Abstract
Mammalian cytosolic sulfotransferases (SULTs) catalyze the sulfation of xenobiotics as well as numerous endogenous molecules. The major aryl (phenol) SULT in rat liver, rSULT1A1, has been used extensively as a model enzyme for understanding the catalytic function of SULTs. Previous studies showed that purified rSULT1A1 displays significant catalytic changes in the presence of GSSG and other oxidants. In the present study, the effects of diamide [1,1′-azobis(N,N-dimethylformamide)] and tert-butyl hydroperoxide (TBHP) on the activity of rSULT1A1 in rat hepatic slices were compared with the effects of these oxidants on a homogeneous preparation of the enzyme. Precision-cut hepatic slices were incubated with 10 μM 7-hydroxycoumarin (7-HC) in the presence of varied concentrations of either diamide or TBHP. Analysis of the 7-hydroxycoumarin sulfate released into the incubation medium indicated that both oxidants significantly increased the sulfation of 7-HC, and this occurred at optimal concentrations of 5 and 10 μM, respectively. Cellular GSH and GSSG levels in the hepatic slices were not significantly altered from control values at these concentrations of diamide and TBHP. Exposure of homogeneous rSULT1A1 to diamide or TBHP also increased the rate of sulfation of 7-HC, although the optimal concentrations of diamide and TBHP were lower (50- and 100-fold, respectively) than those required for effects with the hepatic slices. These results indicate that both diamide and TBHP may modify the rSULT1A1 in intact cells in a manner similar to that observed with the homogeneous purified enzyme.
Introduction
The mammalian cytosolic sulfotransferases (SULTs) contribute to the metabolism of various drugs, carcinogens, environmental contaminants, and other xenobiotics, as well as functioning in the metabolism of endogenous steroids, bile acids, catecholamines, and iodothyronines (Jakoby and Ziegler, 1990; Glatt, 2000; Chapman et al., 2004; Gamage et al., 2006; Alnouti, 2009; Duffel, 2010). The SULTs constitute a superfamily of enzymes that have been organized into families and subfamilies (Blanchard, 2004) on the basis of sequence similarity. Although there are distinct specificities for substrates and inhibitors among SULT families, there is a high degree of structural homology among these enzymes (Negishi et al., 2001), as well as significant overlap in specificity for some substrates (Gamage et al., 2006; Duffel, 2010).
Among the SULTs that have been used extensively in studies on mechanisms, specificity, and inhibition, rSULT1A1, the major family 1 sulfotransferase in rat liver, has been particularly useful in studies focusing on details of the chemical and kinetic mechanisms of reactions catalyzed by sulfotransferases (Duffel and Jakoby, 1981; Duffel et al., 2001; Chapman et al., 2003). One of the more intriguing aspects of the mechanism of rSULT1A1 is the sensitivity of the enzyme to changes in its redox environment (Marshall et al., 2000). Studies with homogeneous preparations of the enzyme have indicated that treatment with thiol oxidants such as diamide and GSSG results in increases in the specific activity of rSULT1A1 with 4-nitrophenol as substrate (Marshall et al., 1997, 1998, 2000). In studies with GSSG as the oxidant, these effects were clearly shown to be attributable to sequential oxidation of cysteine residues in rSULT1A1, with initial formation of an S-glutathionylated protein involving cysteine 66, followed by formation of an intramolecular protein disulfide between cysteines 66 and 232 (Marshall et al., 1997, 2000). Further oxidation resulted in a decrease in catalytic function as a result of formation of additional disulfide bonds in the protein (Marshall et al., 1997).
It was proposed that this sensitivity of rSULT1A1 to its redox environment could have significant implications for sulfation of xenobiotics and physiological substrates under conditions of oxidative stress, wherein Cys66 could serve as a redox switch regulating the specificity and kinetic features of the enzyme (Marshall et al., 1997, 2000; Duffel et al., 2001; Liu et al., 2009). A critical component of evaluation of this possibility is determination of whether oxidation of the enzyme can regulate the rate of reaction within an intact cellular environment. Therefore, we tested the hypothesis that diamide and tert-butyl hydroperoxide (TBHP), two oxidants with differing mechanisms for oxidation of protein thiols, would exert similar effects on the sulfation of 7-hydroxycoumarin (7-HC) in viable, precision-cut, hepatic tissue slices from rats, compared with those seen for the sulfation of 7-HC catalyzed by purified recombinant rSULT1A1.
Materials and Methods
Chemicals.
Diamide [1,1′-azobis(N,N-dimethylformamide)], TBHP, 7-HC, 7-hydroxycoumarin sulfate (7-HCS), lactate dehydrogenase (LDH) assay kit, sulfatase (type H-2 from Helix pomatia), adenosine 3′,5′-diphosphate (PAP)-agarose, d-saccharic acid 1,4-lactone, and HEPES were purchased from Sigma-Aldrich (St. Louis, MO). Williams' medium E (Gibco 12551) was obtained from Invitrogen (Carlsbad, CA). Adenosine 3′-phosphate, 5′-phosphosulfate (PAPS) was obtained from Sigma-Aldrich and was purified, by using a previously described procedure (Sekura, 1981), to >98% purity, as judged with high-performance liquid chromatography. All other chemicals used were of the highest chemical purity commercially available.
Preparation of Liver Slices.
Male Sprague-Dawley rats (280–295 g) were obtained from Harlan Laboratories (Indianapolis, IN). The animals had free access to food and water and were allowed to acclimate for 1 week after arrival at the University of Iowa. All protocols were in compliance with the University of Iowa animal care and use committee and relevant guidelines of the National Research Council for the Care and Use of Laboratory Animals in Research. After carbon dioxide euthanasia of the rat, the liver was removed and immediately placed in a modified Krebs-Henseleit buffer (Barr et al., 1991) at 4°C. This buffer (buffer A) contained 6.87 g of NaCl, 0.4 g of KCl, 0.11 g of MgCl2, 0.10 g of NaH2PO4·H2O, 2.1 g of NaHCO3, 2.0 g of glucose, 0.37 g of CaCl2·2H2O, and 5.96 g of HEPES per liter (adjusted to pH 7.5 with 0.1 N NaOH). Livers were dissected into lobes, and 8.0-mm i.d. cores were produced by using a motor-driven, cylindrical, stainless steel corer (Alabama Research and Development, Munford, AL). The tissue cores were continuously maintained in ice-cold buffer A until the liver slices were prepared, within 2 h. Cores were placed in a Krumdieck tissue slicer (Alabama Research and Development), and tissue slices of ∼250-μm thickness were generated through movement of a weighted tissue core over an oscillating blade. The resulting slices were carried to a collection chamber with a stream of ice-cold buffer A.
Cryopreservation of Liver Slices.
After preparation, slices were washed two times with a cryopreservation medium consisting of Williams' medium E supplemented with 12% (v/v) dimethyl sulfoxide. Tissue slices (two per tube) were placed in 2-ml microcentrifuge tubes (Seal-Rite natural polypropylene; USA Scientific, Ocala, FL) containing 1.0 ml of cryopreservation medium and were kept at −20°C for 6 h, after which the tubes were transferred to storage at −70°C. The slices were thawed by being placed at −20°C for 2 h, followed by transfer of the tubes to a water bath at 37°C and shaking for 2 min. Slices were then removed from the cryopreservation medium and washed three times with buffer A. The viability of the tissue slices was assessed on the basis of LDH release, and this was examined for slices both with and without incubation with 7-HC and oxidant, as described below.
Assay of 7-HC Sulfation.
Studies on the sulfation of 7-HC were performed by using a modification of a previously described method (Thohan et al., 2001). Two cryopreserved liver slices were placed in a 20-ml glass liquid scintillation vial (Research Products International, Mt. Prospect, IL) containing 2.0 ml of buffer A supplemented with 10 μM 7-HC and various indicated concentrations of diamide or TBHP. The open vials were placed on a rotating (1 rpm) incubation platform (Alabama Research and Development) that was contained inside a cell culture incubator (NAPCO 8000DH; Thermo Fisher Scientific, Marietta, OH) maintained at 37°C in the presence of 5% CO2. After a 2-h incubation, 1 ml of the incubation buffer was mixed with 9 ml of an ether/isoamyl alcohol (IAA) mixture (1:0.014), and the extraction mixture was placed on a horizontal reciprocating shaker for 15 min. The organic phase, containing unconjugated 7-HC, was then separated, the ether was removed through evaporation with a stream of nitrogen, and the residue was dissolved in 3 ml of glycine/NaOH buffer (0.2 M, pH 10.3). The amount of 7-HC in this sample was determined with fluorescence spectroscopy (Perkin Elmer LS55 spectrofluorimeter; PerkinElmer Life and Analytical Sciences, Waltham, MA), with excitation and emission wavelengths of 375 and 450 nm, respectively. Authentic standards of 7-HC were used to establish a standard curve (0.078–1.25 μM).
The aqueous phase (1.0 ml) from the ether/IAA extraction was mixed with 1.0 ml of 0.4 M sodium acetate buffer, pH 4.8, to provide a solution with a final pH of 6.8. After addition of sulfatase (final concentration of 20 U/ml) and d-saccharic acid 1,4-lactone (final concentration of 20 mM, for inhibition of any residual glucuronidase activity), each sample was incubated for 2 h at 37°C to hydrolyze 7-HCS to 7-HC. Reactions were terminated with addition of 9 ml of ether/IAA, and the mixture was placed on a horizontal reciprocating shaker for 15 min. The organic phase, containing the 7-HC that had been formed through sulfatase-catalyzed hydrolysis of 7-HCS, was then removed, the solvent was evaporated, and the residue was dissolved in 3 ml of glycine/NaOH buffer (0.2 M, pH 10.3). The resulting amount of 7-HC was determined with fluorescence spectroscopy, as outlined above.
The formation of 7-HCS was normalized with respect to the total protein content of the liver slices in the incubation. After each tissue slice incubation, the tissue was homogenized in buffer A and its protein content, as well as that of a sample of the incubation medium, was determined by using the modified Lowry procedure (Bensadoun and Weinstein, 1976), with bovine serum albumin as the standard.
Determination of Cellular Viability in Liver Slices.
The effects of 7-HC, diamide, and TBHP on cellular viability were examined through measurement of LDH released into the medium. This was performed by using a standard kit for LDH analysis (Tox-7; Sigma-Aldrich), according to the manufacturer's instructions. The change in absorbance at 490 nm was quantified by using a SpectraMAX 190 plate reader (Molecular Devices, Sunnyvale, CA). LDH activities in both the medium and the tissue homogenate were determined, and values of LDH in the medium were normalized as proportions of the total contents of LDH found in the slices and in the incubation medium in the absence of 7-HC and oxidant. LDH release was determined both with and without a 2-h incubation of the tissue slices under the reaction conditions described above.
Sulfation of 7-HC by Purified rSULT1A1.
Recombinant rSULT1A1 was expressed in Escherichia coli BL21(DE3) cells as described previously (Chen et al., 1992). The extraction of rSULT1A1 from the cells and purification through PAP-agarose affinity chromatography were performed as described previously (Liu et al., 2009). Homogeneity of the resulting rSULT1A1 was confirmed through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gel) with Coomassie Brilliant Blue R-250 staining. The purified enzyme was stored at −70°C in pH 7.5 buffer containing 10 mM Tris-HCl, 0.25 M sucrose, 10% (v/v) glycerol, 1 mM dithiothreitol, 1 μM pepstatin A, and 3 μM antipain. Dithiothreitol and other small thiols were removed through pressure filtration dialysis at 4°C (Amicon PM10 membrane; Millipore, Billerica, MA) with the aforementioned buffer without dithiothreitol. The removal of dithiothreitol was verified with an assay for soluble thiols that used 5,5′-dithiobis(2-nitrobenzoic acid) (Jocelyn, 1987). The purified rSULT1A1 (2.5 μg) was preincubated for 1 h at 25°C with various concentrations of diamide or TBHP (0.01, 0.1, 1.0, 10, and 100 μM) in a total volume of 0.5 ml of buffer A. The concentrations of TBHP used were verified with a previously described assay for lipid hydroperoxides (Shertzer et al., 1992; Mihaljevic et al., 1996). A 0.4-ml aliquot of the enzyme (i.e., 2.0 μg) that had been pretreated with either diamide or TBHP was then incubated for 30 min at 37°C in a total volume of 0.5 ml of buffer A containing 10 μM 7-HC and 200 μM PAPS. Reactions were terminated with addition of 6 ml of ethyl ether/IAA (1:0.014), and the extraction mixture was then placed on a horizontal reciprocating shaker for 15 min. Determinations of the 7-HC amounts in the organic phase and the 7-HCS amounts in the aqueous phase were performed by using the analytical procedures described above for 7-HC and 7-HCS in the hepatic tissue slice incubations.
Determination of Intracellular GSH and GSSG Amounts.
After incubation of liver slices with 10 μM 7-HC and varying concentrations of diamide, an aliquot (0.3 ml) of each incubation medium was mixed with 0.3 ml of 5% (w/v) 5-sulfosalicylic acid. Individual hepatic slices were rapidly rinsed with buffer A, and both slices were immediately homogenized with 1.0 ml of 5% (w/v) 5-sulfosalicylic acid. The samples were then centrifuged to remove precipitating proteins, and the supernatant fractions were assayed for total GSH content, as described earlier (Anderson, 1985). The concentration of GSSG was determined through addition of 20 μl of a 1:1 mixture of 2-vinylpyridine and ethanol to 100 μl of the tissue homogenate and incubation for 2 h at room temperature before determination of the concentration of GSSG as described previously (Griffith, 1980). The sulfosalicylic acid-precipitated protein was resuspended in 0.1 N NaOH, and the protein concentration was determined by using a bicinchoninic acid assay kit (Thermo Scientific, Rockford, IL), with bovine serum albumin as the standard. All GSH determinations were normalized with respect to the protein content of the assay.
Statistical Analysis.
Incubations to determine the rate of 7-HCS formation and the contents of GSH and GSSG were conducted in triplicate. For studies with hepatic slices, the three determinations used samples derived from separate animals (two slices from each animal in each incubation mixture). Experiments on the viability of the hepatic slices were also carried out in this way. Data are presented as the mean ± S.E. of determinations from three rats. Results were compared with one-way analysis of variance followed by Bonferroni multiple-comparison analysis. A value of p < 0.05 was used to define statistical significance.
Results
Effects of Diamide and TBHP on Cellular Viability of Liver Slices.
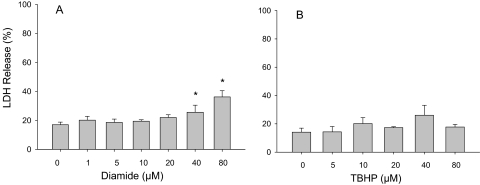
The viability of precision-cut hepatic slices under the conditions of incubation with 7-HC was assessed through determination of the release of LDH into the incubation medium. As shown in Fig. 1, release of LDH is reported as a proportion of the total LDH amounts determined in the incubation medium and the liver slices. Slices from the livers of three rats were incubated for 2 h at 37°C in the presence of 10 μM 7-HC, with and without addition of various concentrations of diamide ranging from 1 μM to 80 μM. At concentrations of diamide of up to 20 μM, there were no significant increases in the release of LDH, compared with the control group (Fig. 1A). In contrast, diamide at concentrations of 40 and 80 μM yielded decreased cellular viability, as indicated by increases in the release of LDH from the slices. When the hepatic slices were incubated with 10 μM 7-HC for 2 h at 37°C in the presence or absence of TBHP (concentrations up to 80 μM), no significant change in the release of LDH was observed.
Fig. 1.
Viability of tissue slices upon treatment with diamide or TBHP. The release of LDH from the tissue slices into the medium was determined after incubation at 37°C for 2 h in buffer A containing 10 μM 7-HC with various concentrations of diamide (A) or TBHP (B). The LDH activity released into the medium is expressed as a proportion of the total (medium plus tissue slice) LDH activity. Data are mean ± S.E. (n = 3). *, significant difference from control incubations without diamide or TBHP (p < 0.05).
Effects of Diamide on Sulfation of 7-HC Catalyzed by Purified rSULT1A1 and by Hepatic Slices.
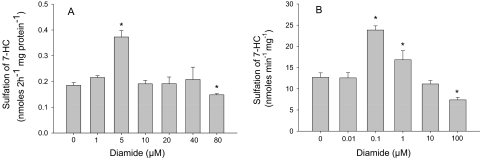
Upon incubation with hepatic slices from three rats, diamide at a concentration of 5 μM significantly increased the rate of sulfation of 7-HC (Fig. 2A). As the concentration of diamide was increased, the rate of sulfation catalyzed by the tissue slices returned to control values, with a significant decrease occurring at 80 μM. This effect of diamide on sulfation in hepatic slices was compared with the effect of this oxidant on homogeneous rSULT1A1 incubated with 7-HC under conditions identical to those used for the tissue slices. These experiments used homogeneous recombinant rSULT1A1 that had been subjected to removal of all small thiols and incubated for 1 h at 25°C with the indicated concentration of diamide, as described under “Materials and Methods.” After this pretreatment of rSULT1A1 with diamide, the enzyme was diluted into an assay containing 10 μM 7-HC and 200 μM PAPS in the same buffer (buffer A) as used for the tissue slice studies. After a 30-min incubation at 37°C, the 7-HCS formed was analyzed with the same method as used for the tissue slices. As shown in Fig. 2B, there were significant increases in the rate of sulfation of 7-HC catalyzed by the homogeneous rSULT1A1 after incubation with either 0.1 or 1.0 μM diamide, with a significant decrease in specific activity at 100 μM diamide.
Fig. 2.
Effects of diamide on the sulfation of 7-HC in hepatic slices and with purified rSULT1A1. A, hepatic slices were incubated for 2 h at 37°C in buffer A containing 10 μM 7-HC with the indicated concentrations of diamide, and aliquots of the medium were analyzed for 7-HCS as described under “Materials and Methods.” Data represent the mean ± S.E. of incubations of two tissue slices from each of three rats. B, purified rSULT1A1 enzyme was preincubated for 1 h at 25°C with the indicated concentration of diamide in a total volume of 0.5 ml of buffer A, pH 7.4. The diamide-pretreated enzyme was then used in an assay mixture containing 10 μM 7-HC and 200 μM PAPS in buffer A. The assay with purified rSULT1A1 was carried out at 37°C for 30 min. Data are the mean ± S.E. of three determinations. *, significant difference from control incubations (p < 0.05).
Effects of TBHP on Sulfation of 7-HC Catalyzed by Purified rSULT1A1 and by Hepatic Slices.
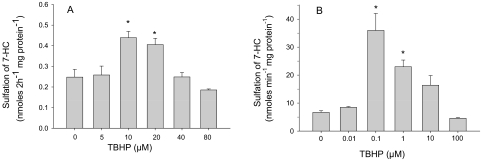
As was seen with diamide, treatment of either hepatic slices or purified rSULT1A1 with TBHP caused an increase in the rate of sulfation (Fig. 3), which then decreased with increasing concentrations of oxidant. When hepatic slices from three rats were treated with increasing concentrations of TBHP, the rate of sulfation of 10 μM 7-HC was significantly increased with either 10 or 20 μM TBHP in the incubation medium. In experiments using the same methods as described above for study of the effects of diamide on purified rSULT1A1, pretreatment of the enzyme with TBHP (instead of diamide), followed by dilution into an assay for sulfation of 10 μM 7-HC, indicated that pretreatment with 0.1 or 1.0 μM TBHP resulted in an increase in specific activity of the enzyme. Additional increases in the concentration of TBHP (i.e., use of either 10 or 100 μM) resulted in rates of sulfation that were not significantly different from the control values.
Fig. 3.
Effects of TBHP on the sulfation of 7-HC in hepatic slices and with purified rSULT1A1. A, hepatic slices were incubated for 2 h at 37°C in buffer A containing 10 μM 7-HC with the indicated concentrations of TBHP, and aliquots of the medium were analyzed for 7-HCS as described under “Materials and Methods.” Data represent the mean ± S.E. of incubations of two tissue slices from each of three rats. B, purified rSULT1A1 enzyme was preincubated for 1 h at 25°C with the indicated concentration of TBHP in a total volume of 0.5 ml of buffer A, pH 7.4. The TBHP-pretreated enzyme was then used in an assay mixture containing 10 μM 7-HC and 200 μM PAPS in buffer A. The assay with purified rSULT1A1 was carried out at 37°C for 30 min. Data are the mean ± S.E. of three determinations. *, significant difference from control incubations (p < 0.05).
Effects of Diamide and TBHP on Contents of GSH and GSSG in Hepatic Slices.
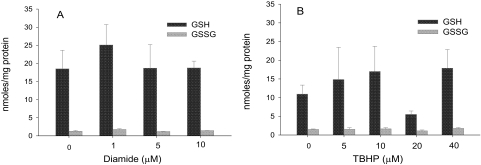
Because one consequence of treatment of hepatic tissue slices with either diamide or TBHP might be an alteration in the cellular GSH/GSSG ratio, we examined the effects of treatment with these oxidants on the concentrations of GSH and GSSG in the slices. Hepatic slices from three rats were challenged with varying concentrations of either diamide or TBHP, such that both the increase and decrease in sulfation seen with these oxidants were included. As shown in Fig. 4A, the concentrations of neither GSH nor GSSG in the hepatic slices deviated significantly from control values after treatment with diamide at concentrations of 1.0, 5, and 10 μM. Although pronounced interindividual variability in the tissue concentrations of GSH was observed after treatment with TBHP (Fig. 4B), these differences were not statistically significant. Moreover, there was no statistically significant alteration in the overall tissue concentration of GSSG with these concentrations of TBHP (Fig. 4B).
Fig. 4.
Measurement of GSH and GSSG levels after treatment of hepatic slices with diamide or TBHP. Liver slices were incubated for 2 h with 10 μM 7-HC and the indicated concentrations of either diamide (A) or TBHP (B). Concentrations of GSH and GSSG in the slices were determined as described under “Materials and Methods.” Results are the mean ± S.E. of three independent determinations.
Discussion
The oxidation of key cysteine residues in SULTs provides a potential mechanism for reversible regulation of the specificity and rate of catalysis for these enzymes. Although this was first described for purified rSULT1A1 (Marshall et al., 1997), investigations of cytosolic and purified preparations of human estrogen sulfotransferase, hSULT1E1, have indicated that the catalytic function of this SULT also can be altered by treatment with GSSG (Maiti et al., 2007). In addition, studies with rats continuously exposed to hyperoxic conditions (i.e., >95% oxygen) showed that there was an increase in the rate of sulfation of 2-naphthol catalyzed by subsequently isolated hepatic and lung cytosolic fractions (Maiti et al., 2005). Although previous experiments with both cytosolic and purified preparations of SULTs indicated that oxidation of key cysteines in these enzymes could regulate catalytic activity, direct comparison of the effects of specific thiol oxidants on a homogeneous SULT with the effects of the same oxidants on the enzyme present in an intact cellular environment had not been explored previously.
The major family 1 sulfotransferase in the livers of male Sprague-Dawley rats, rSULT1A1, represents a useful model SULT for comparison of mechanistic studies with the purified enzyme with effects seen during modification of the enzyme in hepatic tissue. The formation of disulfide bonds in rSULT1A1 affects the specificity and pH optimum of the enzyme for some substrates (Marshall et al., 1997, 2000). Detailed studies on the mechanism of these effects showed that the rate-determining step of the reaction, namely, breakdown of a nonproductive enzyme-PAP-phenol complex, is regulated by the formation of a GSH-Cys66 mixed disulfide and the Cys66-Cys232 disulfide (Marshall et al., 2000; Duffel et al., 2001). Previously published homology modeling of rSULT1A1 (Duffel et al., 2001) showed that the formation of either a Cys66-GSH adduct or a Cys66-Cys232 intramolecular protein disulfide alters the conformation of the PAPS/PAP binding site, but formation of a Cys232-GSH adduct does not (Marshall et al., 1997). The formation of these disulfide bonds involving Cys66 destabilizes the inhibitory, dead-end, ternary complex and increases the rate of reaction. However, further alteration of the structure of the enzyme through cysteine oxidation results in a decrease in reaction velocity (Marshall et al., 1997, 2000; Duffel et al., 2001). It is also important to note in this context that previous mechanistic studies showed that the structure of the phenolic substrate is important in the formation of the dead-end enzyme-PAP-phenol complex (Marshall et al., 2000). Therefore, the extent to which the rate of reaction would be accelerated by disulfide bond formation involving Cys66 also would depend on the structure of the substrate, and this would become an important component in evaluation of the effects of oxidative regulation on the sulfation of individual drugs or other xenobiotics.
To compare the oxidative modification of purified rSULT1A1 with the enzyme in hepatic tissue slices, we chose two oxidants with different structural characteristics (Fig. 5) and different mechanisms of action. Diamide acts through formation of a protein-protein internal disulfide bond after initial formation of a cysteine-substituted hydrazinedicarboxamide intermediate that rapidly reacts with a nearby cysteine (Kosower et al., 1969; Harris, 1979). In the presence of GSH, there would be the possibility of formation of an S-glutathionylated protein, but disulfide interchange then would likely also yield an intramolecular protein disulfide bond. In contrast, alkyl hydroperoxides such as TBHP act through formation of protein sulfenic acid intermediates that can react with GSH to form an S-glutathionylated protein, react with another protein cysteine to form a protein-protein disulfide bond, or react with an amine to form a sulfanylamide (Kettenhofen and Wood, 2010; Roos and Messens, 2011). Our results showed that, regardless of the mechanism for cysteine oxidation, there were similar effects on sulfation of 7-HC in precision-cut hepatic slices and with the homogeneous rSULT1A1. As expected from the increased potential for alternate sites of interaction in tissue slices, as opposed to the purified enzyme, increased concentrations of oxidant were needed for observation of the effect in the tissue slices, relative to that required with the purified enzyme.
Fig. 5.
Structures of oxidants used in these studies. The chemical structures of diamide and TBHP are shown.
Because earlier studies observed the oxidation of purified SULTs with GSSG, we investigated the potential that the effects we observed in tissue slices might be attributable to oxidation of soluble GSH to GSSG, with changes in the cellular GSSG/GSH ratio. Subsequent disulfide exchange reactions might then result in formation of S-glutathionyl protein or protein-protein disulfide bonds. However, the cellular concentrations of GSSG and GSH did not change significantly after treatment of the hepatic slices with the concentrations of diamide and TBHP used in these experiments. Therefore, it is likely that the direct effects of these oxidants seen with the purified enzyme preparation occur within the intact tissues slices as well. However, it should be noted that these specific experiments with hepatic slices would not strictly rule out the possibility of transient localized alterations in the GSSG/GSH ratio, with subsequent disulfide exchange. This did not occur with the purified rSULT1A1, because no GSH was present in those incubations. The changes in the catalytic function of rSULT1A1 also did not correlate with the release of LDH into the medium at concentrations of diamide of up to 20 μM and concentrations of TBHP of up to 80 μM. At the highest concentration of diamide used (i.e., 80 μM), however, it is possible that altered cellular viability, as indicated by LDH release, might have contributed to the lower rate of sulfation observed in tissue slices.
Modifications of proteins through formation of intramolecular protein-protein disulfides, S-glutathionylated proteins, and other oxidation products of cysteine are receiving increased attention because of their potential roles in cellular responses to alterations in redox status, and several reviews highlighted many of those studies (Janssen-Heininger et al., 2008; Dalle-Donne et al., 2009; Jones and Go, 2010). The results presented in this article provide a direct correlation between in vitro mechanistic studies on a purified sulfotransferase and effects seen after treatment of the enzyme with the same oxidant and substrate in viable tissue slices. Therefore, this supplies an important link between studies with the purified sulfotransferase and events that occur within intact cells and tissues. These findings also point to the importance of examination of varied cellular concentrations of the oxidant in observations of these effects, a point that is of key importance in the interpretation of studies that might otherwise be performed with higher concentrations of thiol oxidants.
Although the extent to which oxidative stress attributable to metabolism of other xenobiotics or to disease processes within cells might alter the catalytic function of SULTs through oxidative modification remains to be determined, our results point to the need for further examination of the role that such events may have in the regulation of SULTs. Such studies also would be critical for determining the effects of these oxidative events on in vivo metabolic pathways for specific drugs and other xenobiotics for which sulfation is important in either metabolic activation or detoxication.
Acknowledgments
We appreciate the expert assistance of Dr. Michael McCormick of the Radiation and Free Radical Research Core, Holden Comprehensive Cancer Center, University of Iowa, in the determination of GSH and GSSG concentrations in hepatic tissue slices.
This work was supported by the National Institutes of Health National Cancer Institute [Grant R01-CA038683].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- SULT
- sulfotransferase
- PAP
- adenosine 3′,5′-diphosphate
- PAPS
- adenosine 3′-phosphate, 5′-phosphosulfate
- 7-HC
- 7-hydroxycoumarin
- 7-HCS
- 7-hydroxycoumarin sulfate
- LDH
- lactate dehydrogenase
- TBHP
- tert-butyl hydroperoxide
- IAA
- isoamyl alcohol.
Authorship Contributions
Participated in research design: Dammanahalli and Duffel.
Conducted experiments: Dammanahalli.
Performed data analysis: Dammanahalli and Duffel.
Wrote or contributed to the writing of the manuscript: Dammanahalli and Duffel.
References
- Alnouti Y. (2009) Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci 108:225–246 [DOI] [PubMed] [Google Scholar]
- Anderson ME. (1985) Determination of glutathione in biological tissues, in Handbook of Methods for Oxygen Radical Research (Greenwald RA. ed) pp 317–323, CRC Press, Boca Raton [Google Scholar]
- Barr J, Weir AJ, Brendel K, Sipes IG. (1991) Liver slices in dynamic organ culture. I. An alternative in vitro technique for the study of rat hepatic drug metabolism. Xenobiotica 21:331–339 [DOI] [PubMed] [Google Scholar]
- Bensadoun A, Weinstein D. (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70:241–250 [DOI] [PubMed] [Google Scholar]
- Blanchard RL, Freimuth RR, Buck J, Weinshilboum RM, Coughtrie MW. (2004) A proposed nomenclature system for the cytosolic sulfotransferase (SULT) superfamily. Pharmacogenetics 14:199–211 [DOI] [PubMed] [Google Scholar]
- Chapman E, Best MD, Hanson SR, Wong CH. (2004) Sulfotransferases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew Chem Int Ed Engl 43:3526–3548 [DOI] [PubMed] [Google Scholar]
- Chapman E, Bryan MC, Wong CH. (2003) Mechanistic studies of β-arylsulfotransferase IV. Proc Natl Acad Sci USA 100:910–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang YS, Zheng Y, Martin BM, Duffel MW, Jakoby WB. (1992) Tyrosine-ester sulfotransferase from rat liver: bacterial expression and identification. Protein Expr Purif 3:421–426 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. (2009) Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci 34:85–96 [DOI] [PubMed] [Google Scholar]
- Duffel MW. (2010) Sulfotransferases, in Comprehensive Toxicology, Vol 4: Biotransformation (McQueen CA. ed), pp 367–384, Elsevier, Oxford [Google Scholar]
- Duffel MW, Jakoby WB. (1981) On the mechanism of aryl sulfotransferase. J Biol Chem 256:11123–11127 [PubMed] [Google Scholar]
- Duffel MW, Marshal AD, McPhie P, Sharma V, Jakoby WB. (2001) Enzymatic aspects of the phenol (aryl) sulfotransferases. Drug Metab Rev 33:369–395 [DOI] [PubMed] [Google Scholar]
- Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, McManus ME. (2006) Human sulfotransferases and their role in chemical metabolism. Toxicol Sci 90:5–22 [DOI] [PubMed] [Google Scholar]
- Glatt H. (2000) Sulfotransferases in the bioactivation of xenobiotics. Chem Biol Interact 129:141–170 [DOI] [PubMed] [Google Scholar]
- Griffith OW. (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212 [DOI] [PubMed] [Google Scholar]
- Harris JW. (1979) Mammalian cell studies with diamide. Pharmacol Ther 7:375–391 [DOI] [PubMed] [Google Scholar]
- Jakoby WB, Ziegler DM. (1990) The enzymes of detoxication. J Biol Chem 265:20715–20718 [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. (2008) Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocelyn PC. (1987) Spectrophotometric assay of thiols. Methods Enzymol 143:44–67 [DOI] [PubMed] [Google Scholar]
- Jones DP, Go YM. (2010) Redox compartmentalization and cellular stress. Diabetes Obes Metab 12 (Suppl 2):116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenhofen NJ, Wood MJ. (2010) Formation, reactivity, and detection of protein sulfenic acids. Chem Res Toxicol 23:1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower NS, Kosower EM, Wertheim B, Correa WS. (1969) Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun 37:593–596 [DOI] [PubMed] [Google Scholar]
- Liu Y, Smart JT, Song Y, Lehmler HJ, Robertson LW, Duffel MW. (2009) Structure-activity relationships for hydroxylated polychlorinated biphenyls as substrates and inhibitors of rat sulfotransferases and modification of these relationships by changes in thiol status. Drug Metab Dispos 37:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti S, Dutta SM, Baker SM, Zhang J, Narasaraju T, Liu L, Chen G. (2005) In vivo and in vitro oxidative regulation of rat aryl sulfotransferase IV (AST IV). J Biochem Mol Toxicol 19:109–118 [DOI] [PubMed] [Google Scholar]
- Maiti S, Zhang J, Chen G. (2007) Redox regulation of human estrogen sulfotransferase (hSULT1E1). Biochem Pharmacol 73:1474–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AD, Darbyshire JF, Hunter AP, McPhie P, Jakoby WB. (1997) Control of activity through oxidative modification at the conserved residue Cys66 of aryl sulfotransferase IV. J Biol Chem 272:9153–9160 [DOI] [PubMed] [Google Scholar]
- Marshall AD, Darbyshire JF, McPhie P, Jakoby WB. (1998) A review of the effects of manipulation of the cysteine residues of rat aryl sulfotransferase IV. Chem-Biol Interact 109:107–116 [DOI] [PubMed] [Google Scholar]
- Marshall AD, McPhie P, Jakoby WB. (2000) Redox control of aryl sulfotransferase specificity. Arch Biochem Biophys 382:95–104 [DOI] [PubMed] [Google Scholar]
- Mihaljević B, Katusin-Razem B, Razem D. (1996) The reevaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanistic aspects of the response. Free Radic Biol Med 21:53–63 [DOI] [PubMed] [Google Scholar]
- Negishi M, Pedersen LG, Petrotchenko E, Shevtsov S, Gorokhov A, Kakuta Y, Pedersen LC. (2001) Structure and function of sulfotransferases. Arch Biochem Biophys. 390:149–157 [DOI] [PubMed] [Google Scholar]
- Roos G, Messens J. (2011) Protein sulfenic acid formation: from cellular damage to redox regulation. Free Radic Biol Med 51:314–326 [DOI] [PubMed] [Google Scholar]
- Sekura RD. (1981) Adenosine 3′-phosphate 5′-phosphosulfate. Methods Enzymol 77:413–415 [Google Scholar]
- Shertzer HG, Bannenberg GL, Moldéus P. (1992) Evaluation of iron binding and peroxide-mediated toxicity in rat hepatocytes. Biochem Pharmacol 44:1367–1373 [DOI] [PubMed] [Google Scholar]
- Thohan S, Zurich MC, Chung H, Weiner M, Kane AS, Rosen GM. (2001) Tissue slices revisited: evaluation and development of a short-term incubation for integrated drug metabolism. Drug Metab Dispos 29:1337–1342 [PubMed] [Google Scholar]