Abstract
The interplay between phase II enzymes and efflux transporters leads to extensive metabolism and low bioavailability for flavonoids. To investigate the simplest interplay between one UDP-glucuronosyltransferase isoform and one efflux transporter in flavonoid disposition, engineered HeLa cells stably overexpressing UGT1A9 were developed, characterized, and further applied to investigate the metabolism of two model flavonoids (genistein and apigenin) and excretion of their glucuronides. The results indicated that the engineered HeLa cells overexpressing UGT1A9 rapidly excreted the glucuronides of genistein and apigenin. The kinetic characteristics of genistein or apigenin glucuronidation were similar with the use of UGT1A9 overexpressed in HeLa cells or the commercially available UGT1A9. Small interfering (siRNA)-mediated UGT1A9 silencing resulted in a substantial decrease in glucuronide excretion (>75%, p < 0.01). Furthermore, a potent inhibitor of breast cancer resistance protein (BCRP), 3-(6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester (Ko143), caused, in a dose-dependent manner, a substantial and marked reduction of the clearance (74–94%, p < 0.01), and a substantial increase in the intracellular glucuronide levels (4–8-fold, p < 0.01), resulting in a moderate decrease in glucuronide excretion (19–59%, p < 0.01). In addition, a significant, albeit moderate, reduction in the fraction of genistein metabolized (fmet) in the presence of Ko143 was observed. In contrast, leukotriene C4 and siRNA against multidrug resistance protein (MRP) 2 and MRP3 did not affect excretion of flavonoid glucuronides. In conclusion, the engineered HeLa cells overexpressing UGT1A9 is an appropriate model to study the kinetic interplay between UGT1A9 and BCRP in the phase II disposition of flavonoids. This simple cell model should also be very useful to rapidly identify whether a phase II metabolite is the substrate of BCRP.
Introduction
Interplay between transporters and drug-metabolizing enzymes, first proposed approximately 15 years ago (Wacher et al., 1995), has been postulated to have a major role in determining a drug's absorption and disposition (Custodio et al., 2008; Pang et al., 2009). Originally, this interplay was primarily related to P-glycoprotein and cytochrome P450 3A. More recently, the interplay was expanded to include those between phase II enzymes such as UDP-glucuronosyltransferases (UGTs) and sulfotransferases and other efflux transporters such as breast cancer resistance protein (BCRP) and multidrug resistance protein (MRP) 2 as well as organic anion-transporting polypeptide (Liu and Hu, 2007; Urquhart et al., 2007).
Two of the most common and popular cell lines used to delineate the interplay in epithelial cells are Caco-2 and Madin-Darby canine kidney II cell lines. Both cell lines are polarized cells and suitable for studying phase II metabolism of xenobiotics including flavonoids. However, the above two cell models are still too complex for us to study the interplay between UGTs and efflux transporters because multiple efflux transporters and other phase II enzymes (e.g., sulfotransferase) are also expressed in these cells. Therefore, we modified HeLa cells for the purpose of developing a cell culture model to study the interplay between one UGT isoform and efflux transporters. There were two main reasons for selecting HeLa cells: first, they had no detectable glucuronidation and negligible sulfation activity in metabolizing genistein and apigenin; and second, they expressed a significant amount of BCRP but very little MRP2. The very low expression of MRP2 was consistent with that reported by a different group of investigators (Ahlin et al., 2009). We were interested in these two efflux transporters, because both BCRP and MRP2 are capable of mediating the efflux of glucuronides and sulfates (Enokizono et al., 2007; Lengyel et al., 2008; Sakamoto et al., 2008; Lee et al., 2009).
Human UGT1A9 was the first UGT isoform selected to be overexpressed in HeLa cells for several reasons: first, UGT1A9 was responsible for metabolism of many clinical drugs such as 7-ethyl-10-hydroxycamptothecin (SN-38) and acetaminophen (Court et al., 2001; Paoluzzi et al., 2004); second, and more importantly, our previous data showed that UGT1A9 was able to metabolize many flavonoids at relatively high rates, consistent with results from Pritchett et al. (2008); and third, UGT1A9 was more stable than other isoforms (Kurkela et al., 2003).
Two typical flavonoids, genistein and apigenin, were used as model flavonoids. The flavonoids were selected because previous investigations presumed that it was the interplay between phase II enzymes and efflux transporters that contributed to their low bioavailabilities (Liu and Hu, 2007; Zhu et al., 2010). Low bioavailability impedes the testing of many of their “claimed” beneficial effects in humans including anticancer, anti-inflammatory, and antiviral effects as well as prevention of cardiovascular diseases (Comalada et al., 2005; Benavente-García et al., 2007; Lee et al., 2007; Grassi et al., 2009). We believe that a better understanding of the factors that govern the metabolism of flavonoids holds the key to overcome their oral bioavailability barriers. Moreover, it is likely that the simplest interplay between an efflux transporter and a phase II enzyme will form the basis to understand and delineate the complex interplays between multiple phase II enzymes and efflux transporters that determine the flavonoid bioavailability in vivo. Therefore, the purpose of this research is to study the simplest interplay between UGT1A9 and an efflux transporter in control of glucuronidation of genistein and apigenin and of excretion of their glucuronides.
Materials and Methods
Materials.
HeLa cells and pcDNA3.1(±) were a kind gift from Dr. Yu Rong (University of Texas at Houston, Houston, TX). Apigenin, genistein, and testosterone were purchased from Indofine Chemicals (Hillsborough, NJ). Recombinant human UGT1A9 expressed in baculovirus-infected insect cells (Supersomes) was purchased from BD Biosciences (San Jose, CA). UGT1A9 antibody was purchased from Abnova (Walnut, CA). pCMV6_XL4 vector carrying the UGT1A9 gene was from Origene (Rockville, MD). siRNA of UGT1A9 and scrambled siRNA were purchased from Ambion (Austin, TX). siRNA of MRP2 or MRP3 and 3-(6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester (Ko143) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). LTC4 was purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals and solvents were of analytical grade or better.
Transient Transfection.
HeLa cells were seeded at a density of 1.0 × 105 cells/well in a six-well plate and maintained at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). After 6 to 7 h, vector carrying the UGT1A9 gene (GenBank accession number NM_021027.2) was introduced to the cells using the modified calcium precipitation method (Chen and Okayama, 1988). The medium containing 10% FBS (DMEM with high glucose) was changed to a medium containing 2% FBS on day 2. The transiently transfected HeLa cells were ready for excretion study or UGT activity assay on day 3.
Development of Stably Transfected HeLa Cells.
The UGT1A9 gene (GenBank accession number NM_021027.2) from vector pCMV6_XL4 (Origene) was subcloned into pcDNA3.1(±) vector. Then the vector carrying the UGT1A9 gene was transiently transfected into HeLa cells by using the modified calcium precipitation method (Chen and Okayama, 1988). After transfection, HeLa cells were maintained at 37°C under 5% CO2 in DMEM containing 10% FBS and Geneticin (G418; 1.2 mg/ml). Media were changed every 2 or 3 days until the colonies came out. The colonies were picked up and cultured in a 12-well plate (one colony per well). Once cells reached 100% confluence, the cells from each well of the 12-well plate were split into two wells of the six-well plates and allowed to grow until confluence. Those cells that were able to excrete significant amounts of glucuronides were considered as the positive clones. Positive cloned cells were further cultured for five generations to test the stability of glucuronide production, and stable and highly active cells were then cryopreserved for future use. Each vial of cryopreserved cells was used for 10 passages before a new one was initiated for continued use. The HeLa cells stably transfected with UGT1A9 were called engineered HeLa cells.
Transfection of siRNA.
The engineered HeLa cells were seeded at 0.5 × 105 cells/well in a 12-well plate and maintained at 37°C under 5% CO2 in DMEM containing 10% FBS. On the next day, siRNA of UGT1A9 (sense, 5′-CGAAGUAUAUAUUCUCUAUtt; antisense, 5′-AUAGAGAAUAUAUACUUCGta), scrambled siRNA (30 pmol/well), or an equal volume of water was introduced to the cells by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's protocol (Ee et al., 2004). Cells were ready for experiment 2 days after transfection. Following a similar procedure, siRNA of MRP2 or MRP3 was transfected into the engineered HeLa cells.
RT-PCR.
Cells were collected, and the RNA was extracted by using an RNeasy Mini Kit (QIAGEN, Valencia, CA). RT-PCR was run according to the manufacturer's protocol (OneStep RT-PCR Kit; QIAGEN). In brief, a 50-μl mixture containing 2 μg of total RNA, primers (final 0.6 μM, sequences shown later), QIAGEN OneStep RT-PCR Enzyme Mix (2 μl), dNTP mix (final 400 μM of each dNTP), and QIAGEN OneStep RT-PCR buffer as well as RNase-free water was reverse-transcribed at 50°C for 30 min. Then the mixture was continuously incubated at 95°C for 15 min, followed by 35 cycles of expansion (94°C for 0.5 min, 55°C for 0.5 min, and 72°C for 1 min) and by the final extension at 72°C for 10 min. The forward primer of UGT1A9 is 5′-GTTGCCTATGGAATTTGA, and the reverse primer is 5′-GGGTGACCAAGCAGAT. The forward primer of BCRP is 5′-TTCTCCATTCATCAGCCTCG, and the reverse primer is 5′-TGGTTGGTCGTCAGGAAGA. The forward primer of β-actin is 5′-GAGAAGATGACCCAGATCATGT, and the reverse primer is 5′-TCGTCATACTCCTGCTTGCAG (Ee et al., 2004). All these primers were shown to work previously and were supplied by Sigma-Aldrich. The MRP2 and MRP3 primers were purchased from Santa Cruz Biotechnology, Inc. together with siRNA of MRP2 and siRNA of MRP3, respectively. After RT-PCR, agarose gel electrophoresis and UV visualization were used to determine the relative amounts of PCR products.
Preparation of Cell Lysates.
HeLa cells transiently transfected with UGT1A9 or engineered HeLa cells were grown for 3 to 4 days and then were washed and harvested in 50 mM potassium phosphate buffer (pH 7.4). The collected cells were sonicated in an Aquasonic 150D sonicator (VWR Scientific, Bristol, CT) for 30 min at the maximum power (135 average watts) in an ice-cold water bath (4°C) (Liu et al., 2007). The glucuronidation activity of UGT1A9 was not expected to be affected during sonication process because of its thermal stability (Fujiwara et al., 2007). Then the cell lysates were centrifuged at 4°C (5 min at 6000 rpm). The supernatant to be used in the UGT activity assay or Western blotting was harvested, and its protein concentration was determined by BCA assay (Thermo Fisher Scientific, Waltham, MA).
Western Blotting.
Cell lysates from wild-type and engineered HeLa cells and UGT1A9 Supersomes were boiled at 95°C, and denatured protein was then run on a 10% SDS-polyacrylamide gel electrophoresis gel to test the expression levels of UGTs. After electrophoresis, the separated proteins on the gel were transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA) following a standard protocol. After blocking in 5% nonfat milk for 1 h, the membrane was incubated with UGT1A9 antibody at 1:500 dilution in 3% milk at 4°C overnight. After the membrane was washed three times, it was incubated with horseradish peroxidase-conjugated second antibody (goat host anti-rabbit antibody) (1:2000 dilution) in 3% milk for 1 h. Afterward, the membrane was developed with an enhanced chemiluminescence kit (Thermo Fisher Scientific). The same membrane was stripped for detecting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the loading control. For commercial UGT1A9 Supersomes, GAPDH was not present and could not be detected.
UGT Activity Assay.
The method was modified on the basis of a previously published article (Tang et al., 2010). In brief, we mixed cell lysates (final protein concentration from 0.053 to 0.21 mg/ml) or human UGT1A9 Supersomes (final protein concentration from 0.013 to 0.053 mg/ml), magnesium chloride (0.88 mM), saccharolactone (4.4 mM), and alamethicin (0.022 mg/ml) and different concentrations of genistein (0.5–50 μM) or apigenin (0.5–40 μM) in a 50 mM potassium phosphate solution (pH 7.4) diluted from 100× concentrated stock solutions in organic solvent (DMSO-methanol; 1:4). UDP-glucuronic acid (3.5 mM) was added last to the previous mixture to the final volume of 170 μl, and the mixture was incubated at 37°C for 30, 45, or 60 min. At the end of the reaction, it was stopped by the addition of 50 μl of 100 μM testosterone solution (in 94% acetonitrile-6% glacial acetic acid) as the internal standard. Samples were ready for HPLC or UPLC analysis after centrifugation (15 min at 15,000 rpm).
Excretion Experiments.
The engineered HeLa cells (stably transfected with UGT1A9) were grown on 12-well plates (1 × 105 cells/well) or on 6-well plates (2 × 105 cells/well) for approximately 3 to 4 days. Before experiments were started, the engineered HeLa cells were washed twice with prewarmed (37°C) Hanks' balanced salt solution (HBSS) buffer (pH = 7.4). Then the cells were incubated with HBSS buffer containing genistein or apigenin with or without an inhibitor (defined as “loading solution,” 1 ml/well for 12-well plates and 2 ml/well for 6-well plates) for a predetermined time interval (40, 80, and 120 min) at 37°C. For the experiments on LTC4 and siRNA of MRP2 or MRP3, the time intervals were 60, 120, 180, and 240 min (shown in Supplemental Figs. 2S and 3S). In the loading solution, the proper concentration of genistein (2, 5, 10, 20, and 50 μM) or apigenin (2, 5, 10, and 20 μM) was diluted from 100× concentrated stock solutions in organic solvent (DMSO-methanol; 1:4), the concentration of Ko143 (5 or 10 μM) was diluted from 20 mM stock solutions in DMSO, and LTC4 (0.1 μM) was diluted from 80 μM stock solutions in methanol. The sampling times were selected to ensure that the amounts excreted versus time plots stay in the linear range. At each time point, 200 μl of incubating media from each well were collected, and the same volume of loading solutions was used to replenish each well. The collected incubating media were mixed with a “stop solution” consisting of 94% acetonitrile and 6% acetic acid containing 100 μM testosterone as the internal standard. Supernatants were ready for UPLC analysis after centrifugation (15 min at 15,000 rpm). For transiently transfected HeLa cells, which were used to demonstrate whether intact UGT1A9-overexpressing HeLa cells could excrete amounts of glucuronides (Fig. 1), they were grown on six-well plates and incubated overnight with the loading solution (genistein or apigenin at 10 μM, 2 ml/well). The collected incubating media were mixed with the stop solution and analyzed by HPLC after centrifugation (15 min at 15,000 rpm).
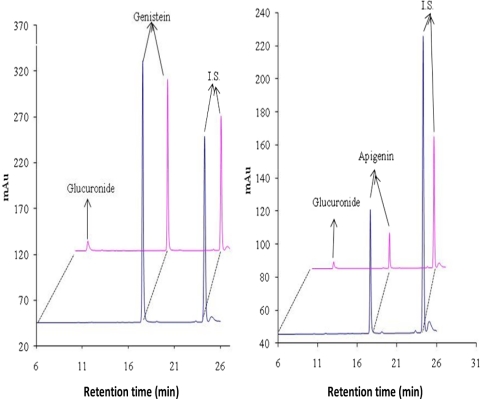
Fig. 1.
The HPLC chromatograms of genistein and apigenin and their glucuronides. The HeLa cells not transfected or transiently transfected with UGT1A9 were treated with 10 μM genistein or apigenin overnight at 37°C. The profile without the glucuronide was derived from the control cells, whereas the profile with the glucuronide was from the transiently transfected cells. The chromatographic profiles were derived from HPLC analysis of the samples. Glucuronides were also previously authenticated with liquid chromatography-MS/MS and authentic standards (Zhu et al., 2010). I.S., internal standard; mAu, milli-absorbance units.
Determination of Intracellular Glucuronides in Engineered HeLa Cells.
The engineered HeLa cells were washed with ice-cold HBSS buffer twice after the excretion experiments at 120 min. Then the cells were collected in 100 or 200 μl of HBSS buffer and sonicated in a Aquasonic 150D sonicator (VWR Scientific) for 30 min at the maximum power (135 average watts) in an ice-cold water bath (4°C) (Liu et al., 2007). UGT1A9 is not expected to function without the cofactors once cells are broken. After centrifugation at 15,500 rpm for 20 min, supernatants were collected and mixed with the stop solution. The samples were ready for UPLC analysis after centrifugation (15 min at 15,000 rpm).
Sample Analysis by HPLC.
The conditions for HPLC analysis of genistein, apigenin, and their glucuronides were modified on the basis of a previously published method (Hu et al., 2003). The conditions were as follows: system, Hewlett Packard 1090 with a diode array detector and Hewlett Packard Chemstation; column, Aqua (5 μm, 150 × 0.45 cm; Phenomenex, Torrance, CA); mobile phase A, 0.1% formic acid plus 0.06% triethylamine (pH 2.6); mobile phase B, 100% acetonitrile; gradient, 0 to 3 min 20% B, 3 to 22 min 20 to 49% B, and 22 to 26 min 49% B; injection volume, 200 μl; and wavelength, 254 nm (for genistein and its glucuronides as well as the internal standard) and 340 nm (for apigenin and its glucuronides). There was a 4-min interval between the end of the run and the next injection to allow the column to be reequilibrated with 20% mobile phase B. The precision was typically better than 5% and accuracy better than 10%. The detection limits were at least 0.2 μM for both alycones and their glucuronides.
Sample Analysis by UPLC.
The conditions for UPLC analysis of genistein, apigenin, and their glucuronides were modified on the basis of a previously published method (Tang et al., 2010; Zhu et al., 2010). The conditions were as follows: system, Acquity with a binary pump and a 2996 DPA diode array detector (Waters, Milford, MA); column, Acquity UPLC BEH C18 column (50 × 2.1 mm, i.d. 1.7 μm; Waters); mobile phase A, 2.5 mM ammonium acetate (pH 7.4); mobile phase B, 100% acetonitrile; gradient, 0 to 2.0 min 10 to 35% B, 2.0 to 3.0 min 35 to 70% B, 3.0 to 3.5 min 70% B; 3.5 to 3.6 min 70 to 90% B; 3.6 to 4.1 min 90% B; and 4.1 to 4.6 min 90 to 10% B; injection volume, 10 μl; and wavelength, 254 nm (for genistein and its glucuronides as well as the internal standard) and 340 nm (for apigenin and its glucuronides). The precision and accuracy were typically within acceptable range (<15%). The detection limits were at least 0.2 μM for both aglycones and their glucuronides. The structures of glucuronides were further confirmed by UPLC-MS/MS as shown previously (Zhu et al., 2010).
Quantification of Glucuronides.
The conversion factors, representing the molar extinction coefficient ratio of glucuronides to aglycones, were used to quantify the amounts of genistein and apigenin glucuronides as described previously (Liu and Hu, 2002; Liu et al., 2007; Tang et al., 2010).
Kinetic Study of UGT1A9.
Rates of metabolism in UGT1A9 Supersomes or HeLa cell lysates were expressed as amounts of glucuronides formed per minute per milligram of protein (or nanomoles per minute per milligram). If the Eadie-Hofstee plot was linear, formation rates (V) of a flavonoid glucuronide at various substrate concentrations (C) were fit to the standard Michaelis-Menten equation (eq. 1):
where Km represents the Michaelis constant and Vmax is the maximum formation rate. If Eadie-Hofstee plots showed characteristic profiles of atypical kinetics such as autoactivation or biphasic kinetics (Houston and Kenworthy, 2000; Hutzler and Tracy, 2002), the data were fit to other corresponding equations (Wang et al., 2006), using an Excel program. Aside from the R2 value, the best-fit model was determined on the basis of the Akaike information criterion (Yamaoka et al., 1978), and the rule of parsimony was applied.
Calculation of fmet Value and Clearance of Efflux Transporter.
Fraction metabolized (fmet) value was defined as the fraction of dose metabolized (eq. 2). The fmet value was considered as the more appropriate parameter to reflect the extent of metabolism in the presence of a transporter-enzyme interplay (Pang et al., 2009).
 |
Clearance of efflux transporter (CL) was used here because intracellular concentrations could be very different from the extracellular concentrations of glucuronides (eq. 3):
where Jmax is the excretion rates of glucuronides, Km′ is the Michaelis constant reflecting affinity of glucuronides to the efflux transporter BCRP, and Ci is the intracellular concentration of glucuronides. The Jmax and Km′ were previously used as two kinetic parameters for transporters (Sun and Pang, 2008). If the average volume of engineered HeLa cells is assumed to be 4 μl/mg protein (Yamaguchi et al., 2000), the intracellular concentrations of glucuronides were calculated after the total amounts of intracellular glucuronides were determined experimentally. Other investigators have estimated volume at a value that was a bit larger than 4 μl/mg protein (Saito et al., 1986), so the intracellular concentrations might be slightly overestimated.
Statistical Analysis.
All the experiments were done in duplicate or triplicate. Data were analyzed by one-way analysis of variance or Student's t test as appropriate, and the level of significance was set at p < 0.05 or p < 0.01.
Results
Excretion of Glucuronides in Control and Transiently Transfected HeLa Cells.
The results indicated (Fig. 1) that there was a new peak eluting before each parent compound in the engineered HeLa cells compared with the control HeLa cells. For genistein, the new peak retention time was approximately 7 min, and for the apigenin, the new peak retention time was approximately 9 min. On the basis of the published data (Liu and Hu, 2002), these new forming peaks represented the glucuronides of each parent compound. On the contrary, only the peak representing genistein or apigenin was detected in control HeLa cells. In brief, a predominant glucuronide of genistein was detected in transiently transfected HeLa cells with pseudo-molecular ion [M − H]− at m/z 445, which was 176 Da higher (characteristic of the addition of glucuronic acid) than that of genistein (m/z 269). Likewise, a predominant glucuronide of apigenin was detected in transiently transfected HeLa cells by using the same UPLC-MS/MS method. The authenticity of the glucuronides was also demonstrated in an earlier article from this laboratory (Zhu et al., 2010).
Characterization of Engineered HeLa Cells Overexpressing UGT1A9.
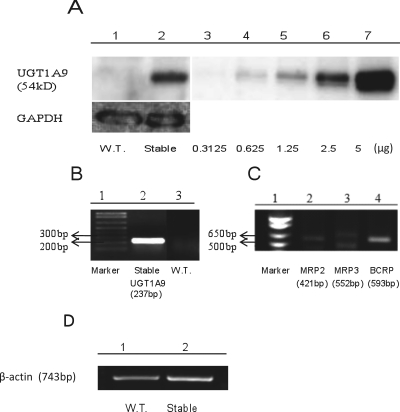
The results (Fig. 2, A and B) showed that UGT1A9 was well expressed at both mRNA and protein levels in engineered HeLa cells with stable transfection. Moreover, mRNA levels of three efflux transporters (BCRP, MRP2, and MRP3) were determined in untransfected HeLa cells (Fig. 2C), because MRP3 was also responsible for the excretion of phytoestrogen glucuronides in addition to MRP2 and BCRP (van de Wetering et al., 2009). This result indicated that BCRP had a relatively higher mRNA expression level than MRP2 or MRP3 in the HeLa cells, consistent with an early observation that MRP2 was poorly expressed in HeLa cells (Ahlin et al., 2009). To determine the stability of UGT1A9 expression in engineered HeLa cells with stable transfection, we measured the UGT1A9 activities for five consecutive generations and found the activities to be fairly stable (Supplemental Fig. 1S).
Fig. 2.
Western blotting (A) and RT-PCR (B–D) of wild-type (W.T.) and engineered HeLa cells. For Western blotting, lane 1 was HeLa cells (wild-type), lane 2 was the engineered HeLa cells stably overexpressing UGT1A9, and lanes 3 to 7 were different amounts of commercially available human UGT1A9 Supersomes (amount, 0.3125–5 μg) used as positive and quality controls. The total amount of protein loaded onto lanes 1 and 2 was 80 μg. B, for RT-PCR, lane 1 was marker, lane 2 was the engineered HeLa cells stably overexpressing UGT1A9, and lane 3 was the control HeLa cells (wild-type). C, for RT-PCR, lane 1 was marker and lanes 2 to 4 were all HeLa cells (wild-type). D, for RT-PCR, lane 1 was HeLa cells (wild-type) and lane 2 was the engineered HeLa cells stably overexpressing UGT1A9. bp, base pairs.
Enzyme Kinetics Study Using UGT1A9 Overexpressed in HeLa Cells.
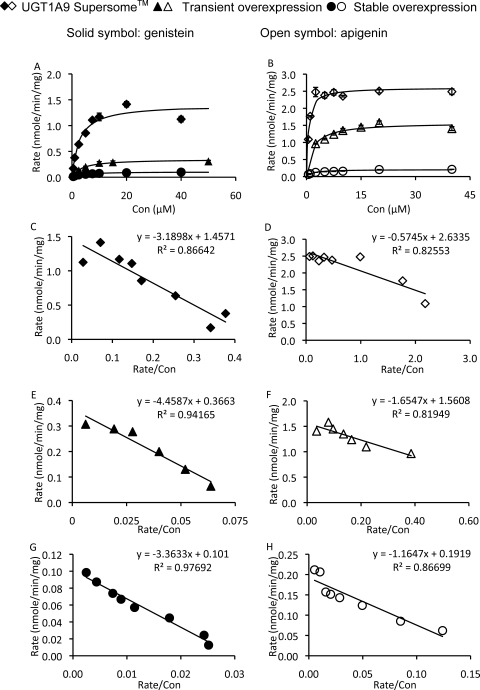
The kinetic profiles of UGT1A9 (transiently or stably overexpressed in HeLa cells)-mediated metabolism of genistein and apigenin were determined (eq. 1) and compared with those of commercially available human UGT1A9 Supersomes. The Km and Vmax values of these kinetic studies are summarized in Table 1, and detailed rates versus concentration plots are shown in Fig. 3. In general, Km values were similar in transiently and stably overexpressed UGT1A9. However, Km values were somewhat higher in UGT1A9 overexpressed in HeLa cells than those derived from UGT1A9 expressed in insect cells (Supersomes). Vmax values from UGT1A9 overexpressed in HeLa cells were not directly comparable to those of human UGT1A9 Supersomes, because the latter was expressed in insect cells and UGT1A9 was membrane-anchored enzyme. UGT1A9 from all the sources followed the classic Michaelis-Menten equation in metabolizing genistein and apigenin (Fig. 3), suggesting that UGT1A9 derived from these various sources were probably the same, although the levels of expression were clearly different.
TABLE 1.
Comparison of kinetics parameters of UGT1A9 from different sources in genistein and apigenin glucuronidation
A Student's t test was used to compare the data. For Supersomes and transient overexpression of UGT1A9, three independent studies (n = 3) were run, whereas for stable overexpression of UGT1A9, only two independent studies (n = 2) were run. The arrows indicate the position for glucuronidation occurring in the engineered HeLa cells.
| Genistein | Apigenin | |||||
|---|---|---|---|---|---|---|
| Source |
 |
 |
||||
| Supersomes | Transient | Stable | Supersomes | Transient | Stable | |
| Km (μM) | 2.72 ± 0.073 | 3.71 ± 0.65 | 3.83 ± 0.18* | 0.51 ± 0.048 | 1.76 ± 0.11* | 1.68 ± 0.037 |
| Vmax (nmol · min−1 · mg−1) | 1.41 ± 0.027 | 0.35 ± 0.025 | 0.10 ± 0.0015 | 2.61 ± 0.023 | 1.58 ± 0.0054 | 0.21 ± 0 |
| AIC | −15.82 | −36.63 | −76.62 | −10.44 | −18.96 | −47.84 |
| R2 | 0.97 | 0.99 | 0.99 | 0.91 | 0.86 | 0.92 |
AIC, Akaike information criterion.
p < 0.05.
Fig. 3.
In vitro metabolism study using human UGT1A9 Supersomes and UGT1A9 overexpressed in HeLa cells (lysates of HeLa cells transiently or stably transfected with UGT1A9). A, genistein kinetics. Rates of metabolism were determined from 0.5 to 50 μM, and reaction time was 60 min. Related Eadie-Hofstee plots are shown for Supersomes (C), transient overexpression (E), and stable overexpression (G). B, apigenin kinetics. Rates of metabolism were determined from 0.5 to 40 μM, and reaction time was 15, 30, or 60 min. Related Eadie-Hofstee plots are shown for Supersomes (D), transient overexpression (F), and stable overexpression (H). Each data point was the average of two (stable overexpression, n = 2) or three (Supersomes and transient overexpression, n = 3) determinations with error bars representing S.D. Con, concentration.
Effects of Concentrations on the Excretion of Glucuronides.
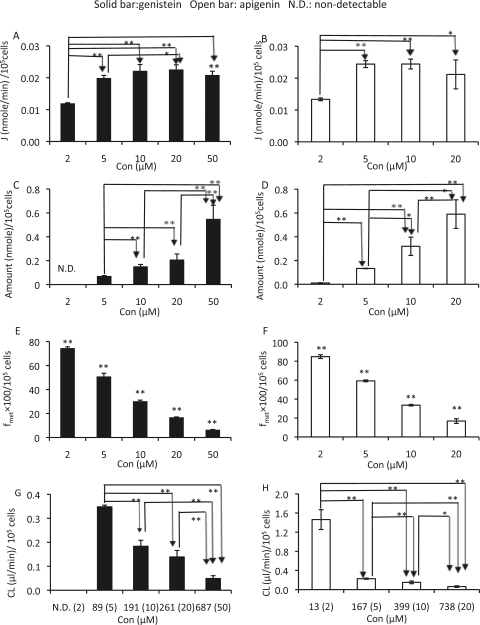
To study whether the concentrations of genistein (2, 5, 10, 20, and 50 μM) or apigenin (2, 5, 10, and 20 μM) had an effect on the excretion of glucuronides, different concentrations of genistein or apigenin were incubated with engineered HeLa cells (Fig. 4). As expected, the rates of excretion of both genistein and apigenin glucuronides increased, whereas their fmet decreased with a rise in concentration. The increase in excretion reached a plateau at a relatively low concentration (5 μM), but the decrease in fmet persisted throughout. In contrast, the intracellular glucuronide concentrations increased faster than the changes in the loading concentrations, which resulted in a significant and substantial decrease in the cellular clearance of these flavonoid glucuronides (eq. 3) as the loading concentration increased.
Fig. 4.
Effects of concentrations (Con) on the excretion rates (A and B), intracellular amounts (C and D), fmet (E and F), and CL (G and H) of genistein or apigenin glucuronide. Different concentrations of genistein (A, C, E, and G) or apigenin (B, D, F, and H) were incubated with engineered HeLa cells grown on 12-well plates, and three samples (200 μl) were taken at 40, 80, and 120 min and replaced with fresh loading solution (200 μl) containing the flavonoid of interest. The excretion rates of glucuronides (A and B) were calculated as slope of amounts versus time (40, 80, and 120 min) curves. The intracellular amounts of glucuronides (C and D) were determined at the end of excretion experiments (i.e., 120 min) after the cells were washed three times with ice-cold buffer. The fmet values (determined at 120 min) are shown in E and F, and the CL values are given in G and H. The calculated intracellular concentrations of glucuronides are shown on the x-axis of G and H followed by the actual loading flavonoid concentrations in parentheses. Each data point is the average of three determinations with error bar representing the S.D. (n = 3). *, p < 0.05; **, p < 0.01. N.D., not detectable.
Effects of siRNA-Mediated UGT1A9 Silencing on the Excretion of Glucuronides.
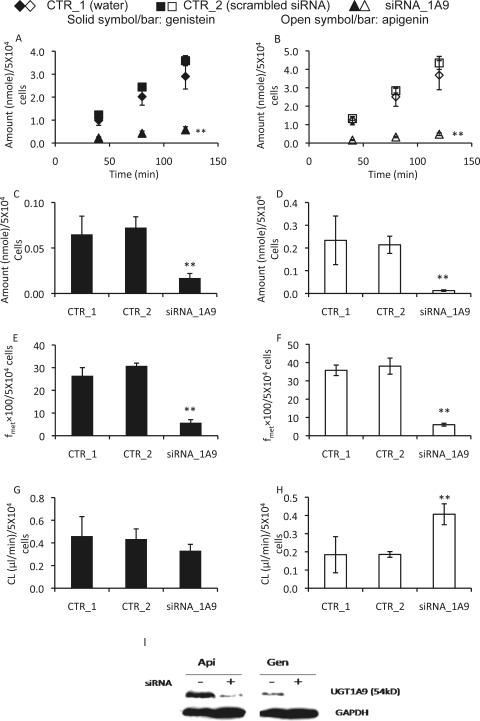
siRNA of UGT1A9 was introduced to the engineered HeLa cells to determine how changes in glucuronidation activity affected cellular glucuronide excretion, because of the unavailability of a specific chemical inhibitor of UGT1A9. Two types of control studies were performed: water transfection and scrambled siRNA transfection. There was no significant difference between these two control groups. Therefore, we used the scrambled siRNA group as the negative control to compare the effects of siRNA against those of UGT1A9. The results indicated that siRNA-mediated UGT1A9 silencing inhibited the excretion of genistein and apigenin glucuronides by 85 and 89%, respectively (Fig. 5, A and B). The intracellular levels of glucuronides in the presence of UGT1A9 siRNA were reduced by 77 and by 94% for genistein and apigenin glucuronides, respectively (Fig. 5, C and D). Likewise, fmet of genistein with siRNA treatment was 81% less than the control, and fmet of apigenin was 84% less than the control (Fig. 5, E and F). For CL of genistein glucuronide, there was no significant difference between control and siRNA of UGT1A9-treated group (Fig. 5G). However, CL of apigenin glucuronide in the presence of siRNA was approximately 2.2-fold higher than that in the control (Fig. 5H). These changes resulting from decreased glucuronidation activities (as demonstrated by a decrease in fmet of both flavonoids) were consistent with the decreases in expression levels of transfected UGT1A9 shown by the Western blotting (Fig. 5I). According to the band intensity ratio (UGT1A9 band intensity relative to GAPDH), the siRNA treatment could inhibit the expression of UGT1A9 by 56% in the engineered HeLa cells.
Fig. 5.
Effects of siRNA-mediated UGT1A9 silencing on the excretion rates, intracellular amounts, fmet, and CL of flavonoid glucuronides. Engineered HeLa cells stably overexpressing UGT1A9 grow on 12-well plates were treated with 10 μM genistein or apigenin in the absence of siRNA (3 μl of water/well) or presence of 30 pmol/well scrambled siRNA or siRNA targeting UGT1A9. The experiment protocols were otherwise the same as those described in the legend to Fig. 4. The amounts of excreting glucuronides as a function of time are shown in A and B, and the intracellular amounts (determined at 120 min) are shown in C and D. fmet values (determined at 120 min) are shown in E and F, and CL values are given in G and H. For the control group treated with an equal volume of water, each data point was the average of six determinations (n = 6); for the control group transfected with scrambled siRNA or the group treated with siRNA targeting UGT1A9, each data point was the average of three determinations (n = 3). The error bar represents S.D. *, p < 0.05; **, p < 0.01. There was no statistically significant difference observed between the scrambled control and water transfection. In I, effects of siRNA-mediated silencing on expression of UGT1A9 are shown. siRNA of UGT1A9 (75 pmol/well of a 6-well plate, corresponding 30 pmol/well of a 12-well plate) was transfected to the engineered HeLa cells. Two days after transfection, cells were incubated with apigenin (Api) and genistein (Gen) at 10 μM for 40 min to maximally mimic the condition of the excretion assay. At the end of the experiments, the cells were collected and lysed for Western blotting, for which 25 μg of protein were loaded into each lane (n = 2).
Effects of siRNA-Mediated MRP2 and MRP3 Silencing on the Excretion of Glucuronides.
siRNA of MRP2 or MRP3 was introduced to the engineered HeLa cells to determine how these treatments might affect the excretion of genistein and apigenin glucuronides, and the results indicated that these two siRNAs had no impact on the excretion of genistein or apigenin glucuronides (Supplemental Fig. S2).
Effects of Ko143 on the Excretion of Glucuronides.
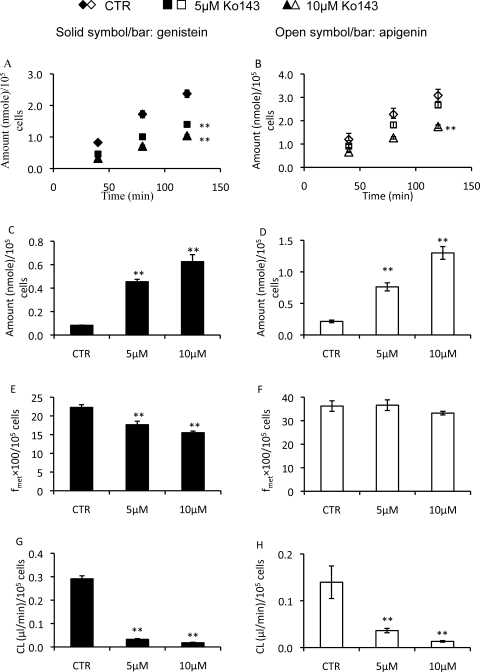
Next, the role of BCRP in excretion of glucuronides in the engineered HeLa cells was determined, using a specific and potent chemical inhibitor, Ko143 (Pick et al., 2010) (Fig. 6). The concentrations of Ko143 (5 and 10 μM) were selected on the basis of references showing that this compound had a Ki value of less than 1 μM (Cooray et al., 2004; Brand et al., 2008). Our data showed that for genistein, the excretion amounts of its glucuronides were reduced by 42 and 59% in the presence of 5 and 10 μM Ko143, respectively. For apigenin, on the other hand, the excretion amounts of its glucuronides were reduced by 19 and 44% in the presence of 5 and 10 μM Ko143, respectively. However, amounts of genistein glucuronides inside the cells were increased by 6 (5 μM)- and 8 (10 μM)-fold comparing with the control, whereas amounts of apigenin glucuronides inside the cells were augmented by 4 (5 μM)- and 6 (10 μM)-fold compared with the control. Additional analysis of the results indicated that Ko143 had limited (or sometimes no) impact on the overall glucuronidation as represented by the fmet value, which was only moderately decreased (Fig. 6, E and F). For fmet of genistein, the decrease was approximately 21% (5 μM, p < 0.01) and 30% (10 μM, p < 0.01), but for apigenin, the decrease was statistically insignificant. Compared with limited reduction of fmet, cellular clearances (eq. 3) were inhibited drastically for both genistein and apigenin glucuronides by Ko143 (Fig. 6, G and H). In the presence of Ko143, CL of genistein glucuronide was reduced by 89% (5 μM) and 94% (10 μM), respectively, whereas CL of apigenin glucuronide was reduced by 74% (5 μM) and 91% (10 μM), respectively.
Fig. 6.
Effects of the BCRP-specific inhibitor Ko143 on the excretion rates, intracellular amounts, fmet, and CL of flavonoid glucuronides. Engineered HeLa cells stably overexpressing UGT1A9 grown on 12-well plates were treated with 10 μM genistein or apigenin in the absence or presence of Ko143 at 5 or 10 μM. The experiment protocols were otherwise the same as those described in the legend to Fig. 4. The amounts of excreted glucuronides as a function of time are shown in A and B, and the intracellular amounts (determined at 120 min) are shown in C and D. fmet values (determined at 120 min) are shown in E and F, and CL values are given in G and H. Each data point is the average of three determinations with the error bar representing the S.D. (n = 3). *, p < 0.05; **, p < 0.01. CTR, control.
Effects of LTC4 on the Excretion of Glucuronides.
We used LTC4, a general inhibitor of MRPs, to determine whether it would affect cellular glucuronide excretion as was demonstrated in Caco-2 cells (Hu et al., 2003). The results indicated that this general MRP inhibitor did not affect the cellular excretion of either glucuronide (Supplemental Fig. 3S).
Discussion
Our results clearly indicate that UGT1A9 overexpressed in the HeLa cells functioned as a conjugating enzyme and was similar to the commercially available human UGT1A9 (Table 1; Fig. 3). Expressed UGT1A9 played a critical role in controlling the concentration-dependent excretion of genistein and apigenin glucuronides in HeLa cells overexpressing UGT1A9 (Fig. 4). On the other hand, the large decreases in clearances for both genistein and apigenin glucuronides in the presence of Ko143 suggested that BCRP was the predominant gatekeeper in the kinetic interplay between UGT1A9 and BCRP in the engineered HeLa cells (Fig. 6, G and H). Therefore, the engineered HeLa cells overexpressing UGT1A9 is an appropriate tool to study the kinetic interplay between UGT1A9 and BCRP and a novel tool for rapidly identifying whether a glucuronide is a BCRP substrate.
The glucuronidation of genistein or apigenin by UGT1A9 overexpressed in the engineered HeLa cells was the rate-determining step in the interplay. The glucuronides were only detectable in the transfected but not in the wild-type HeLa cells (Fig. 1). Furthermore, the excretions of glucuronides and fmet were significantly decreased when siRNA of UGT1A9 was introduced. On the other hand, BCRP was found to be the dominant efflux transporter (i.e., the gatekeeper) in the engineered HeLa cells because the intracellular levels of glucuronides were increased by several fold, and the clearances of glucuronides by efflux transporter were inhibited by greater than 90% in the presence of 10 μM Ko143 (Fig. 6, C, D, G, and H). However, the excretions of glucuronides with Ko143 did not decrease as much as the clearance, as one would have expected (Fig. 6, A and B). This was due to the kinetic compensation; i.e., the increasing levels of intracellular glucuronides compensated for the fact that function of efflux transporter was substantially inhibited by Ko143 (as demonstrated by large decreases in cellular CL). Furthermore, we excluded any major role played by MRP2 and MRP3 in the excretion of flavonoid glucuronides because neither the broad specific MRP inhibitor LTC4 (Supplemental Fig. 3S) nor the potent siRNAs against MRP2 and MRP3 was effective in inhibiting the glucuronide efflux (Supplemental Fig. 2S). Thus, we are able to demonstrate that the observed interplay in the engineered HeLa cells is mainly the result of the kinetic interplay between UGT1A9 and BCRP.
In addition to this application in understanding the kinetic interplay between UGT1A9 and BCRP, the newly engineered HeLa cells are useful for determining whether a glucuronide (e.g., apigenin glucuronide) is a substrate of BCRP. This determination could not be routinely made previously because one would have to use purified glucuronides together with membrane vesicles that overexpress BCRP to positively identify whether a glucuronide is the substrate of BCRP, a tedious and time- and resource-consuming process. As a consequence, very few glucuronides have been positively identified as substrates of BCRP (Chen et al., 2003).
Ideally, it would be desirable to directly extrapolate what we observed in the engineered HeLa cells (i.e., kinetic interplay between UGT1A9 and BCRP) to human hepatocytes or enterocytes. If there was only one pair of interactions between UGT1A9 and BCRP for the metabolism of a particular substrate, such an extrapolation could be done by measuring the relative expression levels of participating proteins (i.e., UGT1A9 and BCRP) in two model systems. Unfortunately, neither of these two compounds undergoes simple metabolism in a complex cellular system (hepatocytes or enterocytes). In these cells, genistein and apigenin are metabolized by both sulfotransferases and UGTs, each by multiple isoforms. Therefore, proper extrapolation of the simple kinetic interplay shown here requires additional development of the model systems such that each of the other possible pairs involved in the complex interplays could be well understood. Then, a cellular model can be built to incorporate all the possible interplays so we can extrapolate what we see in simple model systems to a more complex model system. In other words, the developed HeLa cells are the first simple model, and many more simple models are needed to understand the complex interplays existing in the human enterocytes or hepatocytes.
Not surprisingly, our simple model also has some additional limitations. For example, the capability of BCRP may be exaggerated. The substrates may prefer other efflux transporters, if other options are available. However, here they have to depend on BCRP to get out of the cells because BCRP is the most available efflux transporter in the model. Another limitation is that the compound must be the substrate of UGT1A9, which can be alleviated by using the same approach to build additional HeLa cell models.
In conclusion, the engineered HeLa cells that are stably transfected with UGT1A9 are an appropriate tool to study the kinetic interplay between UGT1A9 and BCRP and a novel tool to rapidly identify the glucuronide substrates of BCRP. Our data obtained from the engineered HeLa cells clearly indicated that the interplay was at least occurring at the kinetic level. At present, more thorough investigations are ongoing to uncover the interplay by using more flavonoids and certain clinically important drugs such as SN-38 because SN-38 is a substrate of UGT1A9 (Paoluzzi et al., 2004) and its glucuronide is the substrate of BCRP (Nakatomi et al., 2001). The present and future studies on the interplay between phase II conjugating enzymes and efflux transporters in the engineered HeLa cells may provide us with the insight necessary to manipulate the oral bioavailability of flavonoids and other therapeutically relevant phenolics.
Supplementary Material
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM70737].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- UGT
- UDP-glucuronosyltransferase
- BCRP
- breast cancer resistance protein
- MRP
- multidrug resistance protein
- SN-38
- 7-ethyl-10-hydroxycamptothecin
- siRNA
- small interfering RNA
- LTC4
- leukotriene C4
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- RT
- reverse transcriptase
- PCR
- polymerase chain reaction
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- DMSO
- dimethyl sulfoxide
- HPLC
- high-performance liquid chromatography
- UPLC
- ultraperformance liquid chromatography
- HBSS
- Hanks' balanced salt solution
- MS/MS
- tandem mass spectrometry.
Authorship Contributions
Participated in research design: Jiang, Yu, and Hu.
Conducted experiments: Jiang and Xu.
Contributed new reagents or analytic tools: Yu and Hu.
Performed data analysis: Jiang, Wu, and Hu.
Wrote or contributed to the writing of the manuscript: Jiang, Wu, and Hu.
References
- Ahlin G, Hilgendorf C, Karlsson J, Szigyarto CA, Uhlén M, Artursson P. (2009) Endogenous gene and protein expression of drug-transporting proteins in cell lines routinely used in drug discovery programs. Drug Metab Dispos 37:2275–2283 [DOI] [PubMed] [Google Scholar]
- Benavente-García O, Castillo J, Alcaraz M, Vicente V, Del Río JA, Ortuño A. (2007) Beneficial action of citrus flavonoids on multiple cancer-related biological pathways. Curr Cancer Drug Targets 7:795–809 [DOI] [PubMed] [Google Scholar]
- Brand W, van der Wel PA, Rein MJ, Barron D, Williamson G, van Bladeren PJ, Rietjens IM. (2008) Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos 36:1794–1802 [DOI] [PubMed] [Google Scholar]
- Chen CA, Okayama H. (1988) Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques 6:632–638 [PubMed] [Google Scholar]
- Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. (2003) Transport of methotrexate, methotrexate polyglutamates, and 17β-estradiol 17-(β-d-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res 63:4048–4054 [PubMed] [Google Scholar]
- Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J, Zarzuelo A. (2005) In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur J Immunol 35:584–592 [DOI] [PubMed] [Google Scholar]
- Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. (2004) Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun 317:269–275 [DOI] [PubMed] [Google Scholar]
- Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, Mackenzie PI. (2001) Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther 299:998–1006 [PubMed] [Google Scholar]
- Custodio JM, Wu CY, Benet LZ. (2008) Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev 60:717–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ee PL, He X, Ross DD, Beck WT. (2004) Modulation of breast cancer resistance protein (BCRP/ABCG2) gene expression using RNA interference. Mol Cancer Ther 3:1577–1583 [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. (2007) Regional expression and activity of breast cancer resistance protein (Bcrp/Abcg2) in mouse intestine: overlapping distribution with sulfotransferases. Drug Metab Dispos 35:922–928 [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Nakajima M, Yamanaka H, Nakamura A, Katoh M, Ikushiro S, Sakaki T, Yokoi T. (2007) Effects of coexpression of UGT1A9 on enzymatic activities of human UGT1A isoforms. Drug Metab Dispos 35:747–757 [DOI] [PubMed] [Google Scholar]
- Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C. (2009) Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des 15:1072–1084 [DOI] [PubMed] [Google Scholar]
- Houston JB, Kenworthy KE. (2000) In vitro-in vivo scaling of CYP kinetic data not consistent with the classical Michaelis-Menten model. Drug Metab Dispos 28:246–254 [PubMed] [Google Scholar]
- Hu M, Chen J, Lin H. (2003) Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther 307:314–321 [DOI] [PubMed] [Google Scholar]
- Hutzler JM, Tracy TS. (2002) Atypical kinetic profiles in drug metabolism reactions. Drug Metab Dispos 30:355–362 [DOI] [PubMed] [Google Scholar]
- Kurkela M, García-Horsman JA, Luukkanen L, Mörsky S, Taskinen J, Baumann M, Kostiainen R, Hirvonen J, Finel M. (2003) Expression and characterization of recombinant human UDP-glucuronosyltransferases (UGTs). UGT1A9 is more resistant to detergent inhibition than other UGTs and was purified as an active dimeric enzyme. J Biol Chem 278:3536–3544 [DOI] [PubMed] [Google Scholar]
- Lee ER, Kang GH, Cho SG. (2007) Effect of flavonoids on human health: old subjects but new challenges. Recent Pat Biotechnol 1:139–150 [DOI] [PubMed] [Google Scholar]
- Lee JK, Abe K, Bridges AS, Patel NJ, Raub TJ, Pollack GM, Brouwer KL. (2009) Sex-dependent disposition of acetaminophen sulfate and glucuronide in the in situ perfused mouse liver. Drug Metab Dispos 37:1916–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel G, Veres Z, Tugyi R, Vereczkey L, Molnár T, Glavinas H, Krajcsi P, Jemnitz K. (2008) Modulation of sinusoidal and canalicular elimination of bilirubin-glucuronides by rifampicin and other cholestatic drugs in a sandwich culture of rat hepatocytes. Hepatol Res 38:300–309 [DOI] [PubMed] [Google Scholar]
- Liu X, Tam VH, Hu M. (2007) Disposition of flavonoids via enteric recycling: determination of the UDP-glucuronosyltransferase isoforms responsible for the metabolism of flavonoids in intact Caco-2 TC7 cells using siRNA. Mol Pharm 4:873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hu M. (2002) Absorption and metabolism of flavonoids in the Caco-2 cell culture model and a perfused rat intestinal model. Drug Metab Dispos 30:370–377 [DOI] [PubMed] [Google Scholar]
- Liu Z, Hu M. (2007) Natural polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab Toxicol 3:389–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi K, Yoshikawa M, Oka M, Ikegami Y, Hayasaka S, Sano K, Shiozawa K, Kawabata S, Soda H, Ishikawa T, et al. (2001) Transport of 7-ethyl-10-hydroxycamptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cells. Biochem Biophys Res Commun 288:827–832 [DOI] [PubMed] [Google Scholar]
- Pang KS, Maeng HJ, Fan J. (2009) Interplay of transporters and enzymes in drug and metabolite processing. Mol Pharm 6:1734–1755 [DOI] [PubMed] [Google Scholar]
- Paoluzzi L, Singh AS, Price DK, Danesi R, Mathijssen RH, Verweij J, Figg WD, Sparreboom A. (2004) Influence of genetic variants in UGT1A1 and UGT1A9 on the in vivo glucuronidation of SN-38. J Clin Pharmacol 44:854–860 [DOI] [PubMed] [Google Scholar]
- Pick A, Klinkhammer W, Wiese M. (2010) Specific inhibitors of the breast cancer resistance protein (BCRP). ChemMedChem 5:1498–1505 [DOI] [PubMed] [Google Scholar]
- Pritchett LE, Atherton KM, Mutch E, Ford D. (2008) Glucuronidation of the soyabean isoflavones genistein and daidzein by human liver is related to levels of UGT1A1 and UGT1A9 activity and alters isoflavone response in the MCF-7 human breast cancer cell line. J Nutr Biochem 19:739–745 [DOI] [PubMed] [Google Scholar]
- Saito H, Inui K, Hori R. (1986) Mechanisms of gentamicin transport in kidney epithelial cell line (LLC-PK1). J Pharmacol Exp Ther 238:1071–1076 [PubMed] [Google Scholar]
- Sakamoto S, Kusuhara H, Horie K, Takahashi K, Baba T, Ishizaki J, Sugiyama Y. (2008) Identification of the transporters involved in the hepatobiliary transport and intestinal efflux of methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-naphthoate (S-8921) glucuronide, a pharmacologically active metabolite of S-8921. Drug Metab Dispos 36:1553–1561 [DOI] [PubMed] [Google Scholar]
- Sun H, Pang KS. (2008) Permeability, transport, and metabolism of solutes in Caco-2 cell monolayers: a theoretical study. Drug Metab Dispos 36:102–123 [DOI] [PubMed] [Google Scholar]
- Tang L, Ye L, Singh R, Wu B, Lv C, Zhao J, Liu Z, Hu M. (2010) Use of glucuronidation fingerprinting to describe and predict mono- and dihydroxyflavone metabolism by recombinant UGT isoforms and human intestinal and liver microsomes. Mol Pharm 7:664–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart BL, Tirona RG, Kim RB. (2007) Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol 47:566–578 [DOI] [PubMed] [Google Scholar]
- van de Wetering K, Feddema W, Helms JB, Brouwers JF, Borst P. (2009) Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology 137:1725–1735 [DOI] [PubMed] [Google Scholar]
- Wacher VJ, Wu CY, Benet LZ. (1995) Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog 13:129–134 [DOI] [PubMed] [Google Scholar]
- Wang SW, Chen J, Jia X, Tam VH, Hu M. (2006) Disposition of flavonoids via enteric recycling: structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab Dispos 34:1837–1848 [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Nakagawa T, Uno T. (1978) Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 6:165–175 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Yano I, Hashimoto Y, Inui KI. (2000) Secretory mechanisms of grepafloxacin and levofloxacin in the human intestinal cell line Caco-2. J Pharmacol Exp Ther 295:360–366 [PubMed] [Google Scholar]
- Zhu W, Xu H, Wang SW, Hu M. (2010) Breast cancer resistance protein (BCRP) and sulfotransferases contribute significantly to the disposition of genistein in mouse intestine. Aaps J 12:525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.