Abstract
Background
Patients with hepatic malignancy have a dismal prognosis with standard therapies. NV1020 is an oncolytic herpes simplex virus that has potential to be a safe and effective therapeutic agent for this disease.
Objective
We set out to discuss the development of NV1020 as an oncolytic agent and explore the potential role of this particular virus in the setting of human hepatic cancer.
Methods
The scope of this review includes an overview of preclinical experience with NV1020, as well as an examination of current standard and developing therapies for liver cancer. The primary focus, however, is on the safety and potential clinical efficacy of NV1020 against hepatic malignancy.
Results/conclusion
We have found that NV1020 is a safe, novel therapeutic agent for treatment of refractory hepatic malignancy.
Keywords: colorectal cancer, hepatic malignancy, herpes simplex virus, oncolytic viral therapy
1. Introduction
Colorectal cancer is the fourth most common cancer in men and the third most common cancer in women worldwide, with over 1.1 million new cases per year. It is also the fourth and fifth leading cause of cancer-related mortality in men and women worldwide, respectively, with an estimated 630,000 deaths in 2007 [1]. While surgical resection offers hope for cure to those patients diagnosed with early colorectal cancer, only about 39% of patients fall into this category. In fact, up to 50% of those patients who undergo surgery will ultimately develop recurrent disease.
Up to 25% of patients with colorectal cancer have liver metastases at the time of presentation. Untreated patients have a median survival of 6 – 13 months. Following hepatic resection, median survival is 24 – 46 months, with a 5-year survival of 20 – 37% [2]. Systemic adjuvant chemotherapy, such as with 5-fluorouracil (5-FU)-based regimens, have been shown to improve 5-year survival, although not drastically [3].
Of those patients who present with hepatic metastases, only a small percentage have resectable disease. For those with unresectable hepatic disease, systemic chemotherapy is one of few therapeutic options available. Regimens such as 5-FU, leucovorin, and oxaliplatin (FOLFOX) and 5-FU, leucovorin, and irinotecan (FOLFIRI) have shown response rates as high as 40 – 60%, but median overall survival with either regimen is still only 18 – 20 months. Hepatic arterial infusion (HAI) of chemotherapeutics, specifically floxuridine (FUDR), is another method developed to deliver chemotherapy directly to liver tumors, but is available only to patients with disease confined to the liver. HAI FUDR significantly improved both disease-free survival and 2-year survival when combined with systemic chemotherapy as opposed to systemic chemotherapy alone after liver resection [4]. Ongoing studies are evaluating the efficacy of HAI chemotherapy relative to systemic therapy alone and in conjunction with surgery.
Surgical resection remains the only possibility for cure for metastatic colorectal cancer (mCRC), as well as for other liver tumors such as hepatocellular carcinoma (HCC) and biliary neoplasms [5,6]. However, this procedure is limited by tumor burden within the liver and estimated degree of functional liver remaining after resection. A clinical risk score (CRS) system was developed to stratify patients by median survival, with implications toward who should be resected [7]. Prognostic factors include: node-positive primary tumor, disease-free interval < 12 months between colon resection and appearance of metastases, tumor > 5 cm, < 1 tumor, and carcinoembryonic antigen (CEA) level > 200 ng/dl.
An estimated 65% of patients with hepatic tumors will recur after surgical resection. These recurrences are thought to originate from microscopic foci of residual disease [5,8]. Hepatic resection results in a burst of hepatocyte regeneration in the immediate postoperative period. This local environment of high mitotic activity is thought to support and enhance growth of residual cancer cells [8]. Novel treatment strategies are necessary to target microscopic residual disease and prevent the development of recurrence after hepatic resection. Several candidate therapies are new in the clinic and in preclinical development.
2. Alternative available therapies for hepatic metastases
Monoclonal antibodies (mAbs) have recently been approved for treatment of mCRC as a therapy that targets the molecular pathways of cancer development. Bevacizumab (Avastin, Genentech, South San Francisco, CA) is an anti-vascular endothelial growth factor (VEGF) mAb that added significant benefit when combined with other chemotherapeutics in several Phase III trials. Based on the Bowel Oncology With Cetuximab Antibody (BOND) study, cetuximab (Erbitux, ImClone Systems, Inc., New York), an anti-epithelial growth factor receptor (EGFR) mAb, was approved for treatment of patients with metastatic disease expressing EGFR refractory to irinotecan-based therapy. Panitumumab (Vectibix, Amgen, Thousand Oaks, CA) is another anti-EGFR mAb approved for therapy in those patients refractory to other chemotherapeutics [9]. While no survival benefit has been shown with mAbs as monotherapy, use is based on the advantages of mAbs in combination with other chemotherapy regimens.
Another therapeutic option for patients with hepatic metastases is tumor ablation. Several ablative options exist, including radiofrequency ablation (RFA) and microwave coagulation. RFA demonstrated a higher recurrence rate compared with hepatic resection, but was associated with an acceptable morbidity rate. Experience with microwave coagulation is limited [2]. A new and promising therapy exists in the form of selective internal radiation therapy (SIRT), in which radioactive microspheres containing yttrium-90, a β-radiation emitter, are delivered via a hepatic arterial catheter. A Phase III trial found favorable results when combining SIRT with HAI therapy [10].
Much like surgical resection, ablative techniques are limited by size, location, and number of tumors in the liver. Chemotherapy and mAbs have associated toxicities that limit use. Despite improved response rates being achieved in mCRC with these therapies, overall 5-year survival for mCRC disease remains < 40%. Novel therapies are needed for patients with mCRC, especially those with recurrent disease, unresectable disease, and disease refractory to current available therapies.
3. Oncolytic viral therapy with herpes simplex virus type-1
Oncolytic viral therapy aims to direct the innate cytolytic ability of viruses to specific killing of cancer cells. A wide variety of viruses have been studied including adenovirus, vaccinia virus, myxoma virus, vesicular stomatitis virus, Newcastle disease virus, and herpes simplex virus [11]. All of these viruses display the ability to infect, replicate in, and lyse host cells, but they differ in innate tumor specificity and in their ability to tolerate genetic manipulation.
Herpes simplex virus type-1 (HSV-1) displays many innate characteristics that make it desirable as a gene therapy vector. The wild-type virus is naturally cytotoxic, destroying host cells by lysis in the course of its life cycle. HSV-1 is able to infect and replicate in a wide variety of cell types by interaction of its surface glycoproteins with cell surface receptors. For example, HSV-1 glycoprotein D (gD) binds the cellular receptor nectin-1 to achieve cell entry. Nectin-1 is not accessible in normal cells because it is present as a component of the adherens junction complex. In cancer cells with decreased intracellular adherence, however, nectin-1 is present in its free form and mediates herpes viral entry [12,13]. Because herpesvirus replicates in cancer cells prior to lysing them, the initial administered dose is progressively amplified over the course of therapy. The HSV-1 genome has an enormous coding capacity for gene delivery. One concern is that HSV-1 is capable of latent infection existing as an episome [14]. However, this virus is susceptible to antiviral therapies and thus has a safeguard against uncontrolled replication [11,15].
The HSV-1 viral genome consists of two unique sequences, unique long and unique short (denoted UL and US, respectively), flanked by inverted repeat sequences, totaling approximately 152 kbp [16]. It has been found that of the 83 open reading frames (ORFs) expressing proteins in the HSV-1 genome, 38 are indispensable for successful viral infection and replication. The remaining 45, however, are not necessary for viral replication in at least some cell types, and can be deleted or modified to improve safety profile and to confer tumor specificity.
Recombinant HSV-1 mutants are under preclinical and clinical investigation for several gene therapy applications, including targeted gene delivery to the central nervous system, vaccination, and targeted anticancer therapy. Several replication-competent HSV-1 mutants have been shown to be effective against a wide variety of human cancer types, including brain, squamous cell, esophageal, gastric, colorectal, pancreatic, hepatocellular, lung, bladder, prostate, breast, melanoma, renal cell, ovarian, soft-tissue (rhabdomyosarcoma), and neural (neuroblastoma and peripheral nerve sheath tumors) [17–37]. These oncolytic herpes vectors have deletions of various viral genes. Host cell proteins that are equivalent or homologous to the missing viral gene products are abundant in malignant cells, but not in normal cells [38]. These deletions, therefore, render the engineered viruses cancer-specific. Table 1 summarizes oncolytic herpes simplex virus constructs that have gone to clinical trial [39–46].
Table 1.
Oncolytic herpes simplex viruses in clinical trial.
| Virus | Disease | Route of Administration and MTD | Toxicity |
|---|---|---|---|
| G207 [39] | Recurrent glioma | Intratumoral, tumor bed | MTD not determined, safe up to 3 × l09 pfu |
| HS VI716 [40–42] | Recurrent and high-grade glioma, melanoma | Intratumoral | MTD not determined, well-tolerated at single dose of 1 × 105 pfu and up to 4 doses of 1 × l03 pfu |
| NV1020 [43] | Liver metastases from colorectal cancer | Intraarterial (Hepatic artery) | MTD not determined; confirmed safe up to 1 × 108 pfu |
| OncoVEXGM-CSF [44] | Cutaneous and subcutaneous metastases from melanoma, breast, head and neck, and colorectal cancers | Intratumoral (multiple dosing) | Dose-limiting local reaction with single admin of 1 × 107 pfu in seronegative patients; well-tolerated up to 1 × 108 pfu after seroconversion from 1 × 106 pfu dose (MTD not determined) |
| HF10 [45,46] | Cutaneous and subcutaneous breast cancer metastases | Intratumoral (multiple dosing) | MTD not determined, well-tolerated up to 3 doses of 5 × 105 pfu |
4. Engineering of tumor specificity in HSV-1 mutant NV1020
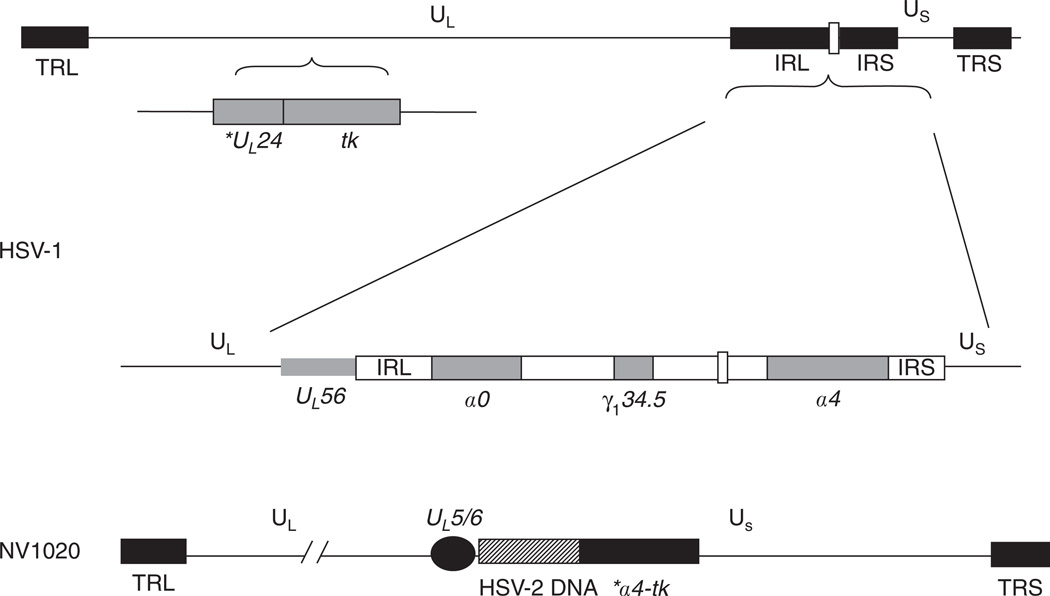
NV1020 is an attenuated, replication-competent, recombinant virus derived from the HSV-1 strain R7020, and was originally designed as a potential HSV-2 vaccine [47]. The construction of the parent strain R7020 was described previously [48], and this virus was proven safe for administration in rodents and primates [48,49]. NV1020 was attenuated by deletion of a 15-kb region at the UL/S junction encompassing one copy of the diploid genes α0, α4, and γ134.5 encoding the proteins ICP0, ICP4, and ICP34.5, respectively, and one copy of UL56, the protein product of which has not been fully characterized but is thought to be involved in neuroinvasiveness of HSV-1 [16,18,45,50]. ICP0 is one of the 45 dispensable gene products but mutants lacking this gene are debilitated at low multiplicity of infection (MOI, number of virus particles per cell) [16]. ICP4 is one of the indispensable gene products; at least one copy of the gene must be present for viral replication. ICP4 blocks apoptosis and positively regulates many other genes in the HSV-1 genome. ICP34.5 is a dispensable gene product. It functions to block the host cell protective mechanism of shutting off protein synthesis in response to viral infection by inhibiting a cellular protein kinase, PKR. Mutants missing this gene are therefore attenuated. In addition, γ134.5 confers the ability of HSV to thrive, particularly in the central nervous system. The mechanism behind this neurovirulence effect of the gene is not known.
NV1020 is further attenuated by a 700-bp deletion encompassing the thymidine kinase (TK) gene locus and the promoter for the gene UL24. This deletion effectively prevents the expression of UL24, which is thought to encode a membrane-associated protein of unknown function [16].
In addition to these deletions, NV1020 carries an exogenous, inserted copy of the tk gene under restrictive control of the ICP4 promoter, and a 5.2-kb fragment of HSV-2 DNA as it was originally designed as an HSV-2 vaccine. Both of these insertions are located at the UL/S junction [18]. A schematic of the modified genome of NV1020 is illustrated in Figure 1.
Figure 1. Construct of oncolytic herpes simplex virus recombinant NV1020.
NV1020 was attenuated by deletion of a 15-kb region at the UL/S junction encoding the proteins ICP0, ICP4, and ICP34.5, and one copy of the gene UL56. In addition, NV1020 has a 700-bp deletion encompassing the thymidine kinase (TK) gene locus and the promoter for the gene UL24. The novel UL/S junction of NV1020 carries a 3.7-kb duplication of UL5/6, a 5.2-kb fragment of HSV-2 DNA, and an exogenous, inserted copy of the tk gene under restrictive control of the ICP4 promoter.
* Denotes promoter for UL24 and a4, not entire gene.
These modifications render NV1020 highly attenuated, so that it can only propagate in transformed cells. Attenuated viruses rely on host cell proteins to compensate for the missing viral gene products, and many of these proteins are expressed only in post-mitotic, replicating cells in G1/S phase [51]. For example, cellular TK is not expressed in quiescent, normal cells, but is expressed abundantly in rapidly dividing malignant cells. Another common alteration in cells that have undergone malignant transformation is constitutive activation of the Ras signaling pathway. Ras activity directly blocks the effects of cellular PKR, so while the viral protein ICP34.5 is required for viral propagation in normal cells, it is not needed in cells with activated Ras [52].
5. Preclinical experience with NV1020
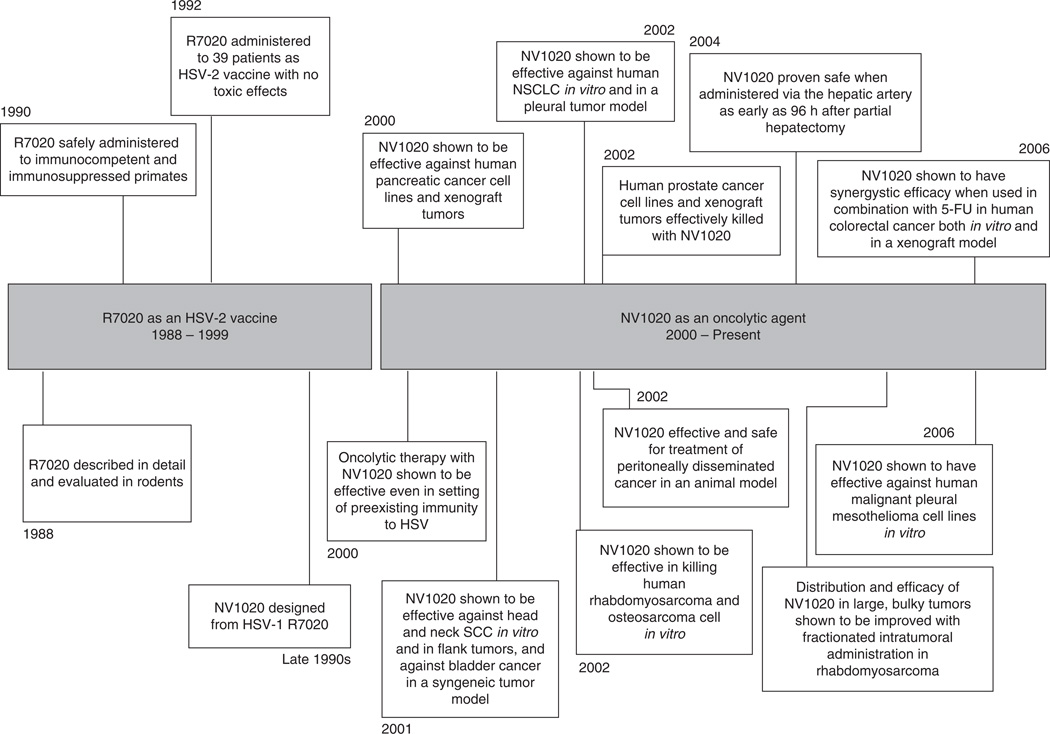
NV1020 has been shown to be efficacious in killing an enormous variety of cancer cell types in vitro and in animal models. It has been tested in pancreatic adenocarcinoma, mesothelioma, rhabdomyosarcoma, osteosarcoma, bladder, lung, gastric, and colorectal cancer, and in metastases to the liver and peritoneum [20,22,23,25,27,35,53,54] (Figure 2).
Figure 2. Timeline of development and preclinical investigation of NV1020.
NV1020 was originally designed as a potential anti-HSV-2 vaccine. It was not until the year 2000, 12 years after the parent strain was designed, that NV1020 came to be investigated as an oncolytic agent. From 2000 to present, multiple preclinical studies have been performed demonstrating the efficacy of this virus against a variety of cancer types and establishing its safety profile.
In 2000, Delman and colleagues reported a study designed to determine the effect of pre-existing immunity to HSV on oncolytic therapy with herpes vectors [6]. In this study, immunocompetent animals were immunized with HSV and tested for antibody titers. A model of hepatic metastasis was developed by splenic injection of CT-26 murine colorectal cancer cells. Animals were then treated with NV1020 administered intravenously or via the hepatic artery. The researchers found that pre-existing immunity somewhat abrogated the oncolytic effect of intravenously administered NV1020, but had no effect when the virus was administered locoregionally. They concluded that when given in reasonable proximity to the tumor target, oncolytic therapy with HSV is still effective against hepatic malignancy in the setting of anti-HSV immunity.
Another animal study reported in 2002 investigated the safety profile of NV1020 after regional and systemic administration [20]. In this study, a xenograft model of carcinomatosis was developed in athymic mice with human gastric cancer cell lines. NV1020 administered intraperitoneally significantly reduced tumor burden (from 1380 ± 310 mg in control animals to 150 ± 40 mg in treated animals; p < 0.005). In addition, brain, liver, kidneys, and tumor were harvested from treated animals for evaluation for HSV biodistribution. There was no HSV staining by immunohistochemisty (IHC) and no necrosis noted in any non-tumor tissues.
Preclinical data up to this point clearly demonstrated NV1020 to be a safe, effective, and tumor-specific oncolytic agent in vivo, and warranted bringing this therapy to clinical trial. Investigators proposed the use of oncolytic HSV in combination with liver resection to enhance killing of hepatic malignancy. This proposal, however, raised the concern that highly metabolically active regenerating hepatocytes in the post-hepatectomy period may themselves be susceptible to infection by oncolytic HSV.
This question was addressed in a study by Delman and colleagues in 2004 [55]. This study demonstrated that in C57Bl/6 mice, hepatocyte mitotic activity peaked 24 – 48 h after 70% hepatectomy and returned to baseline by 96 h. NV1020 was administered via portal vein injection at various time points after hepatectomy. It was found that administration of virus within the peak period of hepatocyte regeneration (24 – 48 h) permitted transient productive viral infection of tissue that does not normally support viral growth. Virus administered after the regenerative peak (≥ 96 h after hepatectomy) was not able to infect liver parenchyma. This data suggest that NV1020 can be administered safely in the adjuvant setting after partial hepatectomy, if given after the peak of hepatic regeneration.
6. Clinical experience with NV1020
Given such promising preclinical data and an excellent safety profile, NV1020 was taken to clinical trial in a Phase I study for administration to patients with refractory mCRC to the liver [43]. All patients had undergone partial hepatectomy and adjuvant chemotherapy prior to participating in this study. NV1020 was administered via HAI to four cohorts of three patients each at progressively escalating doses. The administered doses were 3 × 106, 1 × 107, 3 × 107, and finally 1 × 108 pfu. All patients received a chemotherapy infusion pump and cycles of FUDR plus systemic chemotherapy 1 and 2 months after administration of virus. No complications or adverse events occurred that were severe enough to define a maximum tolerated dose (MTD) for NV1020.
The most frequent adverse events attributable to virus infusion (occurring before pump placement) were pyrexia (50%), headache (50%), and rigors (25%). Only one serious adverse event occurred that was thought to be due to virus administration. This was a transient grade 4 elevation of γ-glutamyltransferase (GGT) in one patient, occurring 12 h after virus administration and normalizing by 24 h. There were no other significant abnormalities in blood chemistries, including liver function tests and serum cytokine levels. Additionally, serial monitoring of hepatic vein blood after virus infusion confirmed that the majority of virus was cleared by the liver. Very little virus reached systemic circulation at the higher doses of 3 × 107 and 1 × 108 pfu. There were no deaths in the study.
Patients underwent radiologic assessment for anti-tumor activity 28 days after administration of virus. Two patients in the higher-dose cohorts exhibited reduction in tumor size (39 and 20%), three exhibited progression of disease, and seven had stable disease.
Long-term follow-up of these 12 patients revealed that they all exhibited reduction in tumor size in response to subsequent chemotherapy. In one patient, NV1020 was detected by polymerase chain reaction (PCR) not only in tumor tissue from the virus-perfused lobe of the liver but also in a tumor from the non-perfused lobe. No patients developed reactivation of herpes or adverse effects as a result of treatment during the course of follow-up. The median survival for the group was 25 months. One patient with the greatest response to NV1020 is still alive at present (62 months after virus administration). The other 11 patients all died of disease progression (Y Fong, unpublished data, 2007).
An additional Phase I/II trial of NV1020 for colorectal cancer hepatic metastases with multiple dosing is underway. The Phase II component of this trial aims to determine the therapeutic effects of the optimally tolerated dose of NV1020 as determined by the Phase I component [56]. Results of this trial are not yet available.
7. Conclusion
In summary, there is demanding need for novel therapies for mCRC as well as for other hepatic malignancies. While surgical resection offers cure, a staggering number of patients are found to have unresectable disease on presentation or develop recurrent disease after resection. For these reasons, survival for hepatic malignancy is dismal.
Multimutated oncolytic herpes simplex viruses are in clinical trials as novel therapies for various malignancies (Table 1). While operating under the same principle of cancer-specific cell lysis, each herpes virus construct is different and each has a disease setting and route of administration with which it works best. NV1020 has been under investigation as an oncolytic agent for decades and has been shown to effectively kill a diversity of human cancer cell lines in vitro and in vivo. Even when given as early as 96 h after hepatectomy, NV1020 does not infect regenerating hepatocytes, which have upregulated metabolic pathways much like malignant cells. In a Phase I clinical trial, NV1020 was safely administered to the hepatic vasculature in 12 patients. The virus produced biologic changes in this toxicity study with single-dose administration.
8. Expert opinion
NV1020 holds great promise as a novel therapeutic agent for refractory hepatic malignancies. The results of the Phase I clinical trial demonstrate a clear biologic response to the virus without approaching a MTD. NV1020, unlike other therapies, remains biologically active once administered to tumor tissue. It is able to replicate in vivo, amplifying the initial administered dose, and is able to spread to tumor tissue adjacent to and even distant from the site of administration. This virus has been proven safe, even in the post-hepatectomy setting of hepatocyte regeneration. In addition, NV1020 has been shown to exhibit a synergistic cancer killing effect when used in combination with other therapies [22].
Preclinical studies have shown that oncolytic HSVs work best when administered in multiple doses [57,58]. Therefore, a logical next step in determining the full therapeutic potential of NV1020 against colorectal cancer liver metastases would be a clinical trial with multiple dosing regimens. The efficacy of NV1020 against other hepatic malignancies such as HCC and biliary neoplasms could also be explored in a similar manner. There could be roles for NV1020 as second- or third-line therapy for unresectable refractory hepatic disease, as adjuvant therapy after hepatic resection, or as neoadjuvant therapy to prevent tumor progression while patients wait for liver transplant.
A foreseeable problem for use of NV1020 and any oncolytic virus in humans is delineating exactly what biologic effects result directly from virus administration, as oncolytic HSVs will be used for patients with refractory disease who have already received and are possibly still receiving standard therapies. In addition, it has been reported that oncolytic HSVs can mutate [59]. Such a mutation would likely further attenuate the virus, and may decrease therapeutic efficacy, but would not likely affect the safety profile, as the virus would still be missing important virulence genes.
If NV1020 is proven to be a safe therapeutic agent at doses that are clinically effective when administered intra-arterially, directly into the blood supply of a vital organ, it would follow that this virus would be safe in less potentially hazardous settings such as intratumoral administration into soft-tissue malignancies and intrapleural and intraperitoneal administration for chest and abdominal disease, respectively. The clinical settings in which NV1020 could potentially be efficacious are unlimited.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- 1.American Cancer Society. [Last accessed 10 March 2009];Cancer facts and figures 2007. Available from: http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf.
- 2.Fahy BN, Jarnagin WR. Evolving techniques in the treatment of liver colorectal metastases: role of laparoscopy, radiofrequency ablation, microwave coagulation, hepatic arterial chemotherapy, indications and contraindications for resection, role of transplantation, and timing of chemotherapy. Surg Clin North Am. 2006;86:1005–1022. doi: 10.1016/j.suc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Parks R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753–761. doi: 10.1016/j.jamcollsurg.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 4.White RR, Jarnagin WR. The role of aggressive regional therapy for colorectal liver metastases. Cancer Invest. 2007;25:458–463. doi: 10.1080/07357900701508561. [DOI] [PubMed] [Google Scholar]
- 5.Fong Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J Clin. 1999;49:231–255. doi: 10.3322/canjclin.49.4.231. [DOI] [PubMed] [Google Scholar]
- 6.Delman KA, Bennett JJ, Zager JS, et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum Gene Ther. 2001;11:2465–2472. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- 7.Morris KT, Song TJ, Fong Y. Recent advancements in diagnosis and treatment of metastatic colorectal cancer to the liver. Surg Oncol. 2006;15(3):129–134. doi: 10.1016/j.suronc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Picardo A, Karpoff HM, Ng B, et al. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery. 1998;124:57–64. [PubMed] [Google Scholar]
- 9.Capdevila J, Saura C, Macarulla T, et al. Monoclonal antibodies in the treatment of advanced colorectal cancer. Eur J Surg Oncol. 2007;33 Suppl 2:S24–S34. doi: 10.1016/j.ejso.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Field K, Lipton L. Metastatic colorectal cancer-past, progress and future. World J Gastroenterol. 2007;13:3806–3815. doi: 10.3748/wjg.v13.i28.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo Y, Adusumilli PS, Fong Y. Advances in oncolytic viral therapy. Curr Opin Investig Drugs. 2006;7(6):549–559. [PubMed] [Google Scholar]
- 12.Yoon M, Spear PG. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J Virol. 2002;76(14):7203–7208. doi: 10.1128/JVI.76.14.7203-7208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Z, Adusumilli PS, Eisenberg DP, et al. Nectin-1 expression by squamous cell carcinoma is a predictor of herpes oncolytic sensitivity. Mol Ther. 2007;15(1):103–113. doi: 10.1038/sj.mt.6300009. [DOI] [PubMed] [Google Scholar]
- 14.Mellerick DM, Fraser NW. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987;158(2):265–275. doi: 10.1016/0042-6822(87)90198-x. [DOI] [PubMed] [Google Scholar]
- 15.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9(12):967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 16.Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci USA. 1996;93(21):11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markert JM, Parker JN, Buchsbaum DJ, et al. Oncolytic HSV-1 for the treatment of brain tumours. Herpes. 2006;13(3):66–71. [PubMed] [Google Scholar]
- 18.Wong RJ, Kim SH, Joe JK, et al. Effective treatment of head and neck squamous cell carcinoma by an oncolytic herpes simplex virus. J Am Coll Surg. 2001;193(1):12–21. doi: 10.1016/s1072-7515(01)00866-3. [DOI] [PubMed] [Google Scholar]
- 19.Stiles BM, Bhargava A, Adusumilli PS, et al. The replication-competent oncolytic herpes simplex mutant virus NV1066 is effective in the treatment of esophageal cancer. Surgery. 2003;134(2):357–364. doi: 10.1067/msy.2003.244. [DOI] [PubMed] [Google Scholar]
- 20.Bennett JJ, Delman KA, Burt BM, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther. 2002;9(11):935–945. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- 21.Kooby DA, Carew JF, Halterman MW, et al. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207) FASEB J. 1999;13(11):1325–1334. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- 22.Gutermann A, Mayer E, Von Dehn-Rothfelser K, et al. Efficacy of oncolytic herpesvirus NV1020 can be enhanced by combination with chemotherapeutics in colon carcinoma cells. Hum Gene Ther. 2006 doi: 10.1089/hum.2006.17.1241. [DOI] [PubMed] [Google Scholar]
- 23.McAuliffe PF, Jarnagin WR, Johnson P, et al. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. J Gastrointest Surg. 2000;4(6):580–588. doi: 10.1016/s1091-255x(00)80106-7. [DOI] [PubMed] [Google Scholar]
- 24.Song TJ, Eisenberg DP, Adusumilli PS, et al. Oncolytic herpes viral therapy is effective in the treatment of hepatocellular carcinoma cell lines. J Gastrointest Surg. 2006;104:532–542. doi: 10.1016/j.gassur.2005.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adusumilli P, Stiles B, Chan M, et al. Imaging and therapy of malignant pleural mesothelioma. J Gene Med. 2006;8(5):603–615. doi: 10.1002/jgm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiles B, Adusumilli P, Bhargava A, et al. Minimally-invasive localization of oncolytic herpes simplex viral therapy of micrometastatic pleural cancer. Cancer Gene Ther. 2006;13(1):53–64. doi: 10.1038/sj.cgt.7700860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cozzi PJ, Malhotra S, McAuliffe P, et al. Intravesical oncolytic viral therapy using attenuated, replication-competent herpes simplex viruses G207 and Nv1020 is effective in the treatment of bladder cancer in an orthotopic syngeneic model. FASEB J. 2001;15(7):1306–1308. doi: 10.1096/fj.00-0533fje. [DOI] [PubMed] [Google Scholar]
- 28.Kelly K, Brader P, Rein A, et al. Attenuated multimutated herpes simplex virus-1 effectively treats prostate carcinomas with neural invasion while preserving nerve function. FASEB J. 2008 doi: 10.1096/fj.07-097808. [DOI] [PubMed] [Google Scholar]
- 29.Eisenberg DP, Adusumilli PS, Hendershott KJ, et al. Real-time intraoperative detection of breast cancer axillary lymph node metastases using a green fluorescent protein-expressing herpes virus. Ann Surg. 2006;243(6):824–832. doi: 10.1097/01.sla.0000219738.56896.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu JC, Booth MJ, Tripuraneni G, et al. A novel HSV-1 virus, JS1/34.5-/47-, purges contaminating breast cancer cells from bone marrow. Clin Cancer Res. 2006;12(22):6853–6862. doi: 10.1158/1078-0432.CCR-06-1228. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe D, Goshima F, Mori I, et al. Oncolytic virotherapy for malignant melanoma with herpes simplex virus type 1 mutant HF10. J Dermatol Sci. 2008 doi: 10.1016/j.jdermsci.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Fu X, Nakamori M, Tao L, Amato R, Zhang X. Antitumor effects of two newly constructed oncolytic herpes simplex viruses against renal cell carcinoma. Int J Oncol. 2007;30(6):1561–1567. [PubMed] [Google Scholar]
- 33.Benencia F, Courreges MC, Conejo-Garcia JR, et al. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther. 2005;16(6):765–778. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 34.Nawa A, Nozawa N, Goshima F, et al. Oncolytic viral therapy for human ovarian cancer using a novel replication-competent herpes simplex virus type I mutant in a mouse model. Gynecol Oncol. 2003;91(1):81–88. doi: 10.1016/s0090-8258(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 35.Currier MA, Adams LC, Mahller YY, Cripe TP. Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther. 2005;12(4):407–416. doi: 10.1038/sj.cgt.7700799. [DOI] [PubMed] [Google Scholar]
- 36.Parikh NS, Currier MA, Mahller YY, et al. Oncolytic herpes simplex virus mutants are more efficacious than wild-type adenovirus Type 5 for the treatment of high-risk neuroblastomas in preclinical models. Pediatr Blood Cancer. 2005;44(5):469–478. doi: 10.1002/pbc.20268. [DOI] [PubMed] [Google Scholar]
- 37.Mahller YY, Vaikunth SS, Currier MA, et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther. 2007;15(2):279–286. doi: 10.1038/sj.mt.6300038. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein DJ, Weller SK. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166(1):41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 39.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 40.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 41.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;96:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 42.MacKie RM, Stewart B, Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet. 2001;357(9255):525–526. doi: 10.1016/S0140-6736(00)04048-4. [DOI] [PubMed] [Google Scholar]
- 43.Kemeny N, Brown K, Covey A, et al. A Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther. 2006;17(12):1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- 44.Hu JC, McNeish I, Shorrock C, et al. A phase I clinical trial with OncoVEXGM-CSF. Proc Am Soc Clin Oncol. 2003;22:185. [Google Scholar]
- 45.Kimata H, Imai T, Kikumori T, et al. Pilot study of oncolytic viral therapy using mutant herpes simplex virus (HF10) against recurrent metastatic breast cancer. Ann Surg Oncol. 2006;13(8):1078–1084. doi: 10.1245/ASO.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 46.Nakao A, Takeda S, Shimoyama S, et al. Clinical experiment of mutant herpes simplex virus HF10 therapy for cancer. Curr Cancer Drug Targets. 2007;7(2):169–174. doi: 10.2174/156800907780058808. [DOI] [PubMed] [Google Scholar]
- 47.Meignier B, Roizman B. Herpes simplex virus vaccines. Antiviral Res. 1985 Suppl 1:259–265. doi: 10.1016/s0166-3542(85)80036-x. [DOI] [PubMed] [Google Scholar]
- 48.Meignier B, Longnecker R, Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988;158(3):602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- 49.Meignier B, Martin B, Whitley RJ, Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed Owl monkeys (Aotus trivirgatus) J Infect Dis. 1990;162(2):313–321. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- 50.Berkowitz C, Moyal M, Rosen-Wolff A, et al. Herpes simplex virus type 1 (HSV-1) UL56 gene is involved in viral intraperitoneal pathogenicity to immunocompetent mice. Arch Virol. 1994;134(1–2):73–83. doi: 10.1007/BF01379108. [DOI] [PubMed] [Google Scholar]
- 51.Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208(2):299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- 52.Farassati F, Yang AD, Lee PW. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol. 2001;3(8):745–750. doi: 10.1038/35087061. [DOI] [PubMed] [Google Scholar]
- 53.Ebright MI, Zager JS, Malhotra S, et al. Replication-competent herpes virus NV1020 as direct treatment of pleural cancer in a rat model. J Thorac Cardiovasc Surg. 2002;124(1):123–129. doi: 10.1067/mtc.2002.122297. [DOI] [PubMed] [Google Scholar]
- 54.Bharatan NS, Currier MA, Cripe TP. Differential susceptibility of pediatric sarcoma cells to oncolysis by conditionally replication-competent herpes simplex viruses. J Pediat Hematol Oncol. 2002;24(6):447–453. doi: 10.1097/00043426-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Delman KA, Zager JS, Bhargava A, et al. Effect of murine liver cell proliferation on herpes viral behavior: implications for oncolytic viral therapy. Hepatology. 2004;39(6):1525–1532. doi: 10.1002/hep.20198. [DOI] [PubMed] [Google Scholar]
- 56.Mosesson MW, Natale PD, Amrani DL, et al. Comparison of plasmic hydrolysis of normal fibrinogen and fibrinogen Giessen I. Thromb Res. 1978;12(4):693–696. doi: 10.1016/0049-3848(78)90260-8. [DOI] [PubMed] [Google Scholar]
- 57.Carew JF, Kooby DA, Halterman MW, et al. A novel approach to cancer therapy using an oncolytic herpes virus to package amplicons containing cytokine genes. Mol Ther. 2001;4(3):250–256. doi: 10.1006/mthe.2001.0448. [DOI] [PubMed] [Google Scholar]
- 58.Delman KA, Zager JS, Bennett JJ, et al. Efficacy of multiagent herpes simplex virus amplicon-mediated immunotherapy as adjuvant treatment for experimental hepatic cancer. Ann Surg. 2002;236(3):337–342. doi: 10.1097/00000658-200209000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dambach MJ, Trecki J, Martin N, Markovitz NS. Oncolytic viruses derived from the gamma34.5-deleted herpes simplex virus recombinant R3616 encode a truncated UL3 protein. Mol Ther. 2006;13(5):891–898. doi: 10.1016/j.ymthe.2006.02.006. [DOI] [PubMed] [Google Scholar]