Abstract
N-Methyl-d-aspartate (NMDA) receptor (NMDAR) hypofunction has been postulated to contribute to the cognitive deficit of schizophrenia. In this study, we examined the effect of lurasidone (Latuda; Dainippon Sumitomo Pharma Co. Ltd., Tokyo, Japan), a newly approved atypical antipsychotic drug (APD), on NMDAR synaptic function in rat frontal cortical pyramidal neurons. In vivo administration of lurasidone produced a significant and selective enhancement of NMDAR-mediated synaptic responses and surface expression of NR2A and NR2B subunits. Lurasidone has high affinity for serotonin 5-HT1A, 5-HT2A, and 5-HT7 receptors and dopamine D2 receptors. In vivo administration of the 5-HT7 receptor antagonist (2R)-1-[(3-hydroxyphenyl)sulfonyl]-2 -(2-(4-methyl-1-piperidinyl)ethyl)pyrrolidine (SB-269970) mimicked the enhancing effect of lurasidone on NMDAR responses, whereas the D2 receptor antagonist haloperidol failed to do so. Previous studies have found that short-term administration of lurasidone reverses the cognitive impairment induced by subchronic administration of phencyclidine (PCP), an NMDAR noncompetitive antagonist. In this study, we found that lurasidone, as well as the prototypical atypical APD clozapine, restored NMDAR-mediated synaptic responses to normal levels in the PCP model of schizophrenia. These results suggest that NMDAR is the potential key molecular target of lurasidone, possibility via antagonizing 5-HT7 receptors, which is consistent with evidence that 5-HT7 receptor antagonism contributes to cognitive enhancement by atypical APDs in patients with schizophrenia.
Introduction
Schizophrenia is characterized by positive symptoms (delusions and hallucinations), negative symptoms (e.g., affective flattening, anhedonia, anergia), abnormalities in mood, and deficits in multiple domains of cognition, including working memory, declarative memory, and executive function, often leading to severe functional impairment from the time of diagnosis (Meltzer et al., 1989; Sawa and Snyder, 2002). Abnormalities in prefrontal cortex (PFC) and temporal cortex are considered to be the most likely basis for the cognitive impairment of schizophrenia (Weinberger et al., 1986). Hypofunction of glutamatergic pyramidal neurons in cortex and hypodopaminergic activity are believed to underlie the cognitive deficit of schizophrenia (Lewis and Lieberman, 2000; Tsai and Coyle, 2002). The evidence for the hypoglutamatergic theory includes the ability of noncompetitive NMDA receptor (NMDAR) antagonists, such as phencyclidine (PCP), 5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine maleate) (MK-801), and ketamine, to produce behavioral symptoms and cognitive dysfunction that have some similarity to schizophrenia in healthy volunteers and to exacerbate positive and negative symptoms in schizophrenia (Javitt and Zukin, 1991). Short-term or subchronic administration of NMDAR antagonists also increases locomotor activity and disrupts prepulse inhibition in rodents, both of which are thought to model schizophrenia symptoms (Jentsch et al., 1997; Jentsch and Roth, 1999). Furthermore, mice with genetic knockdown of the NMDAR subunit NR1, as well as other rodent models in which specific glutamate receptor subtypes are genetically altered, also have phenotypes suggestive of schizophrenia, including increased locomotor activity, stereotypy, and deficits in cognitive and social function (Mohn et al., 1999).
Typical antipsychotic drugs (APDs; e.g., haloperidol and perphenazine) are believed to diminish positive symptoms in patients with schizophrenia through blockade of limbic dopamine D2 receptors (Creese et al., 1976; Sawa and Snyder, 2002), but blockade of D2 receptors in the dorsal striatum produce unwanted extrapyramidal side effects (Meltzer, 1992). Clozapine, the prototypical atypical APD, as well as many other atypical APDs, are more potent serotonin 5-HT2A than dopamine D2 receptor antagonists, which has been suggested to be the basis for some of their advantages over typical APDs, including low extrapyramidal side effects (Meltzer et al., 1989; Meltzer and Huang, 2008). Actions at adrenergic and muscarinic receptors may also contribute to the efficacy of various atypical APDs (Meltzer et al., 1989). Thus, clozapine and related atypical APDs have been referred to as multireceptor antagonists to reflect the contribution of receptors other than 5-HT2A and D2 receptors to their efficacy and side effects (Meltzer and Huang, 2008).
Lurasidone (Latuda; Dainippon Sumitomo Pharma Co. Ltd., Tokyo, Japan) is a novel atypical APD recently approved for treatment of schizophrenia by the U.S. Food and Drug Administration. Lurasidone has potent binding affinities for 5-HT2A, 5-HT7, 5-HT1A, D2, and noradrenaline α2C receptors (Ishibashi et al., 2010). Clinical trials have shown that lurasidone is a safe and effective treatment for patients with schizophrenia and has minimal extrapyramidal, cardiovascular, and metabolic complications (Nakamura et al., 2009; Citrome, 2011; Meltzer et al., 2011a). Lurasidone has been reported to improve short-term MK-801-induced memory impairment in rats (Ishiyama et al., 2007; Enomoto et al., 2008), as well as subchronic PCP-induced impairment in novel object recognition (NOR) (Horiguchi et al., 2011; Meltzer et al., 2011b). The molecular mechanism for the atypical APDs to improve cognition is not fully known. We sought to test the hypothesis that lurasidone and clozapine may reverse NMDAR hypofunction via their 5-HT7 receptor antagonism.
Materials and Methods
Animals.
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of the State University of New York at Buffalo. Sprague-Dawley (3–4-week-old) rats were used in all experiments. Animals were injected with lurasidone (0.1 mg/kg s.c.), clozapine (5 mg/kg s.c.), (2R)-1-[(3-hydroxyphenyl)sulfonyl]-2 -(2-(4-methyl-1-piperidinyl)ethyl)pyrrolidine (SB-269970) (1 mg/kg i.p.), or haloperidol (0.1 mg/kg i.p.). One hour later, animals were anesthetized by halothane inhalation (Sigma-Aldrich, St. Louis, MO) for ∼30 s and decapitated quickly. In some experiments, PCP (Sigma) was administered (5 mg/kg i.p.) once daily for 7 days (Wang et al., 2006). One day after the last administration of PCP, animals were injected with lurasidone (0.1 mg/kg s.c.) or clozapine (5 mg/kg s.c.) and sacrificed 1 h later.
Slice Preparation.
Brains were removed, iced, and blocked, and coronal slices (300 μm) containing prelimbic/infralimbic regions were obtained with a Vibratome (VP1000S; Leica, Wetzlar, Germany) in the presence of a low-Ca2+ HEPES-buffered salt solution (140 mM sodium isethionate, 2 mM KCl, 4 mM MgCl2, 0.1 mM CaCl2, 23 mM glucose, and 15 mM HEPES, pH 7.4; 300–305 mOsM). These slices were then incubated for 1 to 5 h at room temperature (20–22°C) in a NaHCO3-buffered saline bubbled with 95% O2/5% CO2.
Electrophysiological Recordings.
To record evoked synaptic currents in prefrontal cortical slices, the whole-cell voltage-clamp technique was used as described previously (Yuen et al., 2005, 2009). The internal solution contained 130 mM cesium methanesulfonate, 10 mM CsCl, 4 mM NaCl, 1 mM MgCl2, 10 mM HEPES, 5 mM EGTA, 2.2 mM QX-314, 12 mM phosphocreatine, 5 mM MgATP, and 0.5 mM Na2GTP, pH 7.2–7.3; 265 to 270 mOsM. Slices were perfused with ACSF (130 mM NaCl, 26 mM NaHCO3, 3 mM KCl, 5 mM MgCl2, 1.25 NaH2PO4, 1 mM CaCl2, and 10 mM glucose, pH 7.4; 300 mOsM) bubbled with 95% O2/5% CO2. 6-Cyano-2,3-dihydroxy-7-nitroquinoxaline (25 μM) and bicuculline (10 μM) were added when NMDAR-excitatory postsynaptic currents (EPSCs) were recorded, whereas d-2-amino-5-phosphonovalerate (25 μM) and bicuculline (10 μM) were added when AMPAR-EPSCs were measured. Recordings were conducted at room temperature. Neurons were visualized with a 40× water-immersion lens and illuminated with near-IR light. All recordings were performed using a Multiclamp 700A amplifier and digitized with Digidata 1322A (Molecular Devices, Sunnyvale, CA). Tight seals (2–10 GΩ) were generated by applying negative pressure, followed by additional suction to disrupt the membrane and obtain the whole-cell configuration. The access resistance ranged from 8 to 15 MΩ. Evoked currents were generated with a 50-μs pulse from a stimulation isolation unit controlled by a pulse generator (S48; Astro-Med, Inc., West Warwick, RI). A bipolar stimulating electrode (FHC, Inc., Bowdoinham, ME) was positioned ∼100 μm from the neuron under study. The same stimulation intensity was used in individual neurons across groups with various drug treatments, similar to what was described previously (Yuen et al., 2009). For NMDAR-EPSC recording, cells (voltage-clamped at −70 mV) were depolarized to +60 mV for 3 s before stimulation to fully relieve the voltage-dependent Mg2+ block of NMDAR channels. For AMPAR-EPSC recording, cells were constantly held at −70 mV. When miniature EPSC (mEPSC) was recorded, tetrodotoxin (1 μM) was added to ACSF. The capacitance of the recorded neurons ranged from 80 to 120 pF. Signals were acquired at a bandwidth of 20 KHz (EPSC) or 2 KHz (mEPSC) and filtered with a 2-KHz low-pass Bessel filter.
To minimize experimental variations between cells, the following criteria were used: 1) layer V medial prefrontal cortex pyramidal neurons with comparable membrane capacitances were selected; 2) the stimulating electrode was positioned at the same location (layer VI, ∼100 μm horizontally) from the recording neuron, and the electrode tip was cleaned after every recording to allow precise stimulation capacity; and 3) recordings from animals injected with different drugs were interleaved throughout the course of experiments.
Data analyses were performed with Clampfit (Molecular Devices), Mini Analysis (Synaptosoft, Decatur, GA) and Kaleidagraph (Synergy Software, Reading, PA). Rise time was measured from 10 to 90% of peak amplitude. No correlation between EPSC amplitude and rise time was found. Because the distribution of EPSC amplitudes in different animals was often found to be non-normal, statistical analysis was performed with Kruskal-Wallis test. For comparisons of data (two groups) with normal distributions, Student's t test was used.
Biochemical Measurement of Surface and Total Proteins.
The surface AMPA and NMDA receptors were detected as described previously (Yuen et al., 2009). In brief, PFC slices were incubated with ACSF containing 1 mg/ml sulfosuccinimidyl-6-(biotinamido) hexanoate (Thermo Fisher Scientific, Waltham, MA) for 20 min on ice. The slices were then rinsed three times in Tris-buffered saline to quench the biotin reaction, followed by homogenization in modified radioimmunoprecipitation assay buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mM NaPO4, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1 mg/ml leupeptin). The homogenates were centrifuged at 14,000g for 15 min at 4°C, incubated with 50% NeutrAvidin Agarose (Thermo Fisher Scientific) for 2 h at 4°C, and bound proteins were resuspended in SDS sample buffer and boiled. Quantitative Western blots were performed on both total and biotinylated (surface) proteins using antibodies against NR2A (1:500; Millipore, Billerica, MA), NR2B (1:500; Millipore), NR1 (1:500; Cell Signaling Technology, Danvers, MA), GluR1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), GluR2 (1:500; Millipore, Billerica, MA), and actin (1:1000; Santa Cruz Biotechnology).
Results
In Vivo Administration of Lurasidone Induces a Significant Enhancement of NMDAR-Mediated Synaptic Responses.
To examine whether the NMDAR is a target of lurasidone and clozapine, we measured NMDAR-mediated EPSCs in animals injected with either saline, lurasidone (0.1 mg/kg s.c.), or clozapine (5 mg/kg s.c.). As shown in Fig. 1A, mPFC pyramidal neurons from lurasidone- or clozapine-injected rats showed significantly increased NMDAR-EPSC amplitudes, compared with those from saline-injected rats (saline, 112 ± 5.9 pA, n = 12; lurasidone, 238 ± 15.1 pA, n = 13; clozapine: 206 ± 19.8 pA, n = 9; p < 0.001; Kruskal-Wallis test). The amplitudes of NMDAR-EPSC induced by a series of stimulus intensities, as shown in the input/output curves of NMDAR-EPSC, also revealed a significant increase in lurasidone-injected rats (Fig. 1B, 45–77% increase; lurasidone, n = 9; saline, n = 6; p < 0.001, t test). Lurasidone administration did not cause significant changes on the NMDAR-EPSC kinetics (decay time constant; saline, 150 ± 10.1 ms, n = 7; lurasidone, 160 ± 14.9 ms; n = 7; p > 0.05, t test) or the rise time (saline, 7.83 ± 0.23 ms, n = 18; lurasidone, 7.79 ± 0.22 ms, n = 18; p > 0.05, t test). The sensitivity to the NR2B antagonist ifenprodil (5 μM) was also unchanged by lurasidone (Fig. 1, C and D; saline, 32 ± 2.0% reduction, n = 5; lurasidone, 31 ± 2.6% reduction, n = 5; p > 0.05, t test).
Fig. 1.
In vivo administration of lurasidone induces a significant enhancement of NMDAR-EPSC. A, dot plots showing the amplitude of NMDAR-EPSC in PFC pyramidal neurons from rats injected with saline, lurasidone (0.1 mg/kg s.c.), or clozapine (5 mg/kg s.c.). Inset, representative NMDAR-EPSC. Scale bar, 50 pA, 100 ms. B, summarized input-output curves of NMDAR-EPSC in response to a series of stimulation intensity in saline- versus lurasidone-injected rats. *, p < 0.001, Kruskal-Wallis test. C, plot of normalized NMDAR-EPSC showing the effect of ifenprodil (an NR2B antagonist, 5 μM) in PFC neurons from saline- versus lurasidone-injected rats. D, bar graphs summarizing the percentage reduction of NMDAR-EPSC amplitude by ifenprodil in saline- versus lurasidone-injected rats.
In contrast to the significance enhancement of NMDAR-EPSC, AMPAR-mediated excitatory postsynaptic currents (AMPAR-EPSC) were not responsive to either lurasidone or clozapine (Fig. 2A, saline: 146 ± 9.2 pA, n = 12; lurasidone, 144 ± 12.0 pA, n = 10; clozapine, 161 ± 16.7 pA, n = 11; p > 0.05; Kruskal-Wallis test). The input/output curves of AMPAR-EPSC amplitude also showed no change in lurasidone- versus saline-injected rats (Fig. 2B, <5% increase; lurasidone, n = 9; saline, n = 7; p > 0.05, t test). The rise time of AMPAR-EPSC was also unchanged by lurasidone administration (saline, 4.59 ± 0.21 ms, n = 19; lurasidone, 4.52 ± 0.22 ms, n = 20; p > 0.05, t test). The slow EPSC rise time was consistent with previous results recorded in CA1 pyramidal neurons (Xia et al., 2005), which may reflect asynchrony of release rather than heavy filtering or compromised degree of voltage control. Furthermore, mEPSC, an AMPAR-mediated synaptic response resulting from quantal release of single glutamate vesicles, was unchanged by lurasidone administration (Fig. 2, C and D; saline, 24 ± 0.8 pA, 2.1 ± 0.2 Hz, n = 7; lurasidone, 25 ± 0.7 pA, 2.0 ± 0.2 Hz, n = 6; p > 0.05, t test). No change was found on the mEPSC rise time either (saline, 1.52 ± 0.09 ms, n = 5; lurasidone, 1.44 ± 0.05 ms, n = 6; p > 0.05, t test). The lack of changes in evoked or miniature AMPAR-EPSC ruled out the possibility of changes in presynaptic glutamate release by these APDs. The ratio of NMDAR-EPSC to AMPAR-EPSC was significantly higher in individual neurons from lurasidone-injected rats than those from saline-injected rats (Fig. 2E; saline, 1.0 ± 0.1, n = 15; lurasidone, 1.7 ± 0.1, n = 13; p < 0.01, t test). Taken together, our results suggest that in vivo administration of lurasidone or clozapine selectively enhances postsynaptic NMDAR function.
Fig. 2.
In vivo administration of lurasidone does not alter AMPAR-EPSC. A, dot plots showing the amplitude of AMPAR-EPSC in PFC pyramidal neurons from rats injected with saline, lurasidone (0.1 mg/kg s.c.) or clozapine (5 mg/kg s.c.). Inset, representative AMPAR-EPSC traces. Scale bar, 50 pA, 20 ms. B, summarized input-output curves of AMPAR-EPSC in response to a series of stimulation intensity in saline- versus lurasidone-injected rats. C, cumulative plot of mEPSC amplitudes in PFC neurons from saline- versus lurasidone-injected rats. Inset, representative mEPSC traces. Scale bars, 10 pA, 5 s. D, bar graphs showing the mEPSC amplitude and frequency in PFC neurons from saline- versus lurasidone-injected rats. E, bar graphs showing the NMDAR-EPSC/AMPAR-EPSC ratio in PFC neurons from saline- versus lurasidone-injected rats. *, p < 0.01, t test. Inset, representative NMDAR-EPSC and AMPAR-EPSC traces recorded in the same neurons. Scale bars, 50 pA, 100 ms (NMDA), 20 ms (AMPA).
In Vivo Administration of Lurasidone Induces a Significant Enhancement of the Surface Expression of NMDAR Subunits.
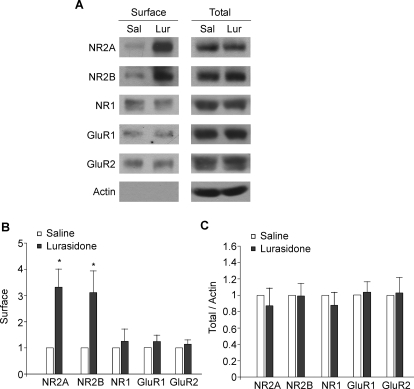
Next we examined the potential mechanism underlying the enhancement of NMDAR-EPSC by lurasidone. One possibility is increased surface delivery of NMDA receptors after administration of this antipsychotic drug. To test it, we performed surface biotinylation and Western blotting experiments to detect the surface and total levels of NMDAR and AMPAR subunits. Animals received injections of saline or lurasidone (0.1 mg/kg s.c.). One hour later, animals were sacrificed and brains were sliced. After 1 h of recovery, cortical slices were harvested for the biochemical assay. As shown in Fig. 3, A–C, lurasidone-injected rats showed a significant increase in surface NR2A and NR2B subunits of NMDA receptors compared with saline-injected rats (surface NR2A, 3.3 ± 0.7-fold of control, n = 7 pairs; surface NR2B, 3.1 ± 0.8-fold of control, n = 5 pairs; p < 0.05, t test). No significant increase was found in surface NR1 subunits (1.2 ± 0.5-fold of control, n = 6 pairs; p > 0.05, t test). The level of surface GluR1 and GluR2 subunits of AMPA receptors was also unchanged by lurasidone (surface GluR1, 1.2 ± 0.2-fold of control, n = 8 pairs; surface GluR2, 1.1 ± 0.2-fold of control, n = 4 pairs; p > 0.05, t test). The total level of these receptor subunits remained similar in saline- versus lurasidone-injected animals (total NR2A/2B/1, 0.9 ± 0.2-fold of control, n = 7 pairs; total GluR1/2, 1.0 ± 0.1-fold of control, n = 6 pairs), which rules out the possibility of new glutamate receptor synthesis. These results suggest that in vivo lurasidone administration selectively increases the surface level of NMDAR NR2 subunits, which may account for the potentiation of NMDAR-mediated synaptic responses in frontal cortex.
Fig. 3.
In vivo administration of lurasidone significantly increases the surface expression of NMDA receptor NR2 subunits. A to C, immunoblots and quantification analysis of the surface and total AMPAR and NMDAR subunits in cortical slices from saline- versus lurasidone-injected rats. *, p < 0.05 t test.
Lurasidone Increases NMDAR Function through 5-HT7 Receptor Antagonism.
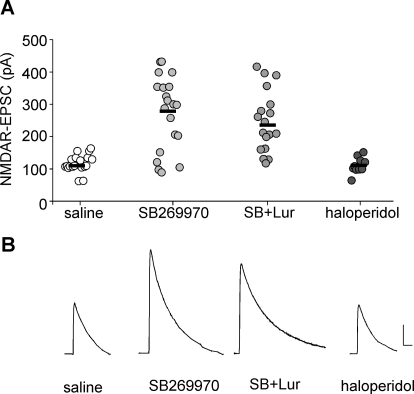
We next examined potential receptors underlying the enhancement by lurasidone of NMDAR-EPSC. In vitro functional assays demonstrate that lurasidone acts as an antagonist at D2 and 5-HT7 receptors and as a partial agonist at the 5-HT1A receptor (Ishibashi et al., 2010). Our previous studies have found that a 5-HT1A agonist decreases NMDAR-EPSC (Yuen et al., 2005), whereas a 5-HT2A agonist or antagonist alone had no effect on NMDAR-EPSC (Yuen et al., 2008). Thus, we focused on the role of 5-HT7 and D2 receptors in the effect of lurasidone on NMDAR-EPSC. As shown in Fig. 4, in vivo administration of the selective 5-HT7 antagonist SB-269970 (1 mg/kg i.p.; Gasbarri et al., 2008) produced significantly increased NMDAR-EPSC (saline, 111 ± 6.2 pA, n = 19; SB-269970: 274 ± 25.5 pA, n = 20; p < 0.001, Kruskal-Wallis test), mimicking the effects of clozapine and lurasidone. Note that a subset of neurons (5 of 20) had no response to the 5-HT7 antagonist, consistent with the previous report that 5-HT7 is not expressed in every mature PFC pyramidal neuron (Béïque et al., 2004). Moreover, coinjection of lurasidone (0.1 mg/kg) plus SB-269970 (1 mg/kg) did not produce an additive enhancement of NMDAR-EPSC (231 ± 32.6 pA, n = 18) at these doses. On the other hand, in vivo administration of the selective D2 antagonist haloperidol (0.1 mg/kg i.p.) failed to change NMDAR-EPSC (112 ± 7.9 pA, n = 10). These results suggest that 5-HT7 antagonism may underlie the lurasidone-induced enhancement of NMDAR function.
Fig. 4.
The enhancing effect of lurasidone on NMDAR-EPSC is mimicked by antagonizing 5-HT7 receptors. A, dot plot showing the amplitude of NMDAR-EPSC in PFC pyramidal neurons from animals injected with saline, the 5-HT7 antagonist SB-269970 (1 mg/kg i.p.), lurasidone (0.1 mg/kg s.c.), plus SB-269970 or the D2 antagonist haloperidol (0.1 mg/kg i.p.). B, representative NMDAR-EPSC traces in rats injected with different agents. Scale bar, 50 pA, 100 ms.
Lurasidone Rescues the NMDAR Hypofunction in a PCP Model of Schizophrenia.
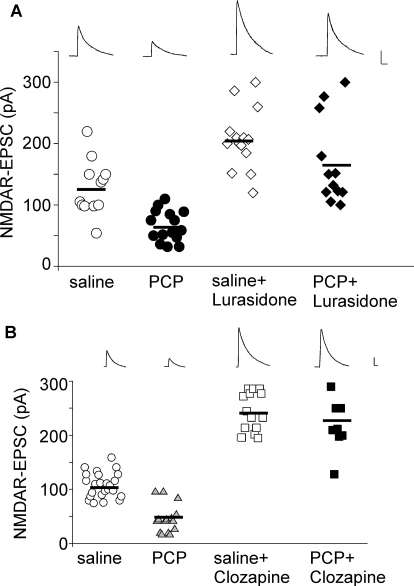
Because lurasidone reversed the effect of subchronic PCP administration on novel object recognition (Horiguchi et al., 2011) and was shown here to potently increase NMDAR function, we further tested its ability to restore NMDAR hypofunction in PCP-treated rats. Thus, we administered lurasidone after subchronic treatment of PCP (5 mg/kg i.p., 7 days). One day after PCP withdrawal, animals were given one injection of lurasidone (0.1 mg/kg s.c.) and tested 1 to 4 h later. As shown in Fig. 5A, PCP-treated animals showed significantly diminished NMDAR-EPSC (saline, 128 ± 12.8pA, n = 12; PCP, 66 ± 6.5 pA, n = 15; p < 0.001, Kruskal-Wallis test). Administration of lurasidone increased NMDAR-EPSC in saline-injected animals (lurasidone, 207 ± 13.3 pA; n = 14), and restored NMDAR-EPSC in the PCP-treated animals (PCP + lurasidone, 168 ± 20.3 pA, n = 12). A similar rescue was observed with in vivo administration of clozapine (5 mg/kg s.c., saline+ clozapine, 244 ± 9.6 pA, n = 14; PCP + clozapine, 227 ± 18.2 pA, n = 9, Fig. 5B). These results suggest that lurasidone, like clozapine, is capable of reversing the NMDAR hypofunction induced by repeated PCP treatment, which is a widely studied animal model of schizophrenia.
Fig. 5.
Lurasidone reverses NMDAR hypofunction in the PCP model of schizophrenia. A and B, dot plots showing the amplitude of NMDAR-EPSC in PFC pyramidal neurons from PCP-treated animals injected with lurasidone (0.1 mg/kg s.c.; A) or clozapine (5 mg/kg s.c.; B). Inset, representative NMDAR-EPSC traces. Scale bar, 50 pA, 100 ms.
Discussion
Despite the ability of lurasidone and clozapine to improve cognition in animal models of schizophrenia (Ishiyama et al., 2007; Enomoto et al., 2008; Nakamura et al., 2009; Snigdha et al., 2010; Horiguchi et al., 2011), little is known about the molecular and cellular mechanism underlying this action. Repeated exposure to lurasidone increases the mRNA and protein levels of brain-derived neurotrophic factor (Fumagalli et al., 2011), an important determinant of synaptic plasticity of glutamatergic synapses, consistent with the idea that antipsychotic treatment may change the expression, trafficking, and interaction of essential components of glutamatergic synapses (Fumagalli et al., 2008; Iasevoli et al., 2010). This study has provided the first electrophysiological evidence showing that in vivo administration of lurasidone or clozapine produces a significant enhancement of NMDAR-mediated EPSC in PFC neurons. Moreover, administration of a single dose of lurasidone or clozapine restored NMDAR responses in subchronic PCP-treated rats.
Similar to the pharmacological profile of clozapine (Meltzer, 1994), lurasidone has high binding affinity to various monoamine receptors, such as 5-HT2A, 5-HT7, 5-HT1A, D2, and α2C receptors (Meyer et al., 2009; Ishibashi et al., 2010). Drugs that affect several 5-HT receptors (e.g., 5-HT2A antagonists and 5-HT2C agonists) are effective to prevent the effects of NMDAR noncompetitive blockers on locomotor activity (Marquis et al., 2007) and to restore NOR in the subchronic PCP model (Meltzer et al., 2011b). Our previous studies have found that selective agonists or antagonists for 5-HT1A or 5-HT2A receptors either reduce NMDAR-EPSC or have no effect (Yuen et al., 2005, 2008). In this study, we show that the selective 5-HT7 antagonist SB-269970 mimics the enhancing effect of lurasidone on NMDAR-EPSC, whereas the D2 antagonist haloperidol (a typical APD) is ineffective. These results suggest that antagonism of 5-HT7 receptors may contribute to the ability of some atypical APDs to potentiate NMDAR function. The electrophysiological results reported here are consistent with the behavioral effects of these compounds in schizophrenia models. Horiguchi et al. (2011) have found that lurasidone, clozapine, and SB-269970, but not haloperidol, improve the impairment in NOR induced by subchronic PCP treatment. Moreover, the ability of lurasidone to reverse the PCP-induced NOR deficit is blocked by the 5-HT7 agonist AS19 (Horiguchi et al., 2011).
Emerging evidence suggests that NMDAR trafficking, which is regulated by interactions with PDZ proteins and tyrosine phosphorylation, plays a key role in controlling NMDAR function at synapses (Wenthold et al., 2003). Our biochemical evidence indicates that the surface levels of NR2A and NR2B subunits of NMDA receptors are selectively and significantly elevated after lurasidone administration. Because the availability of NR2 subunits determines the number of functional NMDARs at synapses, our results suggest that the potential molecular mechanism underlying the enhancing effect of in vivo administration of lurasidone on NMDAR synaptic responses is the increased delivery or decreased internalization of synaptic NMDA receptors.
Pharmacological data suggest that the effect of lurasidone on NMDARs is likely to be through a mechanism involving 5-HT7 receptor antagonism. 5-HT7 is a Gs-coupled GPCR that stimulates type 1 and type 8 Ca2+/calmodulin-sensitive adenylyl cyclases (Baker et al., 1998). The 5-HT7 receptor is enriched in brain regions mediating complex cognitive processes, such as the limbic system, hippocampus, amygdala, and PFC (Ruat et al., 1993; Béïque et al., 2004). 5-HT7 expression and function also correlate with neuronal depolarization in the developing rat PFC (Béïque et al., 2004). 5-HT7 receptor knockout or blockade of the 5-HT7 receptor enhances learning and memory (Gasbarri et al., 2008). A growing body of evidence supports the concept for targeting 5-HT7 antagonism as a possible mechanism for the treatment of cognitive deficits and a potential target for novel anxiolytic and antidepressant drugs (Hedlund et al., 2005; Abbas et al., 2009; Mnie-Filali et al., 2009; Horiguchi et al., 2011). In conclusion, the results reported here suggest that the procognitive effect of 5-HT7 receptor antagonism (Gasbarri et al., 2008; Horiguchi et al., 2011) may result from enhancement of NMDAR function.
Acknowledgments
We thank Xiaoqing Chen for excellent technical support.
This work was supported by grants from the National Institutes of Health National Institute on Mental Health [Grants MH84233, MH55441] (to Z.Y.).
H.Y.M. is a stockholder of ACADIA, Astra Zeneca, and SureGene and has received grant support in the last 3 years from BioLine Rx, Cephalon, Dainippon Sumitomo, Eli Lilly, EnVivo, Janssen, Otsuka, Pfizer, and Sunovion. He is, or has been, a consultant to ACADIA, Alkemes, Astellas, Boehringer Mannheim, Bristol Myers Squibb, Cypress, Janssen, Lundbeck, Ovation, Merck, Novartis, Pfizer, Teva, and Valeant (BioVail).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- PFC
- prefrontal cortex
- NMDA
- N-methyl-d-aspartate
- NMDAR
- N-methyl-d-aspartate receptor
- PCP
- phencyclidine
- MK-801
- 5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine maleate)
- APD
- antipsychotic drug
- NOR
- novel object recognition
- SB-269970
- (2R)-1-[(3-hydroxyphenyl)sulfonyl]-2 -(2-(4-methyl-1-piperidinyl)ethyl)pyrrolidine
- ACSF
- artificial cerebrospinal fluid
- EPSC
- excitatory postsynaptic current
- mEPSC
- miniature excitatory postsynaptic current
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor.
Authorship Contributions
Participated in research design: Yuen, Meltzer, and Yan.
Conducted experiments: Yuen, Li, and Wei.
Performed data analysis: Yuen, Li, and Wei.
Wrote or contributed to the writing of the manuscript: Horiguchi, Meltzer, and Yan.
References
- Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL. (2009) Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl) 205:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LP, Nielsen MD, Impey S, Metcalf MA, Poser SW, Chan G, Obrietan K, Hamblin MW, Storm DR. (1998) Stimulation of type 1 and type 8 Ca2+/calmodulin-sensitive adenylyl cyclases by the Gs-coupled 5-hydroxytryptamine subtype 5-HT7A receptor. J Biol Chem 273:17469–17476 [DOI] [PubMed] [Google Scholar]
- Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. (2004) Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci 24:4807–4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citrome L. (2011) Lurasidone for schizophrenia: a brief review of a new second-generation antipsychotic. Clin Schizophr Relat Psychoses 4:251–257 [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. (1976) Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192:481–483 [DOI] [PubMed] [Google Scholar]
- Enomoto T, Ishibashi T, Tokuda K, Ishiyama T, Toma S, Ito A. (2008) Lurasidone reverses MK-801-induced impairment of learning and memory in the Morris water maze and radial-arm maze tests in rats. Behav Brain Res 186:197–207 [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Calabrese F, Luoni A, Bolis F, Racagni G, Riva MA. (2011) Modulation of BDNF expression by repeated treatment with the novel antipsychotic lurasidone under basal condition and in response to acute stress. Int J Neuropsychopharmacol 24:1–12 [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Frasca A, Racagni G, Riva MA. (2008) Dynamic regulation of glutamatergic postsynaptic activity in rat prefrontal cortex by repeated administration of antipsychotic drugs. Mol Pharmacol 73:1484–1490 [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Cifariello A, Pompili A, Meneses A. (2008) Effect of 5-HT(7) antagonist SB-269970 in the modulation of working and reference memory in the rat. Behav Brain Res 195:164–170 [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. (2005) 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry 58:831–837 [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Huang M, Meltzer HY. (2011) The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther 338:605–614 [DOI] [PubMed] [Google Scholar]
- Iasevoli F, Tomasetti C, Marmo F, Bravi D, Arnt J, de Bartolomeis A. (2010) Divergent acute and chronic modulation of glutamatergic postsynaptic density genes expression by the antipsychotics haloperidol and sertindole. Psychopharmacology (Berl) 212:329–344 [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, et al. (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334:171–181 [DOI] [PubMed] [Google Scholar]
- Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y. (2007) Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur J Pharmacol 572:160–170 [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308 [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. (1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225 [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. (1997) Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology 17:92–99 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28:325–334 [DOI] [PubMed] [Google Scholar]
- Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Jr, Nguyen HQ, Dawson LA, et al. (2007) WAY-163909 ((7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole): a novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther 320:486–496 [DOI] [PubMed] [Google Scholar]
- Meltzer HY. (1992) Dimensions of outcome with clozapine. Br J Psychiatry Suppl (17):46–53 [PubMed] [Google Scholar]
- Meltzer HY. (1994) An overview of the mechanism of action of clozapine. J Clin Psychiatry 55 (Suppl B):47–52 [PubMed] [Google Scholar]
- Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, Kalali AH, Schweizer E, Pikalov A, Loebel A. (2011a) Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry 168:957–967 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Horiguchi M, Massey BW. (2011b) The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology (Berl) 213:289–305 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Huang M. (2008) In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res 172:177–197 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. (1989) Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther 251:238–246 [PubMed] [Google Scholar]
- Meyer JM, Loebel AD, Schweizer E. (2009) Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs 18:1715–1726 [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, Lambas-Señas L, Scarna H, Haddjeri N. (2009) Therapeutic potential of 5-HT7 receptors in mood disorders. Curr Drug Targets 10:1109–1117 [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. (1999) Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98:427–436 [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ogasa M, Guarino J, Phillips D, Severs J, Cucchiaro J, Loebel A. (2009) Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry 70:829–836 [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. (1993) Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA 90:8547–8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. (2002) Schizophrenia: diverse approaches to a complex disease. Science 296:692–695 [DOI] [PubMed] [Google Scholar]
- Snigdha S, Horiguchi M, Huang M, Li Z, Shahid M, Neill JC, Meltzer HY. (2010) Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 332:622–631 [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. (2002) Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42:165–179 [DOI] [PubMed] [Google Scholar]
- Wang X, Gu Z, Zhong P, Chen G, Feng J, Yan Z. (2006) Aberrant regulation of NMDA receptors by dopamine D4 signaling in rats after phencyclidine exposure. Mol Cell Neurosci 31:15–25 [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. (1986) Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 43:114–124 [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. (2003) Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol 43:335–358 [DOI] [PubMed] [Google Scholar]
- Xia YF, Kessler M, Arai AC. (2005) Positive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor modulators have different impact on synaptic transmission in the thalamus and hippocampus. J Pharmacol Exp Ther 313:277–285 [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. (2008) Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of N-methyl-d-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem 283:17194–17204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. (2005) Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J. Neurosci 25:5488–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. (2009) Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA 106:14075–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]