Abstract
ATP-binding cassette (ABC) drug transporters ABCB1 [P-glycoprotein (Pgp)] and ABCG2 are expressed in many tissues including those of the intestines, the liver, the kidney and the brain and are known to influence the pharmacokinetics and toxicity of therapeutic drugs. In vitro studies involving their functional characteristics provide important information that allows improvements in drug delivery or drug design. In this study, we report use of the BacMam (baculovirus-based expression in mammalian cells) expression system to express and characterize the function of Pgp and ABCG2 in mammalian cell lines. BacMam-Pgp and BacMam-ABCG2 baculovirus-transduced cell lines showed similar cell surface expression (as detected by monoclonal antibodies with an external epitope) and transport function of these transporters compared to drug-resistant cell lines that overexpress the two transporters. Transient expression of Pgp was maintained in HeLa cells for up to 72 h after transduction (48 h after removal of the BacMam virus). These BacMam-baculovirus-transduced mammalian cells expressing Pgp or ABCG2 were used for assessing the functional activity of these transporters. Crude membranes isolated from these cells were further used to study the activity of these transporters by biochemical techniques such as photo-cross-linking with transport substrate and adenosine triphosphatase assays. In addition, we show that the BacMam expression system can be exploited to coexpress both Pgp and ABCG2 in mammalian cells to determine their contribution to the transport of a common anticancer drug substrate. Collectively, these data demonstrate that the BacMam-baculovirus-based expression system can be used to simultaneously study the transport function and biochemical properties of ABC transporters.
Introduction
During the preclinical stages of drug development, a new drug under investigation is tested extensively in the laboratory to ensure its safety in further clinical trials. The failure rate of drugs tested in clinical studies still remains high, because of unexpected toxicity or poor pharmacokinetic properties, which prevent them from reaching the intended target in therapeutic doses (Arrowsmith, 2011). This can be credited to the way these tested drugs penetrate biological barriers such as the intestinal wall, blood-brain barrier, or cell membrane (Tsaioun et al., 2009).
The ATP-binding cassette (ABC) drug transporters P-glycoprotein [(Pgp) ABCB1] and ABCG2 are expressed in many such biological barriers including those of the intestine, liver, kidney, brain, placenta, adrenal glands, and testes. These transporters play key roles in determining the absorption, distribution, metabolism, excretion and toxicity properties of the drugs (Borst and Elferink, 2002; Glavinas et al., 2004; Szakács et al., 2008). Developing robust in vitro assays in preclinical studies to characterize absorption, distribution, metabolism, excretion and toxicity properties of candidate drugs specifically related to their interactions with ABC drug transporters can help to improve the pharmacokinetic and pharmacodynamic properties of lead drug molecules. These assays not only help to improve productivity in the field of new drug development but also increase the chances of successful clinical trials with new molecules.
Several established methods are used in preclinical drug development to identify the interactions of a drug with transporters (Lai et al., 2010). These assays can be broadly classified into two categories: cell-based and membrane-based assays (Calcagno et al., 2007; Glavinas et al., 2008). In cell-based assays, transport of the candidate drug across the cell membrane is measured in a polarized monolayer using in vitro cultured cells. This approach closely mimics the small intestine and blood-brain barrier and can potentially identify drug efflux or drug-drug interactions if influenced by ABC transporters. In membrane-based assays, inside-out plasma membrane vesicles are isolated from the cell lines overexpressing ABC transporters, and transport of the drug into the lumen of these vesicles is measured in the presence or absence of ATP, the energy source. This method enables determination of the kinetic parameters of drug interactions with ABC transporters. These two approaches to determining the interactions of candidate drugs with ABC drug transporters rely on two separate in vitro cell-based culture systems that are very different in nature. Caco cells are the most popular for cell-based assays, because these cells can be grown in a monolayer that can be polarized for transport assays. Insect cells (High-Five, SF9), which can be grown in monolayers or in suspension cultures, are capable of overexpressing high levels of transporters for vesicular transport assays (Calcagno et al., 2007).
Quite obviously, the overexpression of transporters in two different expression systems can result in inconsistencies when seeking to develop robust assays during preclinical drug development in the context of ABC drug transporters. The aim of this study was to develop an expression system for both cell-based and membrane-based assays in which ABC transporters can be transiently overexpressed in mammalian cells. We used the BacMam (baculovirus-based expression in mammalian cells) expression system to study the function of two major ABC drug transporters, Pgp and ABCG2 in in vitro-cultured mammalian cell lines. The BacMam system uses modified insect cell baculovirus vectors with a cytomegalovirus promoter instead of a polyhedron promoter to efficiently express genes in mammalian cells with minimal effort and without pleiotropic effects due to exposure to drugs used for selection (Condreay et al., 1999). Several commonly used mammalian cell lines were transduced with BacMam baculovirus-expressing ABCB1 (BacMam-Pgp) or ABCG2 (BacMam-ABCG2) for 24 h. The BacMam baculovirus-transduced cells expressed high levels of functional Pgp and ABCG2 on the cell surface. The expression levels of these transporters were similar to those observed in drug-resistant cell lines that overexpress them. Thus, the baculovirus system can be used for expression of ABC drug transporters either in insect cells to obtain large quantities of purified protein for structural studies or in mammalian cells for mutational analysis and functional characterization in a homologous system. In aggregate, the results demonstrate the efficacy of the BacMam baculovirus system for the transduction of commonly used mammalian cell lines to overexpress the ABC drug transporters Pgp and ABCG2 either individually or simultaneously, which could be used to study drug transporter interactions in preclinical studies.
Materials and Methods
Chemicals.
Rhodamine 123, mitoxantrone, and doxorubicin were obtained from Sigma-Aldrich (St. Louis, MO). Pheophorbide A was from Frontier Scientific (Logan, UT). Calcein-AM was purchased from Invitrogen (Carlsbad, CA). Fumitremorgin C (FTC) was synthesized by Thomas McCloud (Developmental Therapeutics Program, Natural Products Extraction Laboratory, National Cancer Institute, National Institutes of Health, Bethesda, MD). Tariquidar (XR9576) was kindly provided by Dr. Susan Bates (National Cancer Institute, National Institutes of Health). [125I]Iodoarylazidoprazosin (IAAP) (2200 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA).
Cell Lines.
HeLa, MCF7, HEK293, KB-3-1, KB-V1, and MCF7-FLV1000 cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 1% glutamine, and 1% penicillin. KB-V1 and MCF7-FLV1000 cells were selected in 1 μg/ml vinblastine (VBL) and 1 μM flavopiridol, respectively. S1, UACC257, HEMn, Cos-7, LLCPK1, and SKMEL28 were cultured in the media specified by the American Type Culture Collection.
Cloning and Amplification of BacMam-Pgp and BacMam-ABCG2 Viruses.
The expression clones for Pgp and ABCG2 were generated in pDest-625, as described previously (Barsoum et al., 1997). The expression clones were then transformed into Escherichia coli DH10Bac cells from Invitrogen and were plated on selective media containing gentamicin, kanamycin, tetracycline, isopropyl β-d-thiogalactoside, and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) as per the manufacturer's protocols. White colonies were selected from these plates, and bacmid DNA was generated by alkaline lysis plasmid preparation, which was further verified by polymerase chain reaction amplification across the bacmid junctions.
BacMam Transduction.
The BacMam-Pgp or BacMam-ABCG2 virus was added to HeLa cells (2.5 million) at a titer of 50 to 60 viral particles per cell (other virus particles: cell ratios are given in the figure legends) in 3 ml of DMEM and was incubated for 1 h at 37°C. Up to 20 ml of DMEM was then added to these infected cells, and the cells were further incubated for 3 to 4 h. A total of 10 mM butyric acid was then added, and the cells were incubated for another 20 h at 37°C. Similar conditions were also used for the transduction of the other cell lines listed in Table 1. After 24 h, the cells were trypsinized, washed, counted, and analyzed by flow cytometry for cell surface expression and the function of these transporters.
TABLE 1.
Cell surface expression and function of Pgp in multiple commonly used cell lines
| Cell Line | Type of Cells | Pgp Cell Surface Expressiona | Pgp Transport Functionb | ABCG2 Cell Surface Expressiona | ABCG2 Transport Functionb |
|---|---|---|---|---|---|
| HeLa | Cervical cancer | ++++ | ++++ | ||
| MCF7 | Breast cancer | ++++ | ++++ | ||
| S1 | Colon cancer | +++ | +++ | ||
| UACC-257 | Melanoma | ++ | ++ | ||
| HEMn | Epidermal melanocyte | ++ | ++ | ||
| Cos-7 | Monkey kidney fibroblast | ++ | ++ | ||
| LLCPK1 | Porcine kidney | ++ | ++ | ||
| SK-MEL28 | Melanoma | ++ | ++ | ||
| HEK293 | Embryonic kidney | ++ | ++ | ||
| NIH 3T3 | Mouse embryonic fibroblast | ± | ± | ||
| KB-3- 1 | Cervical cancer | ± | ± | ||
| HeLa | Cervical cancer | ++++ | ++++ | ||
| MCF7 | Breast cancer | ++++ | ++++ | ||
| HEK293 | Embryonic kidney | ++ | ++ |
Cell surface expression was measured with MRK16 (for Pgp) or 5D3 (for ABCG2) monoclonal antibodies.
The transport function of Pgp and ABCG2 was determined using calcein-AM or mitoxantrone efflux assays, respectively, as described under Materials and Methods.
++++, highest expression and function [similar to drug-selected Pgp (KB-V1) or ABCG2 (MCF7-FLV1000) cell lines]; +++ and ++, medium expression and function; ±, <10% expression and function.
Flow Cytometry.
Pgp- or ABCG2-mediated transport was determined by flow cytometry using the fluorescent compounds calcein-AM and either pheophorbide A or mitoxantrone, respectively, as described previously (Tiberghien and Loor, 1996; Robey et al., 2004). In brief, cells were trypsinized and incubated with 0.5 μM calcein-AM (for Pgp-mediated transport) for 10 min or 5 μM pheophorbide A or mitoxantrone (for ABCG2-mediated transport) for 45 min in the presence or absence of 2 μM XR9576 (tariquidar, a Pgp inhibitor) or FTC (an ABCG2 inhibitor). Cells were washed with ice-cold phosphate-buffered saline (PBS) before analysis.
Cell surface expression of Pgp and ABCG2 was examined with MRK16 antibody (from Kyowa Medex Company, Tokyo, Japan, for Pgp-expressing cells) or 5D3 antibody, respectively (from eBioscience, San Diego, CA, for ABCG2-expressing cells), as described earlier with minor modifications (Hamada and Tsuruo, 1986; Ozvegy-Laczka et al., 2005). Pgp-expressing cells (200,000 cells) were incubated with MRK16 antibody (1 μg per 100,000 cells for Pgp) for 60 min (for Pgp-expressing cells). Cells were subsequently washed and incubated with fluorescein isothiocyanate (FITC)-labeled anti-mouse secondary antibody (1 μg per 100,000 cells for Pgp; BD Biosciences, San Jose, CA) for 30 min at 37°C. The ABCG2-expressing cells were incubated with 5D3 antibody conjugated with phycoerythrin (1 μg per 100,000 cells) for 45 min at 37°C. The cells were washed with ice-cold PBS and were analyzed.
Calcein, FITC, and phycoerythrin fluorescence was measured on a FACSort flow cytometer (BD Biosciences, San Jose, CA) equipped with a 488-nm argon laser and 530-nm band-pass filter or confocal microscope; mitoxantrone and allophycocyanin fluorescence was measured by a FACSort flow cytometer with a 635-nm red diode laser and a 561-nm long-pass filter or confocal microscope.
[125I]IAAP Transport Assay.
The [125I]IAAP transport assay was performed as described previously (Shukla et al., 2006). In brief, BacMam-Pgp (wild-type) or BacMam-Pgp-EQ (E556Q/E1206Q Pgp mutant that is defective in ATP hydrolysis)-transduced cells (2.5 × 105 cells/well) were grown in a monolayer in a 24-well tissue culture plate at 37°C. The assay was initiated by incubating cells with 1.5 nM [125I]IAAP in the absence or presence of 5 μM XR9576 at room temperature for 60 min in 0.3 ml of complete DMEM. The cells were washed with ice-cold PBS and were lysed by incubation with 0.3 ml of trypsin-EDTA per well at 37°C for 30 min. The cell lysates were transferred to scintillation vials containing 5 ml of Bio-Safe II scintillation fluid (RPI Corp., Mount Prospect, IL), and the radioactivity was measured in a scintillation counter. The accumulation of [125I]IAAP was expressed as picomoles per 1 million cells.
Cytotoxicity Assays.
The BacMam-Pgp-transduced cells were plated at a density of 200,000 cells/well in a 24-well plate and were incubated for 24 h at 37°C in 5% CO2. Various concentrations of doxorubicin were subsequently added, and plates were allowed to incubate for 24 h at 37°C in 5% CO2. Zero point two percent trypan blue dye was then added to cells, and the viable cells were counted as the percentage of cells that excluded trypan blue dye compared with total cells as described (Strober, 2001). The cell viability was then determined by calculating the number of cells that did not take up trypan blue dye. Each concentration was tested in quadruplicate.
Preparation of Crude Membranes from BacMam Baculovirus-Transduced HeLa and Baculovirus-Infected High-Five Insect Cells.
Crude membranes from BacMam-transduced cells were isolated as described previously (Gribar et al., 2000). Crude membranes of Pgp- or ABCG2-expressing High-Five cells were prepared essentially as described previously (Kerr et al., 2001). Crude membranes of HeLa or High-Five cells were stored in aliquots at −70°C.
Photoaffinity Labeling of Pgp with [125I]IAAP.
Crude membranes (1 mg protein/ml) from BacMam-Pgp-transduced HeLa cells and Pgp-expressing High-Five insect cells were photolabeled with [125I]IAAP (2200 Ci/mM) in the absence or presence of 10 μM cyclosporine A and 10 μM XR9576, as described previously (Shukla et al., 2006).
Adenosine Triphosphatase Assay.
Crude membrane protein (100 μg protein/ml) from BacMam-Pgp-transduced HeLa cells and High-Five insect cells expressing Pgp was incubated at 37°C with 30 μM verapamil in the presence and absence of 0.3 mM sodium orthovanadate and the ATP hydrolysis was measured as described previously (Ambudkar, 1998). The vanadate-sensitive Pgp-adenosine triphosphatase (ATPase) activity is expressed as nanomoles Pi per minute per milligram of protein.
SDS-Polyacrylamide Gel Electrophoresis and Western Blot Analysis.
Cell lysates or crude membrane proteins were resolved on 7% Tris-acetate gels. Pgp and ABCG2 in immunoblots were detected with monoclonal antibodies C219 (Kartner et al., 1985) and BXP-21 (Maliepaard et al., 2001), respectively, at 1:2000 dilution.
Results
HeLa Cells Transduced by BacMam-Pgp Virus Overexpress Functional Pgp.
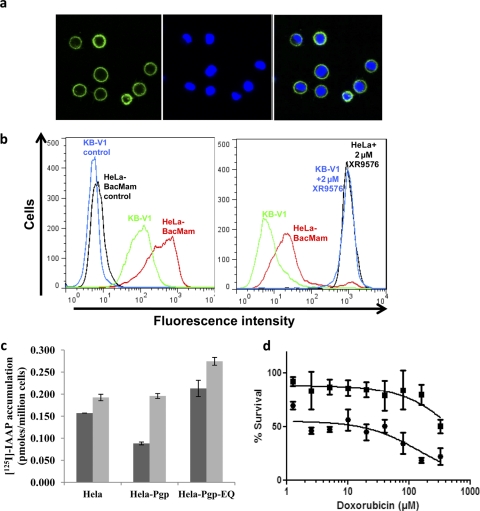
HeLa cells were transduced with BacMam-Pgp virus at a titer of 1:50 to 60 (50–60 viruses/cell) for 24 h as described under Materials and Methods. The cell surface expression of Pgp was monitored by confocal microscopy and flow cytometry using the Pgp-specific MRK16 antibody and as shown in Fig. 1, a and b (left). These transduced cells expressed levels of Pgp comparable to those of KB-V1 (drug-selected HeLa) cells, which are known to overexpress high levels of Pgp (Shen et al., 1986). These Pgp-expressing transduced cells were able to efflux calcein-AM, a known substrate of Pgp, with efficiency similar to that observed in the KB-V1 cells (Fig. 1b, right). The multiplicity of infection for viral infection was titrated to yield maximum Pgp expression with minimal perturbation to cell integrity because of the viral infection. The expression and function of Pgp was optimal and saturated at an infection titer of 50 to 60 viral particles/cells (data not shown). Butyric acid, a histone deacetylase inhibitor, was added, as earlier reports showed that it was able to increase the protein expression in transduced cells (Condreay et al., 1999). Maximal expression and function of Pgp was observed in the presence of 10 mM butyric acid on the basis of a butyric acid titration curve (1–10 mM; data not shown). Thus, this concentration of butyric acid was used in all further experiments.
Fig. 1.
BacMam-Pgp-transduced HeLa cells express functional Pgp. a and b, BacMam-Pgp virus transduced HeLa (a) and KB-V1 cells were incubated with either 0.5 μM calcein-AM for 10 min in the presence and absence of 2 μM XR9576 or MRK16 antibody (1 μg/100,000 cells) for 1 h at 37°C, followed by incubation with FITC-conjugated anti-mouse secondary antibody. The cells were washed and subsequently analyzed by confocal microscopy (a) or flow cytometry (b), as described under Materials and Methods. a, the left panel shows cell surface localization of Pgp as detected by green fluorescence detector. The middle panel shows nuclear staining of cells with 4,6-diamidino-2-phenylindole, a nuclear counter stain, as detected by blue fluorescence detector. The right panel shows an overlay of green and blue fluorescence. b, the histogram shows fluorescence (x-axis) representing surface expression as detected by MRK16 labeling in the left panel and calcein accumulation in the right panel plotted as a function of the number of cells (y-axis). Individual histograms are labeled as shown and represent a single experiment that was done independently at least three times. VBL-selected Pgp-expressing KB-V1 cells (Shen et al., 1986) were used for comparison in both panels. c, [125I]IAAP is transported by HeLa cells transduced with BacMam-Pgp. The control HeLa, HeLa cells expressing wild-type Pgp (HeLa-Pgp), or inactive mutant Pgp (HeLa-Pgp-EQ) cells (0.25 × 106 cells/well) were incubated with 3 to 5 nM [125I]IAAP in the absence (dark gray) or presence (light gray) of 2 μM tariquidar (XR9576) at room temperature for 60 min in DMEM. The amount of radioactive drug that accumulated in the cells was measured by liquid scintillation counting, as described under Materials and Methods. The graph shows the amount of [125I]IAAP that accumulated (picomoles per 1 million cells) in the cells. The mean values from three independent experiments that were performed in duplicate sets are shown here, and error bars indicate the standard deviation. d, BacMam-Pgp-transduced HeLa cells confer resistance to doxorubicin. Cytotoxicity assays for Pgp-expressing transduced HeLa cells (filled squares) and control HeLa cells (filled stars) are shown. Dose-response curves were derived from three independent experiments using the trypan blue viability assay as described under Materials and Methods, and the error bars indicate S.D.
Viral-based induced expression of protein in mammalian cells generally has a cytostatic or cytotoxic effect on the cells that is caused by the viral infection. To ensure that BacMam-Pgp-transduced cells could further be used for functional transport assays using intact cells, the activity of Pgp was monitored in both short- and long-term assays such as transport and cytotoxicity assays, respectively. The data presented in Fig. 1c shows that these cells were able to efflux [125I]IAAP, resulting in decreased intracellular accumulation in a 60-min assay. The total accumulation of [125I]IAAP in Pgp-expressing HeLa cells was 0.088 pM/million cells, which was almost 2-fold lower than the accumulation in control HeLa cells, measured to be 0.157 pM/million cells. This Pgp-mediated efflux of [125I]IAAP was completely inhibited by 2 μM XR9576, an inhibitor of Pgp function. In addition, we also transduced HeLa cells with BacMam-Pgp-EQ virus, which expresses a nonfunctional mutant Pgp (E556Q/E1201Q) with the glutamate residue in the Walker B motif of each nucleotide-binding domain that is changed to glutamine (Sauna et al., 2002). The accumulation of [125I]IAAP in these cells was comparable to that seen in control HeLa cells, suggesting that the viral infection of the cells per se does not have any effect on the permeability properties of the plasma membranes of these cells.
The ability of the intact cells to confer drug resistance in long-term assays such as cytotoxicity assays was also evaluated by trypan blue viability assays. This was assessed at 48 h after viral transduction (24 h after addition of doxorubicin, as described under Materials and Methods). As shown in Fig. 1d, the cells expressing Pgp were resistant to doxorubicin-induced cell death compared to the control, untransfected HeLa cells. These results suggested that the virally transduced Pgp-overexpressing cells were capable of expressing very high levels of functional Pgp up to 48 h after transfection and are suitable for functional assays of Pgp activity. In addition, it was observed that the cells were in fact able to express fairly good amounts of functional Pgp up to 72 h after transfection (Supplemental Fig. 1, a and b) with the half-life of the cell surface Pgp approximately 55 to 60 h in this system.
We also evaluated the use of BacMam-Pgp virus for transducing multiple cell lines that are routinely used in laboratory studies. It was observed that most mammalian cell lines can be transduced to express Pgp, although the expression level of Pgp varies, as shown in Table 1. It should be noted that HeLa and MCF7 cells were able to express the highest level of Pgp, whereas KB-3-1 or NIH 3T3 cells could not be transduced effectively with the BacMam-Pgp virus.
BacMam-Pgp-Transduced Cells for Biochemical Studies.
The data above suggested that BacMam-mediated overexpression of functional Pgp can be achieved in HeLa cells. These cells can be used for functional assays whenever intact cells are required for measuring the activity of the transporters. Other viral-based systems can also be used for such functional studies, but the BacMam-based expression system is advantageous, especially in the case of ABC drug transporters, as this system can also be used for biochemical studies that require high levels of functional protein to be expressed in the membranes of transduced cells.
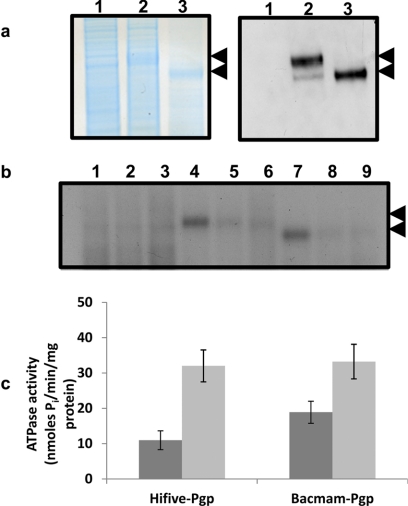
Crude membranes from Pgp-expressing BacMam-Pgp-transduced HeLa cells were isolated by hypotonic lysis and homogenization as described under Materials and Methods. The crude membrane protein, after being resolved in 7% Tris-acetate gel was stained with colloidal blue and probed with C219 (a Pgp-specific monoclonal antibody) for detection of Pgp expression. A high level of Pgp was observed in crude membranes compared to the levels observed in Pgp-expressing High-Five insect cell membranes (Fig. 2a), which are routinely used as a system to study the biochemical properties of functional Pgp. The Pgp expressed in HeLa cells is fully glycosylated but not in insect cells (Fig. 2a). The crude membranes were further evaluated for functional Pgp activity by photolabeling and ATP hydrolysis studies. As shown in Fig. 2b, Pgp could be photo-cross-linked with [125I]IAAP, which is a transport substrate, and this labeling was inhibited in the presence of the competing substrates 20 μM cyclosporine A and 10 μM XR9576. It should again be noted that the levels of photo-cross-linked Pgp were similar to those observed in crude membranes from insect cells. In addition, BacMam-Pgp-expressing crude membranes also showed basal adenosine triphosphatase (ATPase) activity (18.9 nmol Pi · mg−1 · min−1), which was stimulated by approximately 2.5-fold in the presence of 30 μM verapamil (Fig. 2c).
Fig. 2.
Crude membranes of BacMam-Pgp transduced HeLa cells overexpress functional Pgp. a, colloidal blue stain (left) and Western blot analysis (right) of crude membranes (10 μg protein for colloidal blue and 2 μg protein for Western blots) isolated from control HeLa cells (lane 1), HeLa cells transduced with BacMam Pgp (lane 2), and High-Five insect cells expressing Pgp (lane 3). Immunoblotting with C219, a Pgp-specific antibody, was performed as described under Materials and Methods. The arrows represent glycosylated (upper) or nonglycosylated (lower) forms of Pgp. b, Pgp expressed in BacMam-Pgp-transduced HeLa cells can be photolabeled with [125I]IAAP. Crude membranes (500 μg protein/ml) from control HeLa (lanes 1–3), Pgp-expressing HeLa (lanes 4–6), or High-Five insect cells expressing Pgp (lanes 7–9) cells were incubated for 5 min at room temperature in the absence (lanes 1, 4, and 7) or presence of 20 μM cyclosporine A (lanes 2, 5, and 8) or 10 μM tariquidar (lanes 3, 6, and 9) in 50 mM Tris-HCl (pH 7.5) and 3 to 6 nM [125I]IAAP (2200 Ci/mmol) was added. The samples were then photo-cross-linked with [125I]IAAP as described under Materials and Methods. The autoradiogram from a representative experiment is shown, and the arrow represents the position of glycosylated and unglycosylated forms of Pgp. A representative experiment from three independent experiments is shown here. c, Pgp expressed in crude membranes of BacMam-transduced HeLa cells exhibits substrate (verapamil)-stimulated ATPase activity. Crude membranes (100 μg protein/ml) from Pgp-expressing High-Five or HeLa cells were incubated at 37°C in the absence (dark gray) or presence (light gray) of 30 μM verapamil in the presence and absence of 0.3 mM sodium orthovanadate in ATPase assay buffer for 5 min, and the vanadate-sensitive ATPase activity of Pgp was determined as described under Materials and Methods. The histograms represent the ATPase activity (mean values ± S.D.) from three independent experiments.
Expression of ABCG2 in HeLa Cells Using BacMam Baculovirus.
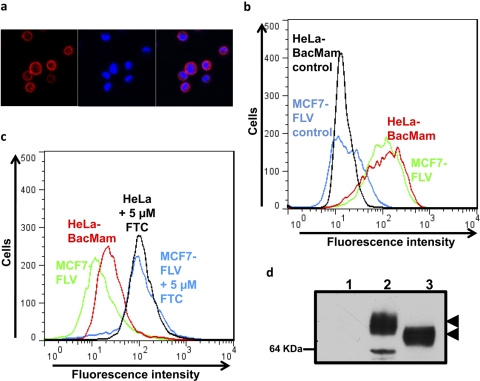
To demonstrate the general applicability of this system, the expression and function of another major ABC drug transporter, ABCG2, was also evaluated by transduction of HeLa cells with BacMam-ABCG2 for 24 h at a titer of 1:50 to 60. Figure 3, a and b, shows that ABCG2 was overexpressed at the cell surface, as detected by the ABCG2-specific 5D3 antibody. The cell surface expression level and function of ABCG2 was similar to that of MCF7-FLV1000 cells, a drug-selected cell line that expresses very high levels of ABCG2 (Robey et al., 2001). As with Pgp, BacMam-ABCG2-transduced cells were able to efflux mitoxantrone, a known substrate of ABCG2, to levels comparable to those of MCF7-FLV1000 cells (Fig. 3c). Total cell expression of ABCG2 was evaluated in cell lysates prepared from BacMam-ABCG2-transduced cells using BXP-21 antibody, and ABCG2 expression was similar to or higher than that in MCF7-FLV1000 cells (Fig. 3d). Similar to Pgp, as shown in Supplemental Fig. 1, the half-life of ABCG2 transporter was also in the range of 50 to 55 h (data not shown).
Fig. 3.
BacMam-ABCG2-transduced HeLa cells express functional ABCG2. a–c, BacMam-ABCG2- virus-transduced HeLa (a) and MCF7-FLV1000 cells were incubated with either 5D3-PE antibody (1 μg/100,000 cells) for 1 h at 37°C or 5 μM mitoxantrone for 45 min in the presence or absence of 5 μM FTC. The cells were washed and were subsequently analyzed by confocal microscopy (a) or flow cytometry (b and c) as described under Materials and Methods. a, the left panel shows cell surface localization of ABCG2 as detected by a red fluorescence detector. The middle panel shows nuclear staining of cells by 4,6-diamidino-2-phenylindole, a nuclear counter stain as detected by a blue fluorescence detector. The right panel shows an overlay of red and blue fluorescence. b and c, the histograms show fluorescence (x-axis) representing surface expression as detected by 5D3 labeling (b) and mitoxantrone accumulation (c) plotted as a function of number of cells (y-axis). Individual histograms are labeled in the figure and represent a single experiment that was done independently three times. d, Western blot analysis of total cell lysates (5 μg protein/lane) isolated from control HeLa cells (lane 1), HeLa cells transduced with BacMam-ABCG2 (lane 2), and MCF7-FLV1000 cells expressing ABCG2 (lane 3). Immunoblotting with BXP-21, an ABCG2-specific antibody, was performed as described under Materials and Methods.
Coexpression of Pgp and ABCG2 in HeLa Cells.
The expression of transporters such as Pgp and ABCG2 can affect drug absorption, distribution, and excretion and can explain mechanisms underlying drug-drug interactions. The regional distribution of Pgp, ABCG2, and SLC transporters along the intestine was reported earlier, but their individual contributions to the transport of specific substrates have not been addressed (Thiebaut et al., 1987; Englund et al., 2006). Therefore, understanding the individual contributions of two or more transporters in one system using in vitro cultured cells or an in vivo system for an experimental drug would help to evaluate clinical drug-drug interactions.
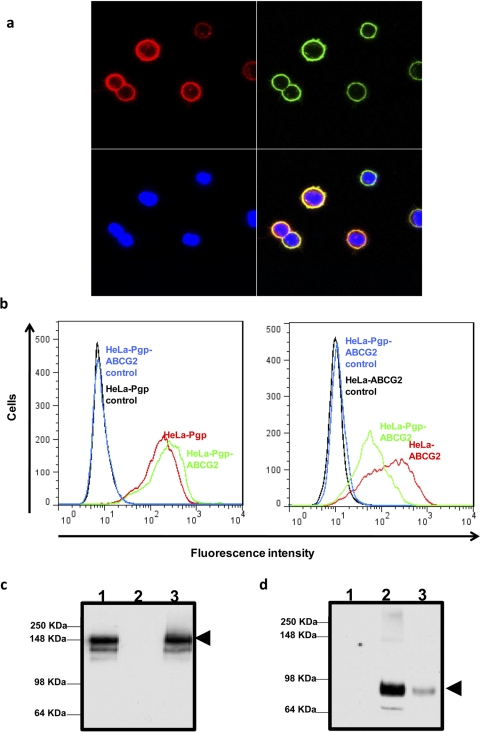
To investigate further, HeLa cells were either transduced with BacMam-Pgp and BacMam-ABCG2 virus individually or cotransduced with BacMam-Pgp and BacMam-ABCG2 virus, as described under Materials and Methods. Cell surface coexpression of Pgp and ABCG2 was monitored by confocal microscopy (Fig. 4a) using MRK16 (green) and 5D3 (red) antibodies, respectively, and by flow cytometry (Fig. 4b), as described under Materials and Methods. The double-transduced cells simultaneously expressed both Pgp and ABCG2 on the cell surface using different titers of the two viruses (1:40 for Pgp and 1:60 for ABCG2) (Fig. 4, a and b). The coexpression of Pgp and ABCG2 was also confirmed by Western blot analysis from the total cell lysates of these cotransfected cells using C219 (Fig. 4c) for Pgp and BXP21 (Fig. 4d) for ABCG2 detection. Both transporters were functional when coexpressed, as determined by their ability to efflux calcein-AM (for Pgp, Fig. 5a) and pheophorbide A (for ABCG2, Fig. 5b) at the same time. It should be noted that the expression of ABCG2 in double-transduced cells (HeLa-Pgp-ABCG2) was lower than that in single-transduced (HeLa-ABCG2) cells (Fig. 4, b and d). However, the efflux of pheophorbide A from both ABCG2- and Pgp-transduced cells was comparable to the efflux observed in the single gene transduced cells (Fig. 5b).
Fig. 4.
Coexpression of both Pgp and ABCG2 in HeLa BacMam-baculovirus-transduced cells: HeLa cells were transduced with both BacMam-Pgp and BacMam-ABCG2 (1:40 virus titer for Pgp and 1:60 virus titer for ABCG2), as described under Materials and Methods, for 24 h. After 24 h, transduced HeLa cells were labeled by 5D3 (top left) or MRK16 (top right) antibodies as described under Materials and Methods, and the cells were analyzed by confocal microscopy. The lower left panel shows nuclear staining of cells by 4,6-diamidino-2-phenylindole as detected by blue fluorescence detector. The lower right panel shows an overlay of red, green, and blue fluorescence. b, the cell surface expression of Pgp (left) and ABCG2 (right) in a single- or double-transduced cells (as indicated on histograms) was detected by MRK16 antibody (for Pgp) and 5D3 antibody (for ABCG2) as described under Materials and Methods. The histograms represent fluorescence intensity (x-axis) plotted as a function of cell number (y-axis) and are representative of three independent experiments. c and d, Western blot analysis of total cell lysates (5 μg protein/lane) from BacMam-Pgp (lane 1), BacMam-ABCG2 (lane 2), and both BacMam-Pgp and BacMam-ABCG2 together (lane 3) transduced HeLa cells, using C219 antibody (c) and BXP-21 antibody (d) was performed as described under Materials and Methods. The arrows represent the position of the Pgp band in a and the ABCG2 band in b.
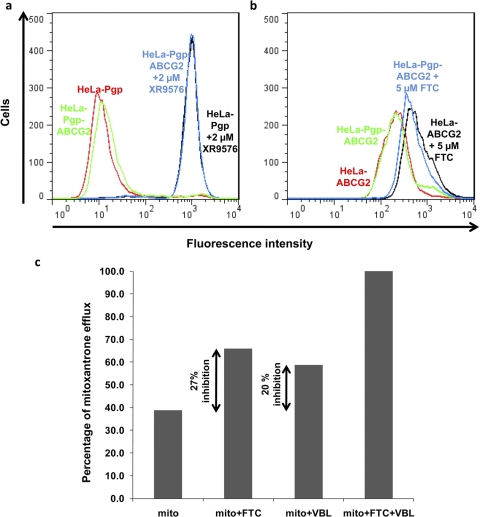
Fig. 5.
Both Pgp and ABCG2 in are functionally expressed in HeLa cells cotransduced with BacMam-Pgp and BacMam-ABCG2 viruses. a and b, HeLa cells were cotransduced with BacMam-Pgp and BacMam-ABCG2 (1:40 virus titer for Pgp and 1:60 virus titer for ABCG2) alone or together, as described under Materials and Methods. The function of Pgp (a) and ABCG2 (b) in single- or double-transduced cells (as indicated on histograms) was evaluated by calcein-AM accumulation assay in the presence or absence of 2 μM XR9576 (for Pgp) and pheophorbide A accumulation assay in the presence or absence of 5 μM FTC (for ABCG2) as described under Materials and Methods. The histograms represent fluorescence intensity (x-axis) plotted as a function of cell number (y-axis) and are representative of three independent experiments. c, determination of contribution of Pgp and ABCG2 in efflux of mitoxantrone from HeLa cells transduced with both Pgp and ABCG2 BacMam virus: HeLa cells were transduced with BacMam-Pgp, BacMam-ABCG2, or both viruses to express/coexpress Pgp, ABCG2, or both Pgp and ABCG2 simultaneously. The Pgp and ABCG2 transport function was detected by incubating cells with 5 μM mitoxantrone for 45 min at 37°C in the dark in the absence or presence of 20 μM vinblastine (as an inhibitor of Pgp transport) and/or 5 μM FTC (as an inhibitor of ABCG2 function). The cells were then washed and were subsequently analyzed by flow cytometry as described under Materials and Methods. The percentage contribution of each transporter to the efflux of mitoxantrone was calculated by subtracting the transport activity observed in double transfectants in the absence of any inhibitor from the activity observed in the presence of 5 μM FTC (a specific ABCG2 inhibitor) or 20 μM VBL (a competitive inhibitor of Pgp) alone. The inhibition of efflux activity in the presence of both FTC and VBL was taken as 100%. Double arrows represent mitoxantrone efflux activity contributed by ABCG2 (difference between mitoxantrone alone and in the presence of 5 μM FTC) or Pgp (difference between mitoxantrone alone and in the presence of 20 μM VBL). Both FTC and vinblastine were added together to obtain complete inhibition of efflux (taken as 100% accumulation). Shown here is a graph from a single experiment that was done independently at least three times. Mito, mitoxantrone.
The two transporters colocalized on the cell surface (as seen by yellow fluorescence in the lower right panel of Fig. 4a), suggesting that these two transporters can simultaneously contribute to the efflux of drugs that are nonoverlapping substrates of each of these transporters. Therefore, this system can also be used to study the contribution of an individual transporter for its ability to efflux a specific substrate and/or overlapping substrates. Along these lines, transport of mitoxantrone (an anticancer agent that is transported by Pgp and ABCG2) was studied in transduced HeLa cells expressing both Pgp and ABCG2 using fluorescence-activated cell sorting assays (Fig. 5c). The percent contribution of each transporter for effluxing mitoxantrone was calculated by subtracting the transport activity observed in double transfectants in the absence of any inhibitor from the activity observed in the presence of 5 μM FTC (a specific ABCG2 inhibitor) or 10 μM VBL (though a substrate, VBL was used here as a competitive inhibitor of Pgp) alone. The inhibition of efflux activity in the presence of both FTC and VBL was considered as 100%. It was observed that whereas the presence of FTC alone inhibited 27% of efflux, the presence of VBL alone resulted in 20% inhibition of mitoxantrone efflux from these cells. These data suggest that the individual activities of two transporters can be measured using a common substrate and specific inhibitors by coexpressing these transporters using the BacMam expression system. It should be mentioned that we have not taken into consideration the affinity differences of mitoxantrone for Pgp and ABCG2 in the above experiment.
Discussion
Pgp and ABCG2 are two major ABC drug transporters that not only have been implicated in clinical drug resistance, but also are now known to have important roles in drug-drug interactions and in the pharmacokinetics of several structurally and pharmacologically diverse substrate drugs. In addition, in a draft guidance issued by the U.S. Food and Drug Administration and the European Medicines Agency, study of the transport function of Pgp and ABCG2 is recommended for in vitro and in vivo drug interaction studies to be conducted during new drug development (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072101.pdf, 2006, and http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/05/WC500090112.pdf, 2010). Therefore, development of robust cellular and biochemical tools that can aid in identifying drug-drug interactions or in characterizing the transport properties of these transporters is required in the field of preclinical drug development.
In an effort to develop a transient expression system that can be used to overexpress ABC drug transporters and that is suitable for both cellular and biochemical assays, we report here the use of a modified baculovirus to express Pgp and ABCG2 in mammalian cells. One major advantage of the BacMam expression system is that gene delivery can be accomplished in many different cell types by simply adding viral inoculums (reviewed in Kost et al., 2005). Using this system, we showed that both Pgp and ABCG2 can be expressed in several routinely used in vitro cultured mammalian cell lines at levels equal to those seen in drug-selected cell lines (Figs. 1 and 3). These transiently transduced cells can also be used to study the function of the transporters in intact cell-based assays such as accumulation assays using fluorescent or radiolabeled substrates or cytotoxicity assays. The virally transduced cells were able to express protein at considerably high levels for up to 72 h after transduction and can therefore be used to study the function and biochemical properties of the transporters in long-term assays.
There are basically two different cell-based assays that are frequently used to determine interactions with transporters. The first is the cellular uptake assay, and the second is an assay that measures bidirectional transport across polarized cell monolayers. We show that the Pgp-expressing transduced cells can be used to measure [125I]IAAP transport (a known substrate) mediated by this transporter. These cells can also be used to measure the uptake of a probe substrate, whether radiolabeled, fluorescent, or one for which the liquid chromatography/tandem mass spectrometry analytical method has been validated. In addition, the uptake of these probes can be monitored in the presence and absence of test compounds to identify potential modulators or drugs that can cause potent drug-drug interactions.
We were also able to coexpress both Pgp and ABCG2 by transducing the cells with BacMam-Pgp and BacMam-ABCG2 virus. The relative contributions of each transporter to the efflux of a common substrate, mitoxantrone, was evaluated in these cells by using specific inhibitors of Pgp and ABCG2 (VBL for Pgp and FTC for ABCG2) function (Fig. 5c). This coexpression system, therefore, can also be used to assess the individual contributions of two or more transporters (that have overlapping substrate specificities) in effluxing clinically important anticancer drugs. It should be noted that the uptake or efflux of many anticancer drugs is influenced by several transporters, and even double-transduced cell lines may not predict the true in vivo situation. A BacMam-based expression system was previously used to coexpress ABCG2 and OATP1B1 in mammalian cells (Hassan et al., 2006). The report showed simultaneous expression of two transporters but did not use the expression system for further biochemical studies of the transporters. Very recently, Poller et al. (2011) reported stable coexpression of human ABCB1 and ABCG2 using a polarized MDCKII cell line and studied the interplay of both transporters by measuring transepithelial transport of clinically used drugs including topotecan, sorafenib, and sunitinib, which are substrates of these transporters. They showed that the relative impact of these transporters can influence the availability of the above drugs at the blood-brain barrier. Although polarized cells can be used to measure direct efflux, setting up polarized cell lines for transport assays can be cumbersome, as relatively few cell lines (Caco-2, MDCK, LLC-PK1, and HT-29) form confluent, polarized monolayers. Moreover, depending on the cell line and the protocol, cells need to be cultured for 1 to 3 weeks to form confluent, polarized monolayers with tight junctions. In contrast, the BacMam-based expression system can not only be used to coexpress these two transporters but could possibly be used in a high-throughput manner to study the transport properties of specific substrates.
For biochemical characterization of the substrate interactions with transporter proteins, generally crude membranes or purified plasma membranes vesicles are isolated from cells overexpressing the transporters, and assays such as ATP hydrolysis, photoaffinity binding assays, and vesicular transport assays are performed. The most commonly used cells for expressing transporter protein and preparing vesicles are insect Sf9 cells (Glavinas et al., 2008). However, the membrane composition of the insect Sf9 cell plasma membrane is different from that of mammalian plasma membranes and has been shown to significantly affect transporter functionality (Pál et al., 2007). Therefore, expression of mammalian ABC drug transporters is most likely to approximate physiological levels when it occurs in human cells. In addition, the membranes isolated from these cells expressed very high levels of transporters compared with the levels observed in insect cells (Fig. 2). An advantage of using the BacMam system is that unlike what happens in insect cells, the overexpressed protein undergoes normal post-translational modification such as glycosylation, as evident from the Western blot analysis in Fig. 2. Crude membranes isolated from Pgp-expressing HeLa cells were also used for both photoaffinity binding assays using [125I]IAAP and ATPase assays and exhibited properties similar to those of Pgp expressed in insect cells. The above data suggest that BacMam-based expression of ABC transporters is not only suitable for intact cell studies, but that membrane vesicles isolated from these cells can also be used to study the biochemical characteristics of the expressed protein. The membrane vesicles can also be used for characterization of kinetic properties and drug interactions, as reported previously (Mutsaers et al., 2011) for ABCG2 and ABCC4. In addition, because of such high levels of expression, this system (see El-Sheikh et al., 2008) can be used for mutational analysis in a homologous system as well as for purification, reconstitution, and possible structural studies of ABC transporters.
There are multiple advantages to using the BacMam-based expression system in mammalian cells (reviewed in Kost et al., 2007). Although the BacMam viruses can transduce many cell types including many primary cells, the proteins can be expressed using different stocks of virus, whereas only a single mammalian cell line has to be maintained. The BacMam-baculovirus-transduced cells show little or no microscopically observable cytotoxic effect, so this system can be used to coexpress multiple proteins. Moreover, the expression levels can be easily controlled by titration of the virus ratio to that of the cells. Baculovirus-mediated gene expression also has the advantage of good biosafety, as the virus replicates only in insect cells and is incapable of replicating in mammalian cells. Our data (Supplementary Fig. 2) further suggest that BacMam-transduced cells can be stored at −80°C or in liquid nitrogen for long periods and can be taken out for immediate usage in case of functional studies. Therefore, the BacMam system can be used to produce quantities of transduced cells (or multiple cells) expressing single or multiple transporters that can be stored in freezers and used later for functional studies as and when needed; maintaining multiple cell lines in culture rooms is not necessary. This could be of particular interest in pharmaceutical industrial applications, as the system provides a platform for high-throughput study of drug-drug interactions with the transporters.
In conclusion, the BacMam-based expression system for ABC drug transporters in human and other mammalian cells can be used for biochemical and functional studies of Pgp and ABCG2 and perhaps other ABC transporters in a single system. We suggest that this system will be very useful to determine the contributions of at least two major ABC drug transporters simultaneously to the efflux of lead drug compounds in preclinical studies.
Supplementary Material
Acknowledgment
We thank George Leiman for editorial assistance.
This work was supported by the Intramural Research program of the National Institutes of Health National Cancer Institute; the National Institutes of Health Center for Cancer Research; and the National Institutes of Health Office of Dietary Supplements [Grant OD-09-101].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- ABC
- ATP-binding cassette
- Pgp
- P-glycoprotein
- Pgp-EQ
- E556Q/E5561206Q nonfunctional Pgp mutant
- BacMam
- baculovirus-based expression in mammalian cells
- FTC
- fumitremorgin C
- IAAP
- iodoarylazidoprazosin
- DMEM
- Dulbecco's modified Eagle's medium
- PBS
- phosphate-buffered saline
- FITC
- fluorescein isothiocyanate
- ATPase
- adenosine triphosphatase
- VBL
- vinblastine
- HEK
- human embryonic kidney.
Authorship Contributions
Participated in research design: Shukla and Ambudkar.
Conducted experiments: Shukla, Schwartz, Kapoor, and Kouanda.
Contributed new reagents or analytic tools: Shukla and Ambudkar.
Performed data analysis: Shukla, Schwartz, Kapoor, and Ambudkar.
Wrote or contributed to the writing of the manuscript: Shukla, Schwartz, Kapoor, and Ambudkar.
References
- Ambudkar SV. (1998) Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol 292:504–514 [DOI] [PubMed] [Google Scholar]
- Arrowsmith J. (2011) Trial watch: phase II failures: 2008–2010. Nat Rev Drug Discov 10:328–329 [DOI] [PubMed] [Google Scholar]
- Barsoum J, Brown R, McKee M, Boyce FM. (1997) Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther 8:2011–2018 [DOI] [PubMed] [Google Scholar]
- Borst P, Elferink RO. (2002) Mammalian ABC transporters in health and disease. Annu Rev Biochem 71:537–592 [DOI] [PubMed] [Google Scholar]
- Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. (2007) ABC drug transporters as molecular targets for the prevention of multidrug resistance and drug-drug interactions. Curr Drug Deliv 4:324–333 [DOI] [PubMed] [Google Scholar]
- Condreay JP, Witherspoon SM, Clay WC, Kost TA. (1999) Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA 96:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh AA, van den Heuvel JJ, Krieger E, Russel FG, Koenderink JB. (2008) Functional role of arginine 375 in transmembrane helix 6 of multidrug resistance protein 4 (MRP4/ABCC4). Mol Pharmacol 74:964–971 [DOI] [PubMed] [Google Scholar]
- Englund G, Rorsman F, Rönnblom A, Karlbom U, Lazorova L, Gråsjö J, Kindmark A, Artursson P. (2006) Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci 29:269–277 [DOI] [PubMed] [Google Scholar]
- Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. (2004) The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv 1:27–42 [DOI] [PubMed] [Google Scholar]
- Glavinas H, Méhn D, Jani M, Oosterhuis B, Herédi-Szabó K, Krajcsi P. (2008) Utilization of membrane vesicle preparations to study drug–ABC transporter interactions. Expert Opin Drug Metab Toxicol 4:721–732 [DOI] [PubMed] [Google Scholar]
- Gribar JJ, Ramachandra M, Hrycyna CA, Dey S, Ambudkar SV. (2000) Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system. J Membr Biol 173:203–214 [DOI] [PubMed] [Google Scholar]
- Hamada H, Tsuruo T. (1986) Functional role for the 170- to 180-kDa glycoprotein specific to drug-resistant tumor cells as revealed by monoclonal antibodies. Proc Natl Acad Sci USA 83:7785–7789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan NJ, Pountney DJ, Ellis C, Mossakowska DE. (2006) BacMam recombinant baculovirus in transporter expression: a study of BCRP and OATP1B1. Protein Expr Purif 47:591–598 [DOI] [PubMed] [Google Scholar]
- Kartner N, Evernden-Porelle D, Bradley G, Ling V. (1985) Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature 316:820–823 [DOI] [PubMed] [Google Scholar]
- Kerr KM, Sauna ZE, Ambudkar SV. (2001) Correlation between steady-state ATP hydrolysis and vanadate-induced ADP trapping in Human P-glycoprotein. Evidence for ADP release as the rate-limiting step in the catalytic cycle and its modulation by substrates. J Biol Chem 276:8657–8664 [DOI] [PubMed] [Google Scholar]
- Kost TA, Condreay JP, Ames RS, Rees S, Romanos MA. (2007) Implementation of BacMam virus gene delivery technology in a drug discovery setting. Drug Discov Today 12:396–403 [DOI] [PubMed] [Google Scholar]
- Kost TA, Condreay JP, Jarvis DL. (2005) Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 23:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Sampson KE, Stevens JC. (2010) Evaluation of drug transporter interactions in drug discovery and development. Comb Chem High Throughput Screen 13:112–134 [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. (2001) Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 61:3458–3464 [PubMed] [Google Scholar]
- Mutsaers HA, van den Heuvel LP, Ringens LH, Dankers AC, Russel FG, Wetzels JF, Hoenderop JG, Masereeuw R. (2011) Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One 6:e18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozvegy-Laczka C, Várady G, Köblös G, Ujhelly O, Cervenak J, Schuetz JD, Sorrentino BP, Koomen GJ, Váradi A, Német K, et al. (2005) Function-dependent conformational changes of the ABCG2 multidrug transporter modify its interaction with a monoclonal antibody on the cell surface. J Biol Chem 280:4219–4227 [DOI] [PubMed] [Google Scholar]
- Pál A, Méhn D, Molnár E, Gedey S, Mészáros P, Nagy T, Glavinas H, Janáky T, von Richter O, Báthori G, et al. (2007) Cholesterol potentiates ABCG2 activity in a heterologous expression system: improved in vitro model to study function of human ABCG2. J Pharmacol Exp Ther 321:1085–1094 [DOI] [PubMed] [Google Scholar]
- Poller B, Wagenaar E, Tang SC, Schinkel AH. (2011) Double-transduced MDCKII cells to study human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) interplay in drug transport across the blood-brain barrier. Mol Pharm 8:571–582 [DOI] [PubMed] [Google Scholar]
- Robey RW, Medina-Pérez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. (2001) Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res 7:145–152 [PubMed] [Google Scholar]
- Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. (2004) Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res 64:1242–1246 [DOI] [PubMed] [Google Scholar]
- Sauna ZE, Müller M, Peng XH, Ambudkar SV. (2002) Importance of the conserved Walker B glutamate residues, 556 and 1201, for the completion of the catalytic cycle of ATP hydrolysis by human P-glycoprotein (ABCB1). Biochemistry 41:13989–14000 [DOI] [PubMed] [Google Scholar]
- Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, Pastan I, Gottesman MM. (1986) Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem 261:7762–7770 [PubMed] [Google Scholar]
- Shukla S, Robey RW, Bates SE, Ambudkar SV. (2006) The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry 45:8940–8951 [DOI] [PubMed] [Google Scholar]
- Strober W. (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix 3:Appendix 3B [DOI] [PubMed] [Google Scholar]
- Szakács G, Váradi A, Ozvegy-Laczka C, Sarkadi B. (2008) The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov Today 13:379–393 [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84:7735–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiberghien F, Loor F. (1996) Ranking of P-glycoprotein substrates and inhibitors by a calcein-AM fluorometry screening assay. Anticancer Drugs 7:568–578 [DOI] [PubMed] [Google Scholar]
- Tsaioun K, Bottlaender M, Mabondzo A, and Alzheimer's Drug Discovery Foundation (2009) ADDME–Avoiding Drug Development Mistakes Early: central nervous system drug discovery perspective. BMC Neurol 9 (Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.