Abstract
The molecular mechanisms involved in uterine quiescence during gestation and those responsible for induction of labor are not completely known. Nitric oxide relaxes uterine smooth muscle in a manner disparate from that for other smooth muscles because global elevation of cGMP after activation of soluble guanylyl cyclase does not relax the muscle. S-Nitrosylation, the covalent addition of an nitric oxide (NO) group to a cysteine thiol is a likely mechanism to explain the ability of NO to relax myometrium. This work is the first to describe the myometrial S-nitrosylproteome in both pregnant and nonpregnant tissue states. Using the guinea pig model, we show that specific sets of proteins involved in contraction and relaxation are S-nitrosylated in laboring and nonlaboring muscle and that many of these proteins are uniquely S-nitrosylated in only one state of the tissue. In particular, we show that S-nitrosylation of the intermediate filament protein desmin is significantly increased (5.7-fold, p < 0.005) in pregnancy and that this increase cannot be attributed solely to the increase in protein expression (1.8-fold, p < 0.005) that accompanies pregnancy. Elucidation of the myometrial S-nitrosylproteome provides a list of mechanistically important proteins that can constitute the basis of hypotheses formed to explain the regulation of uterine contraction/relaxation.
Introduction
Preterm labor affects 12.5% of obstetric practice in the United States and leads to preterm delivery in more than 50% of cases. This inexplicable tragedy (Buxton, 2004; Buxton et al., 2000), in which 20,000 fetuses die annually, seems to be increasing in frequency and disproportionally affects African American (18%) mothers (Behrman and Butler, 2006). Despite decades of interest, more than half of the cases of preterm labor are spontaneous and unexplained. The molecular mechanisms involved in the induction of labor are still unknown. Uterine smooth muscle (USM) has been previously shown to relax in a manner disparate from that of other smooth muscle tissues. Nitric oxide (NO) relaxes uterine smooth muscle in a dose-dependent, cGMP-independent manner (Buxton et al., 2001). The IC50 for the transnitrosylating agent cysteine-NO to relax human myometrium in tissue bath experiments is approximately 1 μM (Buxton et al., 2001). Elevations in cGMP that accompany NO stimulation can be prevented by pretreatment of tissues with inhibitors of soluble guanylyl cyclase such as 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (Buxton et al., 2001), 6-anilino-5,8-quinolinedione (LY 83583), (Kuenzli et al., 1998), or 3,7-bis(dimethylamino) phenothiazin-5-ium (methylene blue), (Kuenzli et al., 1996). Stimulation of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one-treated myometrial tissues with NO donors results in relaxation without cGMP elevation. It is now clearly established from our work and that of others that NO-mediated relaxation of USM is independent of global cGMP elevation or activation of its cognate kinase, protein kinase G type I (Tichenor et al., 2003), no matter whether this is studied in animals (Kuenzli et al., 1996), primates (Kuenzli et al., 1998), or humans (Bradley et al., 1998).
NO could potentially signal as an endogenous tocolytic, but there is no certainty that NO is present as an endogenous myometrial relaxing factor, and this need not be the case for proteomic experiments to be important. NO could be available from uterine arterial endothelium or released from syncytiotrophoblasts (Valdes and Corthorn, 2011). Moreover, Suzuki et al. (2009) have recently suggested that NO is generated in rat uterus. The fact that NO signals in a nonclassic manner in the uterus and that it may selectively S-nitrosylate proteins associated with pregnancy is the compelling feature of our work because there is hope for discovery of therapeutic targets in the myometrium that are absent or disparately regulated in other smooth muscles and thus can permit a reasoned line of investigation to find uterine-specific tocolytic agents.
We have hypothesized that NO-mediated relaxation is dependent on S-nitrosylation of specific and critical proteins involved in the relaxation of USM. We further hypothesize that these critical proteins are S-nitrosylated disparately in pregnant and nonpregnant USM consistent with the development of regulation of contraction/relaxation signaling. S-Nitrosylation is a mechanistically important, NO-dependent, post-translational modification that can alter smooth muscle contraction/relaxation dynamics (Dalle-Donne et al., 2000). The terms nitrosation and nitrosylation are often used interchangeably, and the difference in terminology only refers to the mechanism of formation. Considering that we are not studying the chemistry of the reaction, only the post-translational modification, we use the term S-nitrosylation to refer to the modified proteins that we have identified.
S-Nitrosylation has been shown to alter the function of many proteins including the activity of several enzymes (Hess et al., 2005). On the basis of the striking cGMP independence of NO-mediated USM relaxation (Buxton et al., 2001) and with the benefit of prior research establishing that S-nitrosylation is a source of NO bioactivity (Seth and Stamler, 2011), we propose that NO-mediated relaxation in USM is the result of protein S-nitrosylation.
We used the myometrium from guinea pig, the small animal model proposed as the most appropriate for studies of human parturition (Mitchell and Taggart, 2009), to identify disparate S-nitrosylation between the pregnant and nonpregnant state of the tissue. We show that multiple proteins known to be integral to smooth muscle contraction/relaxation dynamics are S-nitrosylated in USM. Furthermore, our work shows that a large subset of these proteins show disparate abilities to be S-nitrosylated when pregnant and nonpregnant USM tissue are compared.
Materials and Methods
Chemicals.
Sodium ascorbate, HEPES, neocuproine, N-ethylmaleimide (NEM), methyl methanethiosulfonate, CHAPS, SDS, and all other chemicals, unless specified, were obtained from Sigma-Aldrich (St. Louis, MO). N-[6-(Biotinamido)hexyl]-3′-(2′-pyridyldithio) propionamide (biotin-HPDP) was from Thermo Fisher Scientific (Waltham, MA).
Animal Care.
Female virgin Dunkin-Hartley guinea pigs were estrogen-primed as described previously (Smith et al., 1989) and sacrificed the following morning under a protocol approved by the local institutional review board. Term-pregnant Dunkin-Hartley guinea pigs were sacrificed between day 65 and 70 of pregnancy, and the uterine horns were removed and placed in ice-cold phosphate-buffered saline. Muscles were carefully prepared for smooth muscle protein isolation from regions between placentas by dissection of serosa and scraping away endometrium/epithelium to reveal muscle.
Biotin Switch and Streptavidin Pulldown.
Protein isolates from term pregnant and nonpregnant guinea pigs [1.8 ml, 0.8 mg/ml in HEN buffer (25 mM HEPES, pH 7.7, 1 mM EDTA, and 0.1 mM neocuprine)] were incubated with 300 μM GSNO (1746 μl of sample + 54 μl of 10 mM GSNO prepared in the dark) for 20 min at room temperature. At this concentration, GSNO will produce ∼5 μM reactive NO more than 15 to 20 min without reactive NO accumulation (Cleeter et al., 1994). Noncysteinyl nitrosation events would not be recognized because of the reactive chemistry of ascorbate reduction, a nucleophilic attack at the nitroso-nitrogen atom leading to thiol and O-nitrosoascorbate (reaction 1). Moreover, endogenous nitrosylations are preserved and then labeled by our procedure so that we report all possible nitrosylations.
O-Nitrosoascorbate breaks down by various competitive pathways; the dominant one at physiological pH yields dehydroascorbic acid and nitroxyl, which decomposes at physiological pH to nitrous oxide (Kirsch et al., 2009) (reaction 2).
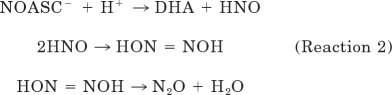 |
Neither biotin-HPDP nor a maleimide dye would lead to false-positive results because the amines or tyrosines would not be labeled even if they were nitrosated. The biotin switch and streptavidin pulldown assays were performed as described previously (Jaffrey and Snyder, 2001). Final protein pellets were washed and dried and then delivered to the Nevada Proteomics Center for LC-MS/MS analysis.
Fluorescent Switch.
The fluorescent switch technique is a variation of the biotin switch pioneered by Jaffrey and Snyder (2001). This technique is used to selectively label S-nitrosylated proteins using thiol reactive dyes. All procedures are performed in the dark in amber tubes. Total protein extracts were S-nitrosylated using 300 μM GSNO (Sigma-Aldrich) for 20 min at room temperature. SDS was added along with NEM (Sigma-Aldrich) to a final 2.0-ml volume with 2.5% SDS and 30 mM NEM. Samples were incubated at 50°C in the dark for 20 min with frequent vortexing. Four volumes of ice-cold acetone (8 ml) were added to each sample, and proteins were precipitated for 1 h at −20°C and collected by centrifugation at 3000g for 10 min. The clear supernatant was aspirated, and the protein pellet was gently washed with 70% acetone (four 1-ml washes). After resuspension in 0.24 ml of HENS buffer (HEN + 1% SDS), the material was transferred to fresh 1.7-ml microfuge tubes containing 100 μM maleimide-Alexa Fluor 555 or 647 dye. The labeling reaction was initiated by addition of 30 μl of 200 mM sodium ascorbate (final 20 mM ascorbate) with gentle shaking at room temperature for 1 h. Unreacted dye was removed by acetone precipitation, and proteins were pelleted and washed with 70% acetone (four 1-ml washes) and air-dried.
Nitrosyl-DIGE.
Dried samples were lyophilized for 15 min and 360 μl of EB3 was added. The samples were vortexed a number of times and sonicated for 10 min over a period of 1 h 50 min. The samples were spun at 10,000g and 22°C for 2 min. The supernatants, along with one-third dilutions of each supernatant in EB3 ReadyPrep Sequential Extraction Reagent 3 (Bio-Rad Laboratories, Hercules, CA), were assayed by EZQ Protein Quantification (Invitrogen, Carlsbad, CA). Proteins were equilibrated to 244 μg of total protein/300 μl of a mixture containing protein, EB3, DeStreak reagent (GE Healthcare, Uppsala, Sweden), and 0.1% bromphenol blue. Equal amounts of total protein from laboring, nonlaboring, and total control samples (containing a mixture of all samples) were used to rehydrate 17-cm IPG strips (pH 3–1) by overnight passive rehydration. The strips were transferred to an isoelectric focusing plate and run as follows: 250 V, linear, 20 min; 10,000 V, linear, 2 h 30 min; 10,000 V, rapid until 40,000 V-h; and 500 V, rapid, 24 h. The strips were equilibrated for electrophoresis and placed on 8 to 16% Protean II gels (Bio-Rad Laboratories, Hercules, CA), and electrophoresis was performed under the following conditions: 5 mA constant, 30 min; 16 mA constant, 30 min; and 24 mA constant, 4 h 45 min. Gels were then transferred to low fluorescence plates and scanned on the Typhoon Trio (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Images were analyzed using DeCyder software (GE Healthcare), and proteins of interest were excised, trypsin-digested, and analyzed by either MS or LC-MS/MS at the Nevada Proteomics Center.
Protein Digestion and Mass Spectrometry.
The Nevada Proteomics Center analyzed selected proteins by trypsin digestion and MALDI TOF/TOF or LC-MS/MS. Spots were digested using a previously described protocol with some modifications (Rosenfeld et al., 1992). Samples were washed twice with 25 mM ammonium bicarbonate and 100% acetonitrile, reduced and alkylated using 10 mM dithiothreitol and 100 mM iodoacetamide, and incubated with 75 ng of sequencing-grade modified porcine trypsin (Promega, Madison, WI) in 25 mM ammonium bicarbonate for 6 h at 37°C. Samples were spotted onto a MALDI target with ZipTip μ-C18 pipette tips (Millipore Corporation, Billerica, MA). Samples were eluted with 70% acetonitrile and 0.2% formic acid and overlaid with 0.5 μl of 5 mg/ml MALDI matrix (α-cyano-4-hydroxycinnamic acid and 10 mM ammonium phosphate). All mass spectrometric data were collected using an ABI 4700 Proteomics Analyzer MALDI TOF/TOF mass spectrometer (Applied Biosystems, Foster City, CA), using their 4000 Series Explorer software version 3.6. The peptide masses were acquired in reflectron-positive mode (1-keV accelerating voltage) from a mass range of 650 to 4000 Da; 1250 laser shots were averaged for each mass spectrum. Each sample was internally calibrated on trypsin's autolysis peaks 842.51 and 2211.10 to within 20 ppm. Any sample failing to internally calibrate was analyzed under default plate calibration conditions of 150 ppm. Raw spectrum filtering/peak detection settings were S/N threshold of 3, cluster area S/N optimization enabled at S/N threshold of 10, and baseline subtraction enabled at peak width 50. The 20 most intense ions from the MS analysis, which were not on the exclusion list, were subjected to MS/MS. The MS/MS exclusion list included known trypsin masses: 842.51, 870.54, 1045.56, 1126.56, 1420.72, 1531.84, 1940.94, 2003.07, 2211.10, 2225.12, 2239.14, 2283.18, 2299.18, 2678.38, 2807.31, 2914.51, 3094.62, 3337.76, and 3353.75. For MS/MS analysis, the mass range was 70 to precursor ion with a precursor window resolution of −1 to +4 Da with an average 2500 laser shots for each spectrum, collision-induced dissociation on and metastable suppressor on. Raw spectrum filtering/peak detection settings were S/N threshold of 5, cluster area S/N optimization enabled at S/N threshold 6, baseline subtraction enabled at peak width 50. The data were then stored in an Oracle database (schema version 3.19.0; data version 3.90.0).
MALDI Data Analysis.
Peak lists were also created using 4000 Series Explorer software version 3.6 Peaks to Mascot feature. MS peak filtering included mass range 650 to 4000 Da, minimum S/N filter 10, and a peak density filter of 50 peaks/200 Da with a maximum number of peaks set to 200. MS/MS peak filtering included a mass range of 60 to 20 Da below each precursor mass, minimum S/N filter 10, peak density filter of 50 peaks/200 Da, and cluster area filter used with maximum number of peaks 200. The filtered data were searched by Mascot (version 2.1.03; Matrix Science Inc., Boston, MA) using the National Center for Biotechnology Information database (20090319), containing 8,080,522 sequences. Searches were performed without restriction to protein species, Mr, or pI and with variable oxidation of methionine residues and carbamidomethylation of cysteines. Maximum missed cleavage was set to 1 and limited to trypsin cleavage sites. Precursor mass tolerance and fragment mass tolerance were set to 20 ppm and ±0.2 Da, respectively.
MALDI data were collected for both trypsin-digested spots from nitrosyl-DIGE as well as streptavidin pulldown of biotin-labeled samples. We acquired LC-MS/MS capability during this study, and, therefore, identification of samples was initially done only with MALDI and then with LC-MS/MS when it was available. Single protein identifications were recorded for the six spots cut, and the additional proteins identified by MALDI in Supplemental Table S1 are from the streptavidin pulldown samples.
LC-MS/MS Data Analysis.
For LC-MS/MS analysis, trypsin digestion was performed as detailed above for MALDI TOF/TOF. The labels introduced in the Biotin Switch are removed when the disulfide linking biotin-HPDP to the protein is cleaved after addition of β-mercaptoethanol and is therefore not considered during database searching. The samples were digested in solution, as outlined under Protein Digestion and Mass Spectrometry, and the samples were reduced and alkylated using 10 mM dithiothreitol and 100 mM iodoacetamide. These steps lead to carbamidomethylation of cysteines. Peptides were first separated using a paradigm multidimensional liquid chromatography instrument (Magic C18AQ. 3-μm, 200-Å, 0.2 × 50 mm column; Michrom Bioresources Inc., Auburn, CA) with an ZORBAX 300SB-C18 5-μm (5 × 0.3 mm) trap (Agilent Technologies, Santa Clara, CA). The gradient used 0.1% formic acid in water (pump A) and 0.1% formic acid in acetonitrile (pump B) [time in minutes, flow in micromoles per minute, pump B percentage]: (0.00, 4.00, 5.00), (5.00, 4.00, 5.00), (95.00, 4.00, 45.00), (95.10, 4.00, 80.00), (96.10, 4.00, 80.00), and (96.20, 4.00, 5.00). Eluted peptides were analyzed using a Thermo Fisher Scientific LTQ-Orbitrap using Xcalibur version 2.0.7. MS spectra (m/z 300–2000) were acquired in the positive ion mode with resolution of 60,000 in profile mode. The top six data-dependent signals were analyzed by MS/MS with collision-induced dissociation activation, a minimum signal of 50,000, an isolation width of 3.0, and a normalized collision energy of 35.0. The reject mass list included known trypsin fragments: 323.2040, 356.0690, 371.1010, 372.1000, 373.0980, 374.0970, 445.1200, 523.2840, 536.1650, 572.5680, 747.3510, 824.4870, and 930.1760. Dynamic exclusion settings were used with a repeat count of 2, repeat duration of 10 s, exclusion list size of 500, and exclusion duration of 30 s.
Criteria for Protein Identification.
Scaffold (version 3.00.07; Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm (Keller et al., 2002). Protein identifications were accepted if they could be established at greater than 95.0% probability and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al., 2003). Proteins that contained similar peptides and could not be differentiated on the basis of MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Western Blot.
Pregnant and nonpregnant USM protein isolated for nitroso-DIGE and the biotin switch was used to measure total levels of desmin. Protein isolates were separated by SDS-polyacrylamide gel electrophoresis (10%), transferred to nitrocellulose and blotted with rabbit anti-desmin (Y266; Abcam, Inc., Cambridge, MA), and labeled with anti-rabbit Alexa-Fluor 680 (Invitrogen). Blots were visualized using the Odyssey imaging system (LI-COR Biosciences, Lincoln, NE).
Identification of Previously Characterized S-Nitrosylated Proteins.
The lack of a central comprehensive online database for the classification of characterized S-nitrosylated proteins makes classification of novel S-nitrosylated proteins difficult. We used two methods to determine whether our identified proteins had been previously classified as being S-nitrosylated. First, we methodically searched the compiled database of Xue et al. (2010) that was generated for their SNO identification algorithm. We included hits from both the experimentally identified database as well as the inferred database. Second, we performed a search in PubMed and Google Scholar using the protein of interest as a key word with “nitrosylation or nitrosation” as the second keyword. Results are listed in Table 1 with the PubMed identification number of the most relevant literature citation that hit to each keyword search.
TABLE 1.
The myometrial nitrosylproteome
A total of 118 unique proteins were identified, 62 of which have not been previously described as being S-nitrosylated, 75 proteins were disparately S-nitrosylated only during pregnancy and 10 were disparately S-nitrosylated only during non-pregnancy, and 33 proteins were S-nitrosylated in both pregnancy and the nonpregnant tissue.
| Protein | Biological Process | Previously Identified | NP | P | UniProt | No. of Peptides | Coverage |
|---|---|---|---|---|---|---|---|
| % | |||||||
| 40S ribosomal protein S11a | Protein binding | Y C131 (PMID: 20585580) | Y | Y | P62280 | 3 | 22.8 |
| 40S ribosomal protein S28a | Protein binding | N | Y | N | P62857 | 2 | 33 |
| 4-Trimethylamino-butyraldehyde dehydrogenasea | Metabolism | Y C(74, 173, 443) (PMID: 20585580) | N | Y | P49189 | 3 | 8.65 |
| 60-kDa Heat shock protein, mitochondriala | Protein folding | Y C(237, 442, 447) (PMID: 20585580) | N | Y | P10809 | 2 | 9.95 |
| 60S ribosomal protein L8a | rRNA binding | N | Y | N | P62917 | 2 | 12.7 |
| 6-Phosphogluconate dehydrogenase, decarboxylationa | Metabolism | N | N | Y | P52209 | 2 | 11.88 |
| Actin, α skeletal musclea | Cytoskeletal | Y (PMID: 10961840) | Y | Y | P68133 | 4 | 13.3 |
| Actin, cytoplasmic 1a | Cytoskeletal | Y C(217, 272, 285) (PMID: 20585580) | Y | Y | P60709 | 29 | 76.13 |
| ADP-ribosylation factor 4a | Transport | N | N | Y | P18085 | 3 | 36.67 |
| Aflatoxin B1 aldehyde reductase member 2a | Metabolism | N | N | Y | O43488 | 2 | 10.46 |
| Alcohol dehydrogenase [NADP+]a | Metabolism | Y (PMID: 19738628) | N | Y | P14550 | 5 | 28.62 |
| Aldehyde dehydrogenase, mitochondriala | Metabolism | Y C66 (PMID: 20585580) | N | Y | P05091 | 2 | 8.83 |
| Aldose reductasea | Metabolism | Y C299 (PMID: 20585580) | N | Y | P15121 | 3 | 28.48 |
| α-2-HS-glycoproteina | Endocytosis | N | Y | N | P02765 | 2 | 10.61 |
| α-Actinin-1a,b | Cytoskeletal | Y C332 (PMID: 20585580) | N | Y | P12814 | 4 | 7.85 |
| α-Aminoadipic semialdehyde dehydrogenasea | Metabolism | N | N | Y | P49419 | 2 | 10.57 |
| α-Enolasea | Metabolism | Y C(119,357) (PMID: 20585580) | Y | Y | P06733 | 11 | 34.79 |
| Ankyrin-3a | Cytoskeletal membrane linker | Y C(1123, 1246, 1369, 2096, 2835, 3395, 3958, 3980, 4233, 4321) (PMID: 20585580) | Y | N | Q12955 | 2 | 0.25 |
| Annexin A1a | Exocytosis and phospholipase A2 regulation | N | N | Y | P04083 | 8 | 35.84 |
| Annexin A2a,b | Cytoskeletal adapter | Y (PMID: 12199706) | N | Y | P07355 | 16 | 53.69 |
| Annexin A3a,b | Inhibitor of phospholipase A2 | N | N | Y | P12429 | 5 | 23.53 |
| Annexin A4a | Exocytosis, membrane fusion | N | N | Y | P09525 | 2 | 14.33 |
| Annexin A5a | Blood coagulation | N | N | Y | P08758 | 2 | 11.29 |
| ATP synthase subunit α, mitochondriala | Transport | Y (PMID: 20585580) | N | Y | P25705 | 2 | 4.73 |
| Calpain small subunit 1a | Cytoskeletal remodeling and signal transduction | N | N | Y | P04632 | 4 | 34.98 |
| Calponin-1a | Smooth muscle contraction; interaction of calponin with actin inhibits actomyosin Mg-ATPase | N | Y | N | P51911 | 2 | 17.67 |
| Cell division cycle 42 GTP binding protein, 25 kDaa | Cell division | N | N | Y | Q5JYX0 | 5 | 28.8 |
| Chloride intracellular channel protein 1a | Ion transport (redox regulated) | N | N | Y | O00299 | 2 | 18.26 |
| Cofilin-1a | Cytoskeletal actin binding | N | Y | Y | P23528 | 6 | 56.02 |
| Corticosteroid-binding globulina | Transport | N | N | Y | P08185 | 2 | 14.07 |
| Creatine kinase B-typea | Metabolism | Y (PMID: 18670085) | N | Y | P12277 | 8 | 50.66 |
| Cysteine- and glycine-rich protein 1a | Zinc ion binding | N | Y | Y | P21291 | 2 | 27.98 |
| Cysteine sulfinic acid decarboxylasea | Metabolism | N | N | Y | Q9Y600 | 5 | 16.53 |
| Cysteine-rich protein 2a | Zinc ion binding; interacts with TGFB1I1 | N | Y | Y | P52943 | 7 | 7.5 |
| Cytochrome c oxidase subunit 2b | Electron transport | Y (PMID: 15561762) | N | Y | P00403 | 4 | 19.38 |
| Desmina,b | Cytoskeletal | Y C333 (PMID: 20585580) | Y | Y | P17661 | 18 | 50.21 |
| Destrina,b | Actin depolymerization | Y (PMID: 18670085) | N | Y | P60981 | 9 | 46.06 |
| % | |||||||
| Dihydropyrimidinase-like 2a | Hydrolase | N | N | Y | Q86U75 | 2 | 11.07 |
| Dihydropyrimidinase-related protein 2a | Cell signaling | Y C504 (PMID: 16418269) | Y | N | Q16555 | 2 | 3.03 |
| Elongation factor 1-α1a | Protein biosynthesis | Y C(3234, 411) (PMID: 20585580) | N | Y | P68104 | 4 | 21.54 |
| Elongation factor 1-γa | Protein biosynthesis | Y C194 (PMID: 20585580) | N | Y | P26641 | 2 | 11.67 |
| Elongation factor 2a | Translation elongation | Y C(41, 567) (PMID: 20585580) | Y | Y | P13639 | 7 | 15.02 |
| Eukaryotic initiation factor 4A-Ia | Protein biosynthesis | N | N | Y | P60842 | 5 | 19.6 |
| Fatty acid-binding protein, epidermala | Transport | N | N | Y | Q01469 | 2 | 15.56 |
| Filamin-Aa | Cytoskeletal scaffold actin binding | Y C717 (PMID: 20585580) | Y | Y | P21333 | 33 | 19.74 |
| Filamin-Ca | Cytoskeletal actin cross-linker | Y C (805, 1680, 2096, 2660) (PMID: 20585580) | N | Y | Q14315 | 10 | 6.93 |
| Galectin-1a | Regulation of apoptosis, cell proliferation/differentiation | Y C61 (PMID: 20585580) | N | Y | P09382 | 5 | 39.26 |
| Glutaredoxin-1a | Transport and thiol reduction | Y (PMID: 17355958) | N | Y | P35754 | 2 | 38.68 |
| Glutathione transferase Mu 2a | Metabolism | Y (PMID: 20585580) | N | Y | P28161 | 5 | 23.96 |
| Glutathione transferase Mu 3a | Metabolism | Y C3 (PMID: 20585580) | N | Y | P21266 | 2 | 16.59 |
| Glutathione transferase Pa | Metabolism | Y C(47, 101) (PMID: 11533048) | Y | Y | P09211 | 8 | 59.52 |
| Glyceraldehyde-3-phosphate dehydrogenasea,b | Metabolism | Y C(152, 156, 247) (PMID: 20585580) | N | Y | P04406 | 12 | 59.46 |
| Glycerol-3-phosphate dehydrogenase 1-like proteina | Regulation of sodium current | N | N | Y | Q8N335 | 2 | 10.8 |
| Gi subunit α-2a | Signal transduction | N | N | Y | P04899 | 2 | 9.58 |
| Heat shock 105 kDa/110 kDa protein 1a | Chaperone | N | N | Y | Q5TBM3 | 2 | 4.42 |
| Heat shock protein 90 kDa α cytosolic, class B member 1a | Chaperone | N | Y | N | Q5T9W8 | 2 | 5.75 |
| Heat shock protein β-1a,b | Cytoskeletal remodeling and signal transduction | Y (PMID: 18670085) | Y | Y | P04792 | 11 | 84.5 |
| Heat shock protein β-6a | Stress response (HSP20 family) | N | N | Y | O14558 | 4 | 51.85 |
| Heat shock protein HSP90-αb | Chaperone | Y C(481, 597, 598) (PMID: 20585580) | Y | Y | P07900 | 10 | 14 |
| Hemoglobin subunit αa,b | Oxygen transport | Y C105 (PMID: 20585580) | Y | Y | P69905 | 8 | 57.04 |
| Hemoglobin subunit βa,b | Oxygen transport | Y C93 (PMID: 16594178) | Y | Y | P68871 | 13 | 90.48 |
| Histidine-rich glycoproteina | Blood coagulation | N | N | Y | P04196 | 2 | 3.68 |
| Kinesin family member 17a | Microtubule-based movement | N | N | Y | A2A3Q7 | 2 | 4.91 |
| Lipoma-preferred partnera | Cell signaling and protein scaffold | N | Y | Y | Q93052 | 14 | 31.97 |
| l-Lactate dehydrogenase A chaina | Metabolism | Y C163 (PMID: 20585580) | N | Y | P00338 | 10 | 54.14 |
| Malate dehydrogenase, cytoplasmicb | Metabolism | Y C(137, 154) (PMID: 20585580) | Y | Y | P40925 | 12 | 194 Mowsec |
| Myosin light chain kinase, smooth musclea | Smooth muscle contraction | N | N | Y | Q15746 | 3 | 3.45 |
| Myosin light polypeptide 6a,b | Smooth muscle contraction | ? (PMID: 20585450) | Y | Y | P60660 | 9 | 51.66 |
| Myosin regulatory light polypeptide 9a | Smooth muscle contraction | ? (PMID: 20585450) | N | Y | P24844 | 4 | 45.35 |
| Myosin-11a | Smooth muscle contraction | ? (PMID: 20585450) | N | Y | P35749 | 6 | 5.27 |
| Neutral α-glucosidase ABb | Metabolism | N | Y | Y | Q14697 | 4 | 3.91 |
| Nucleoside diphosphate kinase Aa | Metabolism and cell signaling | N | N | Y | P15531 | 2 | 25.47 |
| Oligoribonuclease, mitochondriala | Exonuclease | N | N | Y | Q9Y3B8 | 2 | 18.2 |
| PDZ and LIM domain protein 1a | Cytoskeletal adapter (interacts with α-actinins 1, 2, and 4) | N | N | Y | O00151 | 3 | 19.82 |
| Peptidyl-prolyl cis-trans isomerase Aa | Protein folding | N | Y | Y | P62937 | 7 | 51.52 |
| Peroxiredoxin-1a | Redox regulation | Y C(52, 173) (PMID: 20585580) | Y | Y | Q06830 | 6 | 36.14 |
| Phosphofructokinase, plateleta | Metabolism | N | N | Y | Q5VSR7 | 3 | 4.8 |
| Phosphoglucomutase-like protein 5a | Cytoskeletal; interacts with cytoskeletal proteins dystrophin and utrophin | N | N | Y | Q15124 | 2 | 7.14 |
| Phosphoglycerate kinase 1a | Metabolism | Y C50 (PMID: 20585580) | N | Y | P00558 | 9 | 42.82 |
| Phosphoglycerate mutase 1a | Metabolism | Y C55 (PMID: 20585580) | N | Y | P18669 | 2 | 13.39 |
| Prefoldin subunit 3a | Protein folding | N | N | Y | P61758 | 2 | 28.43 |
| Preproalbumin precursora | Guinea pig specific | N | Y | Y | Q6WDN9 | 39 | 65.46 |
| % | |||||||
| Profilin-1a | Cytoskeletal remodeling | Y (PMID: 18283105) | N | Y | P07737 | 3 | 22.86 |
| Prolow-density lipoprotein receptor-related protein 1a | Endocytosis and cell signaling | N | N | Y | Q07954 | 2 | 0.9 |
| Prorelaxin H1a | Signal transduction and tissue remodeling | N | N | Y | P04808 | 3 | 51.25 |
| Protein DJ-1a | Transcriptional activator | N | N | Y | Q99497 | 5 | 38.62 |
| Protein S100-A10a | Protein binding; induces the dimerization of ANXA2/p36 | N | N | Y | P60903 | 2 | 37.5 |
| Protein S100-A11a,b | Calcium binding and signal transduction | Y C13 (PMID: 20585580) | N | Y | P31949 | 7 | 53.92 |
| Protein S100-A4a,b | Calcium binding | N | N | Y | P26447 | 2 | 11.88 |
| Protein S100-A9a | Signal transduction | Y (PMID: 18832721) | N | Y | P06702 | 3 | 51.26 |
| Purine nucleoside phosphorylaseb | NAD biosynthesis | N | N | Y | P00491 | 4 | 28.82 |
| Pyruvate kinase isozymes M1/M2a | Metabolism | Y C(326, 358, 423, 474) (PMID: 20585580) | Y | Y | P14618 | 14 | 47.27 |
| Ras-related protein Rab-1Aa | Transport | N | N | Y | P62820 | 3 | 35.12 |
| Ras-related protein R-Rasa | Cytoskeletal remodeling | N | N | Y | P10301 | 3 | 26.15 |
| Serotransferrina | Transport | Y C(58, 67, 137, 246, 387, 396, 469, 596, 615) (PMID: 20585580) | Y | Y | P02787 | 6 | 10.08 |
| Serpin peptidase inhibitor, clade B ovalbumin, memb0.6a | Peptidase inhibitor | N | N | Y | Q5TD02 | 11 | 48.99 |
| S-Formylglutathione hydrolasea | Metabolism | N | N | Y | P10768 | 2 | 15.6 |
| SH3 domain-binding glutamic acid-rich-like proteinb | SH3 binding | No Cys in Human | Y | Y | O75368 | 2 | 17.2 |
| Stress-70 protein, mitochondriala | Chaperone | Y C487 (PMID: 20585580) | N | Y | P38646 | 2 | 4.28 |
| Talin-1a | Cytoskeletal membrane connector | N | Y | Y | Q9Y490 | 13 | 10.27 |
| Testis derived transcript 3 LIM domainsa | Zinc ion binding | N | N | Y | A4D0U5 | 6 | 18.89 |
| Thioredoxinb | S-Nitrosylation regulation | Y (62, 69, 73) (PMID: 20585580) | Y | Y | P10599 | 7 | 32 |
| Transforming growth factor β-1-induced transcript 1 proteina | Molecular adapter at focal adhesion complexes | N | Y | Y | O43294 | 3 | 13.3 |
| Transforming protein RhoAa | Signal transduction | N | N | Y | P61586 | 2 | 13.99 |
| Transgelina,b | Actin binding | N | N | Y | Q01995 | 10 | 59.7 |
| Transgelin-2a | Protein binding | N | Y | Y | P37802 | 7 | 40.09 |
| Transitional endoplasmic reticulum ATPasea,b | Transport | Y C(77, 209, 695) (PMID: 20585580) | Y | N | P55072 | 2 | 11 |
| Transthyretina | Transport | Y (PMID: 16101296) | Y | N | P02766 | 2 | 26.19 |
| Tropomyosin α-4 chaina | Smooth muscle contraction is regulated by interaction with caldesmon | Y C170 (PMID: 18992711) | N | Y | P67936 | 3 | 16.53 |
| Tropomyosin β chaina,b | Smooth muscle contraction is regulated by interaction with caldesmon | Y C170 (PMID: 19447776) | Y | Y | P07951 | 4 | 29.58 |
| Tryptophanyl-tRNA synthetase, cytoplasmica | Protein biosynthesis | N | N | Y | P23381 | 2 | 12.13 |
| Tubulin β chaina,b | Cytoskeletal | Y (PMID: 16418269) | Y | Y | P07437 | 18 | 54.05 |
| Tubulin β-2B chaina | Cytoskeletal | N | N | Y | Q9BVA1 | 15 | 46.07 |
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, β polypeptidea | Protein binding | N | N | Y | Q59EQ2 | 2 | 24.39 |
| Vimentina,b | Cytoskeletal remodeling | Y C328 (PMID: 20585580) | Y | Y | P08670 | 18 | 50.22 |
| Vinculina | Cytoskeletal actin binding | Y (PMID: 17704302) | N | Y | P18206 | 3 | 5.11 |
| Vitamin D-binding proteina | Transport | N | Y | N | P02774 | 2 | 5.88 |
| Zinc finger protein 641a | Transcriptional activator | N | Y | Y | Q96N77 | 13 | 40.69 |
NP, nonpregnant estrogen-primed guinea pig; P, pregnant near term 60- to 67-day timed pregnant; Y, yes; N, no; PMID, PubMed identification number.
Proteins were identified by LTQ-Orbitrap LC-MS/MS.
Proteins were identified by MALDI TOF/TOF.
As identified by spot cutting of Nitrosyl-DIGE gels and MALDI/TOF/TOF analysis.
Controls.
Stringent controls were performed to avoid false-positive identification of non-S-nitrosylated cysteines that could have been mislabeled during the experimental procedures. These controls are standard when the biotin switch procedure is performed and include removal of GSNO and/or ascorbate during the biotin or fluorescent switch. A small number of proteins were shown to be constitutively S-nitrosylated and were labeled without the addition of GSNO; this is a common and expected result. Removal of ascorbate during the biotin or fluorescent switch removed any signal that was seen when ascorbate was present. The fluorescent switch or streptavidin purification of biotin switched proteins removes any signal from naturally biotinylated proteins, and these are therefore not present in our analysis. Streptavidin purification and LC-MS/MS analysis of ascorbate-negative samples were shown to only contain the contaminating keratin proteins, trypsin and serum albumin. Therefore, our ascorbate-positive sample identification contains almost no nonspecific binding proteins. Dye swapping and calibration with identical samples was performed on nitrosyl-DIGE samples, and it was determined that differences more than 1.5-fold as found by DeCyder software were real.
Data Analysis.
All data were analyzed using Prism 5 and Instat 3 (GraphPad Software, San Diego, CA). Student's t test and one-way analysis of variance were used with p < 0.05 considered significant.
Results
The first step in identifying differentially S-nitrosylated proteins in USM was to elucidate the overall USM S-nitrosylproteome. We used the biotin switch technique (Fig. 1) coupled with streptavidin pulldown to isolate all S-nitrosylated proteins from both pregnant and nonpregnant USM tissue. Isolated samples were identified on an LTQ-Orbitrap LC-MS/MS system; all biological samples were examined in triplicate to avoid false-positive results. Three different biological samples were used for each group (nonpregnant and pregnant). Each of these samples was subjected to multiple analysis involving nitrosyl-DIGE and LC-MS/MS after biotin switch and streptavidin pulldown. Only proteins that were present in a minimum of two of the three LC-MS/MS analyses or present in all nitrosyl-DIGE gels are included in Table 1. Note that endogenously biotinylated proteins such as CoA-carboxylase remain attached to the beads after the proteins of interest are eluted and are thus not seen (see Materials and Methods). To identify proteins that show disparate levels of S-nitrosylation, we used a technique that we are calling “nitrosyl-DIGE.” In brief, S-nitrosylation of pregnant and nonpregnant samples is induced with the biologically relevant NO donor GSNO, and samples are “fluorescently switched” so that the induced S-NO is exchanged for S-maleimide dye as described under Materials and Methods. The labeled samples are then separated using two-dimensional in-gel electrophoresis and analyzed for increases/decreases in spot intensity compared with a control sample that contains a mixture of every sample in the entire experiment. Differences greater than 1.5-fold were determined to be statistically significant on the basis of rigorous controls. Spots of interest (Fig. 2) were excised, trypsin-digested, and analyzed by MS/MS or LC-MS/MS and identified using Mascot and Scaffold software. Spots that were repeatedly identified from multiple nitrosyl-DIGE gels with high confidence are included in Table 1.
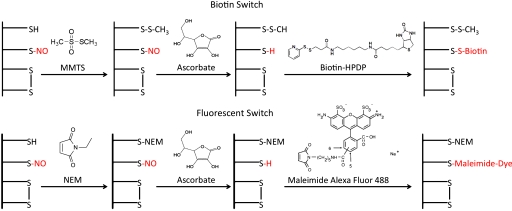
Fig. 1.
Representative schematic diagram of the biotin switch and the fluorescent switch. Proteins that have been previously S-nitrosylated can be either labeled with biotin-HPDP or an Alexa Fluor-maleimide dye. Step 1 involves blocking of all free thiols with either methyl methanethiosulfonate (MMTS) for the biotin switch or NEM for the fluorescent switch. Step 2 involves the reduction of all S-nitrosylated thiols with ascorbate. Reduced thiols, which were originally S-nitrosylated, are then labeled with biotin-HPDP or an Alexa Fluor-maleimide dye. The Alexa Fluor dye in step 3 of the fluorescent switch can also be 555 or 647 maleimide dyes.
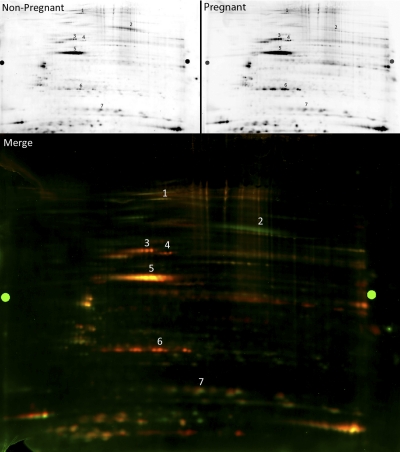
Fig. 2.
Nitrosyl-DIGE analysis of nonpregnant and pregnant guinea pig USM. A representative nitrosyl-DIGE showing individual channels (red fluor, pregnant; green fluor, nonpregnant) as well as the two-dye overlay. Numbered spots were cut, trypsin-digested, and identified by MS analysis. 1, transitional endoplasmic reticulum ATPase; 2, serotransferrin; 3, vimentin; 4, desmin; 5, actin; 6, HSP27; 7, transgelin.
Our list of S-nitrosylated USM proteins is the first from USM tissue and includes a number of interesting and functionally important proteins involved in USM contraction/relaxation dynamics (Table 2). Of the 118 unique S-nitrosylated proteins that we identified, 51 are novel targets of S-nitrosylation not previously described and several, including desmin, transgelin, myosin light polypeptide 9, and myosin light chain kinase (MYLK) are known to have important roles in smooth muscle contraction/relaxation dynamics (Tang, 2008; Han et al., 2009).
TABLE 2.
S-Nitrosylated proteins identified in guinea pig USM that are involved in contraction/relaxation dynamics
A total of 34 S-nitrosylated proteins were identified that are known to play a role in either cytoskeletal rearrangement or the regulation of contraction/relaxation in smooth muscle tissues, 15 of these are novel targets of S-nitrosylation not previously identified and 20 are selectively S-nitrosylated during pregnancy.
| Protein | Biological Process | Previously Identified | NP | P | UniProt |
|---|---|---|---|---|---|
| Transgelina,b | Actin binding, contraction | N | N | Y | Q01995 (TAGL_Hu) |
| Destrina,b | Actin depolymerization | Y (PMID: 18670085) | N | Y | P60981 (DEST_Hu) |
| Tubulin β chaina,b | Cytoskeletal | Y (PMID: 16418269) | Y | Y | P07437 (TBB5_Hu) |
| α-Actinin-1a,b | Cytoskeletal | Y C332 (PMID: 20585580) | N | Y | P12814 (ACTN1_Hu) |
| Desmina,b | Cytoskeletal | Y C333 (PMID: 20585580) | Y | Y | P17661 (DESM_Hu) |
| Actin, cytoplasmic 1a | Cytoskeletal | Y (PMID: 20585580) | Y | Y | P60709 (ACTB_Hu) |
| Actin, α skeletal musclea | Cytoskeletal | Y (PMID: 10961840) | Y | Y | P68133 (ACTS_Hu) |
| Tubulin β-2B chaina | Cytoskeletal | N | N | Y | Q9BVA1 (TBB2B_Hu) |
| Vinculina | Cytoskeletal actin binding | Y (PMID: 17704302) | N | Y | P18206 (VINC_Hu) |
| Cofilin-1a | Cytoskeletal actin binding | N | Y | Y | P23528 (COF1_Hu) |
| Filamin-Ca | Cytoskeletal actin cross-linker | Y (PMID: 20585580) | N | Y | Q14315 (FLNC_Hu) |
| Annexin A2a,b | Cytoskeletal adapter | Y (PMID: 12199706) | N | Y | P07355 (ANXA2_Hu) |
| PDZ and LIM domain protein 1a | Cytoskeletal adapter (interacts with α-actinins 1, 2, and 4) | N | N | Y | O00151 (PDLI1_Hu) |
| Profilin-1a | Cytoskeletal remodeling | Y (PMID: 18283105) | N | Y | P07737 (PROF1_Hu) |
| Vimentina,b | Cytoskeletal remodeling | Y C328 (PMID: 20585580) | Y | Y | P08670 (VIME_Hu) |
| Ras-related protein R-Rasa | Cytoskeletal remodeling | N | N | Y | P10301 (RRAS_Hu) |
| Calpain small subunit 1a | Cytoskeletal remodeling and signal transduction | N | N | Y | P04632 (CPNS1_Hu) |
| Heat shock protein β-1a,b | Cytoskeletal remodeling and signal transduction | Y (PMID: 18670085) | Y | Y | P04792 (HSPB1_Hu) |
| Filamin-Aa | Cytoskeletal scaffold actin binding | Y C717 (PMID: 20585580) | Y | Y | P21333 (FLNA_Hu) |
| Phosphoglucomutase-like protein 5a | Cytoskeletal; interacts with dystrophin and utrophin | N | N | Y | Q15124 (PGM5_Hu) |
| Ankyrin-3a | Cytoskeletal-membrane linker | Y (PMID: 20585580) | Y | N | Q12955 (ANK3_Hu) |
| Talin-1a | Cytoskeletal-membrane connector | N | Y | Y | Q9Y490 (TLN1_Hu) |
| Kinesin family member 17a | Microtubule-based movement | N | N | Y | A2A3Q7 (A2A3Q7_Hu) |
| Transforming growth factor β-1-induced transcript 1 proteina | Molecular adapter at focal adhesion complexes | N | Y | Y | O43294 (TGFI1_Hu) |
| Protein S100-A10a | Protein binding, induces the dimerization of ANXA2/p36 | N | N | Y | P60903 (S10AA_Hu) |
| Gi subunit α-2a | Signal transduction | N | N | Y | P04899 (GNAI2_Hu) |
| Myosin regulatory light polypeptide 9a | Smooth muscle contraction | ? (PMID: 20585450) | N | Y | P24844 (MYL9_Hu) |
| Myosin-11a | Smooth muscle contraction | ? (PMID: 20585450) | N | Y | P35749 (MYH11_Hu) |
| Myosin light polypeptide 6a,b | Smooth muscle contraction | ? (PMID: 20585450) | Y | Y | P60660 (MYL6_Hu) |
| Myosin light chain kinase, smooth musclea | Smooth muscle contraction | N | N | Y | Q15746 (MYLK_Hu) |
| Tropomyosin β-chaina,b | Smooth muscle contraction is regulated by interaction with caldesmon | Y C170 (PMID: 19447776) | Y | Y | P07951 (TPM2_Hu) |
| Tropomyosin α-4 chaina | Smooth muscle contraction is regulated by interaction with caldesmon | Y C170 (PMID: 18992711) | N | Y | P67936 (TPM4_Hu) |
| Calponin-1a | Smooth muscle contraction | N | Y | N | P51911 (CNN1_Hu) |
NP, nonpregnant estrogen-primed guinea pig; P, pregnant near term 60- to 67-day timed pregnant; Y, yes; N, no; Hu, human; PMID, PubMed identification number.
Proteins were identified by LTQ-Orbitrap LC-MS/MS.
Proteins were identified by MALDI TOF/TOF.
Two methods were used to identify disparate protein S-nitrosylation in USM. The “biotin switch” coupled with streptavidin pulldown and LC-MS/MS analysis of proteins labeled in nonpregnant and pregnant USM was used to identify the complete S-nitrosylproteome of each tissue. Of the 118 total proteins identified, 10 were found to be unique to nonpregnant tissue, 75 were found to be unique to pregnant tissue, and 33 were found to be present in both nonpregnant and pregnant tissue (Table 1). This list contains numerous proteins that are integral to smooth muscle contraction/relaxation dynamics including desmin, vimentin, heat shock protein β-1 (HSP27), transgelin, myosin, actin, MYLK, and Giα (Table 1).
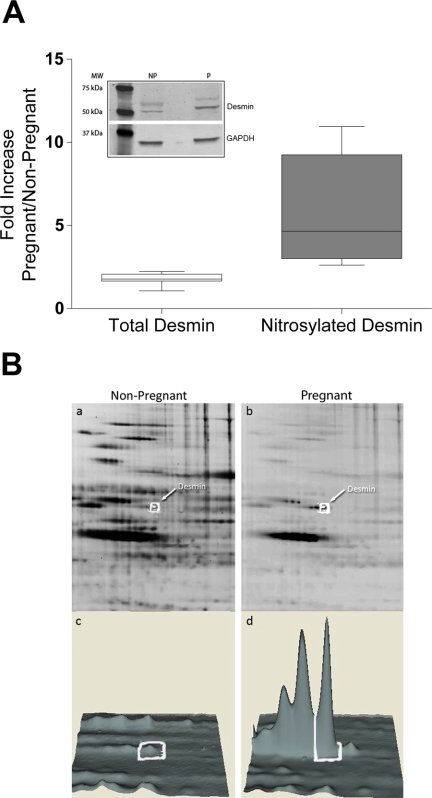
The nitrosyl-DIGE technique identified several candidate proteins that exceeded the significant 1.5-fold difference in spot intensity. In particular, we determined that desmin (5.9-fold increase in pregnancy), vimentin (3.8-fold increase in pregnancy), and transgelin (4.6-fold increase in pregnancy) all showed a statistically significant difference in S-nitrosylated state when pregnant and nonpregnant USM tissue were compared. Western blot analysis of total levels of desmin protein in pregnant and nonpregnant samples showed an approximately 2-fold increase in total protein levels of desmin; this is in agreement with previous analysis of intermediate filament (IF) increases during pregnancy (Leoni et al., 1990). However, the increase in protein level was not sufficient by itself to account for the near 6-fold increase in the level of S-nitrosylation seen (Fig. 3). Desmin has a single cysteine (Cys-333) that could undergo S-nitrosylation, and, therefore, any increase in measured levels indicates an increase in total S-nitrosylated desmin rather than an increase in the number of S-nitrosylation events per protein.
Fig. 3.
Comparison of increased levels of total desmin protein versus total levels of S-nitrosylated desmin. A, total desmin protein level from nonpregnant (NP) and pregnant (P) USM was separated by SDS-polyacrylamide gel electrophoresis, and a Western blot was performed using a desmin-specific antibody. Total desmin was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the fold increase was calculated to be 1.792. B, total desmin S-nitrosylation was determined from three independent nitrosyl-DIGE experiments using DeCyder software, and the fold increase was calculated to be 5.733. A Student's t test was performed to analyze the difference in means between groups, and the result was shown to be statistically significant with p = 0.0005. MW, molecular weight.
Discussion
Elucidation of the guinea pig USM S-nitrosylproteome is the first step in identifying mechanistically important proteins regulated by S-nitrosylation. The novel relaxation cascade induced by NO in USM argues for a tissue-specific role of S-nitrosylation. To our knowledge this is the first work examining the S-nitrosylproteome in USM tissue.
We have used GSNO at a seemingly high concentration if one compares the effort to S-nitrosylate target proteins in this work with the classic action of NO as an activator of purified soluble guanylyl cyclase. This, however, is not the best comparison for the nonclassic effects of NO in nitrosylating proteins on cysteine residues. Direct effects of NO on purified soluble guanylyl cyclase are in the nanomolar range (Cary et al., 2006), but nanomolar stimulation of cGMP accumulation in tissues by NO donors in vitro is ineffective because of the many factors associated with time-dependent reactive NO delivery (estimated for 300 μM GSNO at 5 nmol · min−1 · ml−1, exhausted after 15 min) (Cleeter et al., 1994), the presence or absence of thiol reagents, and penetration into the tissue. Our experiments using 300 μM GSNO with tissue lysates will expose proteins to 5 μM reactive •NO (•NO does not accumulate). Some effects of NO such as those of GSNO on mitochondrial function require concentrations from 100 to 500 μM (Cleeter et al., 1994). The relaxant actions of NO donors in smooth muscle are half-maximal in the 1 to 10 μM range (Norman and Cameron, 1996; Buxton et al., 2001; Modzelewska and Kostrzewska, 2005), although, notably, a 300 μM median effective dose of NO donor was required to relax rat myometrium in studies by Buhimschi et al. (1997).
In examining the nonclassic effects of NO, it is not possible to readily predict the levels of NO as liberated from NO donors at the protein level. However, one can model the levels of NO that may be available physiologically. The model prepared by Lim et al. (2008) predicts [GSNO] ranging from 1 to 5 μM. These predictions for [GSNO] are reasonable given the micromolar levels determined in various tissues from humans and animal models of human disease. For example, Kluge et al. (1997) measured [GSNO] of 6 to 8 μM in normal rat cerebellum, whereas Stamler et al. (1992) have found nitrosothiol levels, in general, ranging from 7 μM in blood to 15 to 20 μM in pulmonary fluids (Gaston et al., 1993). This range does not factor in possible compartmentalization or increased levels of nitrosothiols during nitrosative stress or activated NO signaling. Higher concentrations are likely in the cell and are necessary for some physiological effects of S-nitrosylation. Padgett and Whorton (1995) found that half-maximal inhibition of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation using GSNO occurred between 200 and 700 μM and increasing in the presence of dithiothreitol. There is also evidence that GSNO, GSH, and S-nitrosothiols are in a dynamic equilibrium and a high concentration of GSNO will push the balance toward the highest number of S-nitrosylated proteins. To identify specificity as well as the maximum number of candidate S-nitrosylated proteins, we chose to use 300 μM GSNO.
Desmin and vimentin are IF proteins that have been shown to be involved in regulation of smooth muscle contraction/relaxation in USM cells (Leoni et al., 1990). Smooth muscles are unique in that they are able to adjust their contraction/relaxation status by reorganizing the actin cytoskeleton and the IF network (Tang, 2008). We have shown that levels of S-nitrosylated desmin increase in a statistically significant manner in pregnant tissue compared with that in nonpregnant tissue (Fig. 3). We propose that one method of regulation of contraction/relaxation is through selective S-nitrosylation of desmin and reorganization of the cytoskeleton. Vimentin is thought to be involved in force development in smooth muscle tissues (Wang et al., 2006), which argues that regulation by S-nitrosylation could involve inhibition of the contractile state and therefore promotion of relaxation.
HSP27 has been shown to have a regulatory role in smooth muscle contraction (Somara et al., 2009). Its regulatory interaction with thin filaments has been studied in several smooth muscle systems with postsecondary modifications (i.e., phosphorylation) being the main process of regulation (Kostenko and Moens, 2009). HSP27 has also been shown to be S-nitrosylated under various conditions, leading to the possibility that regulation of HSP27 could be dependent on its nitrosylated state (Shi et al., 2008). HSP27 has also been shown to be highly induced in the myometrium during pregnancy and labor (White et al., 2005). Therefore, we consider HSP27 to be a prime candidate for future studies regarding the possible role of S-nitrosylation as a regulatory mechanism for HSP27 action.
Transgelin (also designated SM22α and p27) is a 22-kDa smooth muscle protein that physically associates with cytoskeletal actin filament bundles in contractile smooth muscle cells. Studies in transgelin knockout mice have demonstrated a pivotal role of transgelin in the regulation of Ca2+-independent contractility (Je and Sohn, 2007). Transgelin has also been implicated in induction of actin polymerization and/or stabilization of F-actin and is proposed to be necessary for actin polymerization and bundling (Han et al., 2009). We show S-nitrosylation of transgelin in USM tissue and also that it is only able to be S-nitrosylated during pregnancy. This observation argues for a plausible role of modulating the contractile state of USM through S-nitrosylation of transgelin during pregnancy.
Myosin and actin have both been shown to be S-nitrosylated in skeletal muscle with myosin being reversibly inhibited by S-nitrosylation with GSNO (Nogueira et al., 2009). Exposure of skeletal and cardiac myosins to physiological concentrations of nitrogen oxides, including the endogenous nitrosothiol S-nitroso-l-cysteine, reduced the velocity of actin filaments over myosin in a dose-dependent and oxygen-dependent manner, caused a doubling of force as measured in a laser trap transducer, and caused S-nitrosylation of cysteines in the myosin heavy chain (Evangelista et al., 2010). Inhibition of the Mg2+-ATPase activity of myosin and actomyosin by GSNO provides a plausible explanation for the functional effects of SNO donors in muscle fibers (Nogueira et al., 2009). We show for the first time that myosin-11 and myosin regulatory light peptide 9 are able to become S-nitrosylated during pregnancy but not in nonpregnant USM. Calponin-1 was shown to be S-nitrosylated only in nonpregnant USM, and its interaction with actin and inhibition of actomyosin Mg2+-ATPase activity argue for a plausible role in the regulation of relaxation during pregnancy.
MYLK is a serine/threonine protein kinase whose role is to phosphorylate myosin regulatory light chain, which in turn initiates actin-myosin ATPase on myosin heavy chains and thus myometrial cross-bridge cycling and contraction (Kamm and Stull, 1989). We have shown that MYLK is able to be S-nitrosylated in pregnant USM but not in nonpregnant USM. The effect of this modification is currently unknown, and it could plausibly play a role in inhibiting phosphorylation of myosin light chain and hence promote relaxation of pregnant USM during periods of quiescence. Further research should be conducted to probe this interesting question to identify the mechanistic role of MYLK S-nitrosylation.
One of the hypothesized modes for maintaining quiescence in the uterus is by activation of adenylate cyclases and the production of cAMP. cAMP is thought to promote the relaxation of myometrial and other smooth muscle cells via activation of cAMP-dependent protein kinase and downstream phosphorylation of MYLK (Price and Bernal, 2001). Giα is an inhibitor of adenylate cyclases shown to be present in the myometrium and hence promotes contraction (Yuan and Lopez Bernal, 2007). We have shown for the first time that Giα is S-nitrosylated in USM and that it is selectively S-nitrosylated only during pregnancy. This leads to the possibility that S-nitrosylation of Giα could maintain the uterus in a quiescent state by preventing inhibition of adenylate cyclase and hence production of cAMP. This possibility notwithstanding, a major role for cAMP action in uterine quiescence is controversial because it is well known that β2 agonists such as ritodrine (Yutopar) have little effect as tocolytic agents and no longer have an U.S. Food and Drug Administration indication for the treatment of preterm labor. Yutopar has been discontinued by the manufacturer.
We unambiguously identified 118 proteins of which 10 were found to be unique to nonpregnant tissue, 75 were found to be unique to pregnant tissue, and 33 were found to be present in both nonpregnant and pregnant tissue. Of these 118 proteins we believe that 51 are novel targets of S-nitrosylation not previously identified in the searchable literature. This list also includes an interesting subset of proteins known to be involved in smooth muscle contraction/relaxation dynamics. Work is presently being performed to isolate biologically significant SNO proteins that will then be used to unambiguously localize S-nitrosylation sites. However, the identification of the overall S-nitrosylproteome first is important because it allows investigators to identify proteins of interest to further study mechanistic changes induced by S-nitrosylation.
Further research into what role these pregnancy state-dependent S-nitrosylations play in the regulation of contraction/relaxation needs to be completed to understand how they affect the progression of pregnancy. Our work described here provides a starting point.
Acknowledgments
We are grateful to Professor David A. Schooley for critical review of the manuscript and to Sara B. Thompson for expert technical assistance.
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant R01-HD053028]; National Institutes of Health National Center for Research Resources [Grant P20-RR016464]; and a Gates Grand Challenges Grant (to I.L.O.B.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- USM
- uterine smooth muscle
- NO
- nitric oxide
- LY 83583
- 6-anilino-5,8-quinolinedione
- NEM
- N-ethylmaleimide
- CHAPS
- 3-(3-cholamidopropyl)dimethylammonio-1-propanesulfonate
- biotin-HPDP
- N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio) propionamide
- GSNO
- S-nitroso-glutathione
- LC
- liquid chromatography
- MS/MS
- tandem mass spectrometry
- DIGE
- two-dimensional in-gel electrophoresis
- MS
- mass spectrometry
- MALDI
- matrix-assisted laser desorption ionization
- TOF/TOF
- tandem time of flight
- S/N
- signal/noise ratio
- SNO
- S-nitrosothiol
- MYLK
- myosin light chain kinase
- HSP27
- heat shock protein β-1
- IF
- intermediate filament.
Authorship Contributions
Participated in research design: Ulrich and Buxton.
Conducted experiments: Ulrich, Quilici, Schegg, Woolsey, and Nordmeier.
Performed data analysis: Ulrich and Buxton.
Wrote or contributed to the writing of the manuscript: Ulrich and Buxton.
References
- Behrman RE, Butler AS, editors (2006) Preterm Birth: Causes, Consequences, and Prevention, National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- Bradley KK, Buxton IL, Barber JE, McGaw T, Bradley ME. (1998) Nitric oxide relaxes human myometrium by a cGMP-independent mechanism. Am J Physiol 275:C1668–C1673 [DOI] [PubMed] [Google Scholar]
- Buhimschi C, Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. (1997) Contrasting effects of diethylenetriamine-nitric oxide, a spontaneously releasing nitric oxide donor, on pregnant rat uterine contractility in vitro versus in vivo. Am J Obstet Gynecol 177:690–701 [DOI] [PubMed] [Google Scholar]
- Buxton IL. (2004) Regulation of uterine function: a biochemical conundrum in the regulation of smooth muscle relaxation. Mol Pharmacol 65:1051–1059 [DOI] [PubMed] [Google Scholar]
- Buxton IL, Crow W, Mathew SO. (2000) Regulation of uterine contraction: mechanisms in preterm labor. AACN Clin Issues 11:271–282 [DOI] [PubMed] [Google Scholar]
- Buxton IL, Kaiser RA, Malmquist NA, Tichenor S. (2001) NO-induced relaxation of labouring and non-labouring human myometrium is not mediated by cyclic GMP. Br J Pharmacol 134:206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary SP, Winger JA, Derbyshire ER, Marletta MA. (2006) Nitric oxide signaling: no longer simply on or off. Trends Biochem Sci 31:231–239 [DOI] [PubMed] [Google Scholar]
- Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345:50–54 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Milzani A, Giustarini D, Di Simplicio P, Colombo R, Rossi R. (2000) S-NO-actin: S-nitrosylation kinetics and the effect on isolated vascular smooth muscle. J Muscle Res Cell Motil 21:171–181 [DOI] [PubMed] [Google Scholar]
- Evangelista AM, Rao VS, Filo AR, Marozkina NV, Doctor A, Jones DR, Gaston B, Guilford WH. (2010) Direct regulation of striated muscle myosins by nitric oxide and endogenous nitrosothiols. PLoS One 5:e11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston B, Reilly J, Drazen JM, Fackler J, Ramdev P, Arnelle D, Mullins ME, Sugarbaker DJ, Chee C, Singel DJ. (1993) Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci USA 90:10957–10961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Dong LH, Zheng B, Shi JH, Wen JK, Cheng Y. (2009) Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci 84:394–401 [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6:150–166 [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. (2001) The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001:pl1. [DOI] [PubMed] [Google Scholar]
- Je HD, Sohn UD. (2007) SM22alpha is required for agonist-induced regulation of contractility: evidence from SM22alpha knockout mice. Mol Cells 23:175–181 [PubMed] [Google Scholar]
- Kamm KE, Stull JT. (1989) Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol 51:299–313 [DOI] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74:5383–5392 [DOI] [PubMed] [Google Scholar]
- Kirsch M, Büscher AM, Aker S, Schulz R, de Groot H. (2009) New insights into the S-nitrosothiol-ascorbate reaction. The formation of nitroxyl. Org Biomol Chem 7:1954–1962 [DOI] [PubMed] [Google Scholar]
- Kluge I, Gutteck-Amsler U, Zollinger M, Do KQ. (1997) S-Nitrosoglutathione in rat cerebellum: identification and quantification by liquid chromatography-mass spectrometry. J Neurochem 69:2599–2607 [DOI] [PubMed] [Google Scholar]
- Kostenko S, Moens U. (2009) Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci 66:3289–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzli KA, Bradley ME, Buxton IL. (1996) Cyclic GMP-independent effects of nitric oxide on guinea-pig uterine contractility. Br J Pharmacol 119:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzli KA, Buxton IL, Bradley ME. (1998) Nitric oxide regulation of monkey myometrial contractility. Br J Pharmacol 124:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni P, Carli F, Halliday D. (1990) Intermediate filaments in smooth muscle from pregnant and non-pregnant human uterus. Biochem J 269:31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CH, Dedon PC, Deen WM. (2008) Kinetic analysis of intracellular concentrations of reactive nitrogen species. Chem Res Toxicol 21:2134–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BF, Taggart MJ. (2009) Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 297:R525–R545 [DOI] [PubMed] [Google Scholar]
- Modzelewska B, Kostrzewska A. (2005) The influence of methylene blue on the spontaneous contractility of the non-pregnant human myometrium and on the myometrial response to DEA/NO. Cell Mol Biol Lett 10:389–400 [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75:4646–4658 [DOI] [PubMed] [Google Scholar]
- Nogueira L, Figueiredo-Freitas C, Casimiro-Lopes G, Magdesian MH, Assreuy J, Sorenson MM. (2009) Myosin is reversibly inhibited by S-nitrosylation. Biochem J 424:221–231 [DOI] [PubMed] [Google Scholar]
- Norman JE, Cameron IT. (1996) Nitric oxide in the human uterus. Rev Reprod 1:61–68 [DOI] [PubMed] [Google Scholar]
- Padgett CM, Whorton AR. (1995) S-Nitrosoglutathione reversibly inhibits GAPDH by S-nitrosylation. Am J Physiol 269:C739–C749 [DOI] [PubMed] [Google Scholar]
- Price SA, Bernal AL. (2001) Uterine quiescence: the role of cyclic AMP. Exp Physiol 86:265–272 [DOI] [PubMed] [Google Scholar]
- Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. (1992) In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem 203:173–179 [DOI] [PubMed] [Google Scholar]
- Seth D, Stamler JS. (2011) The SNO-proteome: causation and classifications. Curr Opin Chem Biol 15:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Feng J, Qu H, Cheng YY. (2008) A proteomic study of S-nitrosylation in the rat cardiac proteins in vitro. Biol Pharm Bull 31:1536–1540 [DOI] [PubMed] [Google Scholar]
- Smith MA, Silverstein JL, Westfall DP, Buxton IL. (1989) Dissociation between adenosine receptors and adenylate cyclase in the smooth muscle of guinea pig myometrium. Cell Signal 1:357–365 [DOI] [PubMed] [Google Scholar]
- Somara S, Gilmont R, Bitar KN. (2009) Role of thin-filament regulatory proteins in relaxation of colonic smooth muscle contraction. Am J Physiol Gastrointest Liver Physiol 297:G958–G966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. (1992) Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA 89:7674–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Mori C, Yoshikawa H, Miyazaki Y, Kansaku N, Tanaka K, Morita H, Takizawa T. (2009) Changes in nitric oxide production levels and expression of nitric oxide synthase isoforms in the rat uterus during pregnancy. Biosci Biotechnol Biochem 73:2163–2166 [DOI] [PubMed] [Google Scholar]
- Tang DD. (2008) Intermediate filaments in smooth muscle. Am J Physiol Cell Physiol 294:C869–C878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichenor SD, Malmquist NA, Buxton IL. (2003) Dissociation of cGMP accumulation and relaxation in myometrial smooth muscle: effects of S-nitroso-N-acetylpenicillamine and 3-morpholinosyndonimine. Cell Signal 15:763–772 [DOI] [PubMed] [Google Scholar]
- Valdes G, Corthorn J. (2011) Review: The angiogenic and vasodilatory utero-placental network. Placenta 32 (Suppl 2):S170–S175 [DOI] [PubMed] [Google Scholar]
- Wang R, Li Q, Tang DD. (2006) Role of vimentin in smooth muscle force development. Am J Physiol Cell Physiol 291:C483–C489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BG, Williams SJ, Highmore K, Macphee DJ. (2005) Small heat shock protein 27 (Hsp27) expression is highly induced in rat myometrium during late pregnancy and labour. Reproduction 129:115–126 [DOI] [PubMed] [Google Scholar]
- Xue Y, Liu Z, Gao X, Jin C, Wen L, Yao X, Ren J. (2010) GPS-SNO: computational prediction of protein S-nitrosylation sites with a modified GPS algorithm. PLoS One 5:e11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Lopez Bernal A. (2007) Cyclic AMP signalling pathways in the regulation of uterine relaxation. BMC Pregnancy Childbirth 7 (Suppl 1):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]