: 10.1080/10611860500107401. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kojima H, Yamamoto H, Kawashima Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J Control Release. 2001;75(1-2):83–91. doi: 10.1016/s0168-3659(01)00368-6. [DOI] [PubMed] [Google Scholar]
- Torchilin V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin Drug Deliv. 2008;5(9):1003–25. doi: 10.1517/17425247.5.9.1003. [DOI] [PubMed] [Google Scholar]
- Vandegriff KD, Malavalli A, Minn C, Jiang E, Lohman J, Young MA, Samaja M, Winslow RM. Oxidation and haem loss kinetics of poly(ethylene glycol)-conjugated haemoglobin (MP4): dissociation between in vitro and in vivo oxidation rates. Biochem J. 2006;399(3):463–71. doi: 10.1042/BJ20060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegriff KD, Malavalli A, Wooldridge J, Lohman J, Winslow RM. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43(4):509–16. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- Vangala A, Bramwell VW, McNeil S, Christensen D, Agger EM, Perrie Y. Comparison of vesicle based antigen delivery systems for delivery of hepatitis B surface antigen. J Control Release. 2007;119(1):102–10. doi: 10.1016/j.jconrel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang X, Zhang Y, Yang S, Wang J, Zhang X, Zhang Q. RGD-modified polymeric micelles as potential carriers for targeted delivery to integrin-overexpressing tumor vasculature and tumor cells. J Drug Target. 2009;17(6):459–67. doi: 10.1080/10611860902974085. [DOI] [PubMed] [Google Scholar]
- Zhang N, Palmer AF. Development of a dichloroacetic acid-hemoglobin conjugate as a potential targeted anti-cancer therapeutic. Biotechnol Bioeng. 2011;108(6):1413–20. doi: 10.1002/bit.23071. [DOI] [PubMed] [Google Scholar]