Abstract
Human organic anion-transporting polypeptide (OATP) 2B1 (OATP-B; SLCO2B1) is expressed in the apical membrane of the small intestine and the hepatocyte basolateral membrane and transports structurally diverse organic anions with a wide spectrum of pH sensitivities. This article describes highly pH-dependent OATP2B1-mediated antifolate transport and compares this property with that of sulfobromophthalein (BSP), a preferred OATP2B1 substrate. At pH 5.5 and low substrate concentrations (∼2.5 μM), only [3H]pemetrexed influx [in contrast to methotrexate (MTX), folic acid, and reduced folates] could be detected in OATP2B1-transfected HeLa R1-11 cells that lack endogenous folate-specific transporters. Influx was optimal at pH 4.5 to 5.5, falling precipitously with an increase in pH >6.0; BSP influx was independent of pH. Influx of both substrates at low pH was markedly inhibited by the proton ionophore 4-(trifluoromethoxy)phenylhydrazone; BSP influx was also suppressed at pH 7.4. At 300 μM MTX, influx was one-third that of pemetrexed; influx of folic acid, (6S)5-methyltetrahydrofolate, or (6S)5-formyltetrahydrofolate was not detected. There were similar findings in OATP2B1-expressing Xenopus laevis oocytes. The pemetrexed influx Km was ∼300 μM; the raltitrexed influx Ki was ∼70 μM at pH 5.5. Stable expression of OAPT2B1 in HeLa R1-11 cells resulted in substantial raltitrexed, but modest pemetrexed, growth inhibition consistent with their affinities for this carrier. Hence, OATP2B1 represents a low-affinity transport route for antifolates (relative affinities: raltitrexed > pemetrexed > MTX) at low pH. In contrast, the high affinity of this transporter for BSP relative to antifolates seems to be intrinsic to its binding site and independent of the proton concentration.
Introduction
Antifolates, the first antimetabolites introduced into the clinics, remain an important class of agents in cancer chemotherapy. Methotrexate (MTX), a dihydrofolate reductase inhibitor, continues to play an essential role in the treatment of acute lymphoblastic leukemia, lymphomas, and solid tumors worldwide. Raltitrexed, a thymidylate synthase inhibitor was introduced outside of the United States in the 1990s. Pemetrexed, primarily a thymidylate synthase inhibitor but with additional pharmacologic activities associated with inhibition of purine synthesis, was introduced in 2004. Most recently, pralatrexate, a dihydrofolate reductase inhibitor with enhanced properties relative to MTX, was approved for the treatment of cutaneous T-cell lymphoma. These agents are all hydrophilic and diffuse poorly across the lipid membrane of cells; hence, they require for activity utilization of processes that facilitate the transport of physiological folates into cells. The evolution and current status of the antifolates were the subject of a recent review (Goldman et al., 2010).
The reduced folate carrier (RFC) is the major route of transport of the physiological folate, 5-methyltetrahydrofolate (5-methylTHF), and antifolates into systemic tissues and human tumor cells. This is an organic anion antiporter that functions optimally at neutral pH. Early on, the critical role that RFC plays as a determinant of MTX activity and drug resistance was recognized. The proton-coupled folate transporter (PCFT) is also expressed to varying extents in normal and malignant human cells and is the mechanism by which folates and antifolates are absorbed in the acidic milieu at the apical brush-border membrane of the proximal small intestine. Folate receptor α represents a third highly specific endocytic process expressed primarily at the proximal renal tubule, choroid plexus, retinal pigment epithelium, the placenta and, broadly, in malignant cells; the β-isoform is expressed in placenta and malignant cells of hematopoietic origin. The mechanisms of transport mediated by these folate-specific processes have been the subjects of several recent reviews (Matherly and Hou, 2008; Zhao et al., 2009, 2011).
There is little information regarding other less specific facilitative mechanisms that transport folates and antifolates into cells and/or are involved in their intestinal absorption. Identification of these transporters has relied on the utilization of folic acid or MTX, usually at neutral pH, an approach that might overlook routes with selective higher affinities for other folates and/or antifolates and/or low pH optima. For instance, PCFT was identified because of its high affinity for pemetrexed at low pH (Qiu et al., 2006). A number of members of the organic anion transporting polypeptide have been noted to have prominent activities at low and neutral pH (Leuthold et al., 2009). In initial studies, we screened these carriers for their ability to transport a variety of folates and antifolates under acidic conditions and identified one, OATP2B1-SLCO2B1, with pemetrexed transport activity that is the subject of this report. This transporter was of particular interest because it is expressed at the apical brush-border membrane of the small intestine and might therefore represent a route, distinct from PCFT, of absorption of folates and/or antifolates (Kobayashi et al., 2003; Nozawa et al., 2004). Studies in human cells were made possible by the availability of a unique HeLa cell line (HeLa R1-11), that lacks expression of the major endogenous folate-specific transporters (Zhao et al., 2004; Diop-Bove et al., 2009), allowing careful characterization of what turned out to be a relatively lower affinity, but highly selective, antifolate transport process.
Materials and Methods
Reagents.
[3H]Folic acid, [3H](6S)5-formyltetrahydrofolate [(6S)5-formylTHF], [3H](6S)5-methylTHF, [3H]MTX, and [3H]pemetrexed were obtained from Moravek Biochemicals (Brea, CA), and purity was monitored by high-performance liquid chromatography as described previously (Zhao et al., 2000). (6S)5-FormylTHF and (6S)5-methylTHF were purchased from Schircks Laboratories (Jona, Switzerland). Folic acid and MTX were obtained from Sigma-Aldrich (St. Louis, MO), pemetrexed was obtained from the pharmacy, and raltitrexed (Tomudex) was from AstraZeneca Pharmaceuticals LP (Wilmington, DE). FCCP (4-(trifluoromethoxy)phenylhydrazone) was purchased from Thermo Fisher Scientific (Waltham, MA). Sulfobromophthalein (BSP) and [35S]BSP were provided by Dr. Alan Wolkoff (Albert Einstein College of Medicine, Bronx, NY).
Cell Lines, Cell Culture, and Transfection Conditions.
HeLa R1-11 cells that lack RFC and do not express PCFT (Zhao et al., 2004; Diop-Bove et al., 2009) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA), 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. Cells were transiently transfected with the pCMV6-XL4/SLCO2B1 construct generously provided by Dr. Bruno Hagenbuch (University of Kansas Medical Center). Transient transfection was performed in HeLa R1-11 cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Stable HeLa R1-11 OATP2B1-expressing cells (HeLa R1-11-2B1) were generated by Lipofectamine 2000 transfection, followed by hygromycin B selection (300 μg/ml; Calbiochem, San Diego, CA) and were maintained in RPMI 1640 medium with the selection agent.
Subcloning of OATP2B1 into pcDNA3.1(+)hygro.
To isolate the human OATP2B1 open-reading frame (ORF) from the pCMV6-XL4 vector, the original construct was digested with NotI enzyme (New England BioLabs, Ipswich, MA). The pcDNA3.1(+)hygro vector (Invitrogen) was linearized by NotI and dephosphorylated with alkaline phosphatase (Invitrogen). The OATP2B1 ORF and the linearized pcDNA3.1(+)hygro plasmid were purified from a 1% agarose gel by the QIAquick gel extraction kit (QIAGEN GmbH, Hilden, Germany). The isolated insert was ligated into the pcDNA3.1(+)hygro using T4 ligase enzyme (Promega, Madison, WI). The insert was verified by sequencing in the Albert Einstein Cancer Center Genomics Shared Resource.
Total RNA Extraction and Reverse Transcriptase-Polymerase Chain Reaction of OATP2B1.
Total RNA was isolated from cultured cells using TRIzol (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized from 5 μg of total RNA using oligo(dT) primers and Superscript Reverse Transcriptase II (Invitrogen). The final cDNA was used for the subsequent polymerase chain reaction (PCR). Primers for PCR were designed using the Primer3 Input (version 0.4.0; http://frodo.wi.mit.edu/primer3/). The sequence of the forward primer was 5′-CACAGAAACCCAGCATCTGA-3′; the sequence of the reverse primer was 5′-CCATCSTGGTCACTGCAAAC-3′. Expression was evaluated on a 1% agarose gel.
cRNA Synthesis for Expression in Xenopus laevis Oocytes.
pCMV6-XL4 containing the OATP2B1-coding sequence and the X. laevis expression vector pXoon were digested with NotI enzyme. The OATP2B1 ORF and the linearized pXoon were purified from a 1% agarose gel by the QIAquick gel extraction Kit (QIAGEN GmbH). The isolated insert was ligated into pXoon using the T4 ligase enzyme (Promega) and verified by sequencing. The pXoon/OATP2B1 construct was linearized with XbaI enzyme (New England BioLabs) for the synthesis of capped sense OATP2B1 cRNA using the mMESSAGE mMachine system (Ambion, Austin, TX).
Assessment of Initial Rates of BSP, Folate, and Antifolate Uptake.
Initial uptake of tritiated folates/antifolates and BSP in HeLa R1-11 cells was measured using a protocol designed for rapid uptake determinations in cells (Sharif and Goldman, 2000). Forty-eight hours after transfection and growth in monolayer culture at the bottom of glass vials, the medium was aspirated, 1 ml of HBS buffer (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM dextrose, adjusted with 1 N NaOH to achieve pH levels of ≥7.0) was added, and the vials were incubated in a 37°C water bath for 20 min. Buffer was then aspirated, and uptake cocktail containing the labeled reagents was added. MBS uptake buffer (20 mM MES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM dextrose, adjusted with 1 N HCl, was used to achieve pH levels of 7.0). Uptake was stopped after 4 min by injection of 10 volumes of ice-cold HBS buffer at pH 7.4; the cells then were washed three times in this buffer. When intracellular BSP was assessed, the first wash was with ice-cold HBS containing 5% bovine serum albumin. Cells were digested with 0.2 N NaOH at 65°C for 45 min, and then portions of the lysate (400 and 10 μl, respectively) were taken for assessment of intracellular radioactivity on a liquid scintillation spectrometer and protein content by the bicinchoninic acid protein assay (Thermo Fisher Scientific). In all experiments, OATP2B1-independent uptake was determined in mock-transfected cells and is either shown or subtracted from total uptake to quantify OATP2B1-mediated uptake. Influx is expressed as picomoles of tritiated folate or BSP per milligram of protein per minute.
Intracellular Accumulation of Antifolates.
To evaluate antifolate accumulation, HeLa R1-11 cells stably transfected with OATP2B1 (HeLa R1-11-2B1) and mock-transfected cells were trypsinized and grown for 1 to 6 days in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, GAT (0.2 mM glycine, 0.1 mM adenosine, and 0.01 mM thymidine), and 1 μM [3H]pemetrexed or [3H]MTX. After incubation, cells were washed three times in ice-cold HBS and processed as described above.
Growth Inhibition Assay.
Cells stably transfected with OATP2B1 were seeded in 96-well plates at a density of 1 to 2 × 103 cells/well in a medium containing a spectrum of pemetrexed, MTX, or raltitrexed concentrations. Growth rates were quantified after 6 days by sulforhodamine B staining. Absorbance was measured at 540 nm with the VERSAmax plate reader (GE Intelligent Platforms, Charlottesville, VA).
Uptake Studies in X. laevis Oocytes.
Defolliculated oocytes were injected with 50 nl of water or OATP2B1 cRNA (50 ng) and maintained at 16°C in 82.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 2.3 mM CaCl2, and 5 mM HEPES with 5% horse serum at pH 7.5 as described previously (Qiu et al., 2006). Transport was assessed 3 days after cRNA injection in oocytes incubated with buffer consisting of 90 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 10 mM MES, at pH 5.5. Ten oocytes were incubated with 300 μM [3H]folates for 30 min at room temperature, after which the oocytes were washed 10 times in buffer containing 10 mM HEPES, 90 mM NaCl, 1 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2 at pH 7.4. OATP2B1-independent uptake was determined in water-injected oocytes. Oocytes were solubilized in 10% SDS for 2 h, after which fluor was added and radioactivity assessed on a liquid scintillation spectrometer. Influx is expressed as picomoles of tritiated folate per oocyte per 30 min.
Electrophysiological Analyses in X. laevis Oocytes.
For these experiments, X. laevis oocytes were prepared as described previously and maintained in OR3 buffer [containing one pack of powdered Leibovitz L-15 media (in 2.0 liters) with l-glutamine (Invitrogen), 100 ml of solution containing 10,000 U of penicillin and 10,000 U of streptomycin in 0.9% NaCl (Sigma), and 5 mM HEPES (pH 7.5; 195–200 mOsmol/kg)]. At 16°C (Romero et al., 1998). Oocytes that expressed OAT2B1 were perfused with ND90 (90 mM NaCl, 1 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM Tris base, pH 7.5, adjusted with 1 N Tris base) and clamped to −60 mV. The bath pH was then decreased to 5.5 (ND90 with 5 mM MES replacing Tris base, adjusted to pH 5.5 with 1 M MES), and BSP or pemetrexed was added. After 5 min, the oocytes were perfused with drug-free buffer at pH 5.5 for another 5 min, after which the buffer pH was returned to 7.5. Intracellular pH and current were monitored as described previously (Unal et al., 2009).
Using a two-electrode voltage clamp to −60mV, current as a function of voltage (I-V curve) was assessed in OATP2B1 cRNA- or water-injected oocytes. In brief, oocytes were first perfused with ND90, pH 7.5, after which the pH in the ND90 buffer was decreased to pH 5.5; 5 min later, pemetrexed was added to the perfusate. With each solution change, the oocyte was perfused until the current stabilized. Changes in voltage were achieved with an OC-725C voltage clamp (Warner Instruments, Hamden, CT), filtered at 2 to 5 kHz, and digitized at 10 kHz. Output was recorded with Pulse software (HEKA, Lambrecht/Pfalz, Germany) as described previously. Pemetrexed current (IPMX) was calculated as IPMX 5.5 − IpH5.5. The data were analyzed using the PulseFit program (HEKA). When I-V protocols were not being executed, oocytes were clamped at a holding potential (Vh) of −60 mV, and current was continuously monitored and recorded at 1.0 Hz. The I-V protocol consisted of 200-ms intervals from Vh in 20-mV steps between −160 and +60 mV.
Statistics.
Statistical analyses were performed with the two-tailed Student's paired t test using Prism, version 3.0 for Windows (GraphPad Software, Inc., La Jolla, CA).
Results
Functional Properties of OATP2B1.
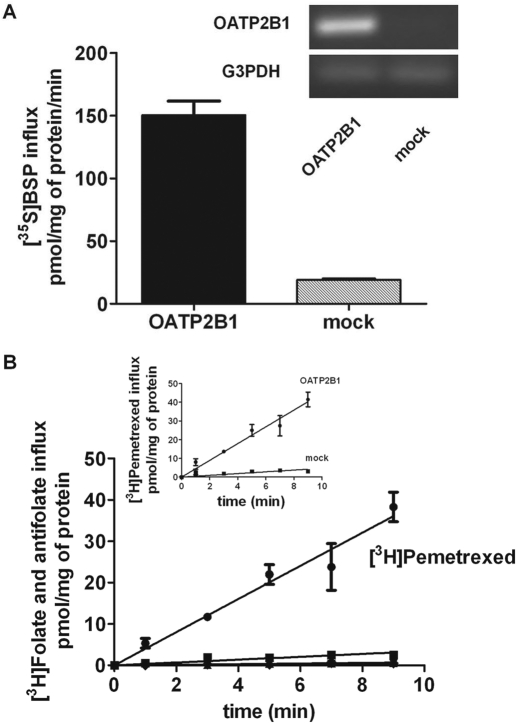
Expression of OATP2B1 in HeLa R1-11 cells transiently transfected with the pCMV6-XL4/SLCO2B1 vector was verified by RT-PCR (Fig. 1A, inset); function was assessed by uptake of 2.5 μM [35S]BSP over 5 min at pH 7.4 (Fig. 1A). BSP is a preferred substrate for this transporter with an influx Km of ∼ 0.7 μM (Kullak-Ublick et al., 2001). Figure 1B illustrates the time course of uptake of tritiated folates and antifolates over an interval of 9 min at an extracellular concentration of 2.5 μM at pH 5.5. The increase in intracellular pemetrexed as a function of time is linear, and the uptake slope extrapolates to the point of origin. Hence, uptake over this interval reflects the unidirectional flux of pemetrexed into these cells. The inset shows uptake in mock-transfected cells, which was trivial under these conditions (Fig. 1B). MTX uptake was slightly higher than, but not significantly different from, uptake in mock-transfected cells (P = 0.08). Uptake of (6S)5-methylTHF, (6S)5-formylTHF, and folic acid was not perceptible.
Fig. 1.
Impact of OATP2B1 expression in HeLa R1-11 cells on BSP and folate transport. A, uptake of 2.5 μM [35S]BSP over 5 min at pH 7.4. The inset shows the OATP2B1 mRNA levels assessed by reverse transcriptase-PCR. glyceraldehyde-3-phosphate dehydrogenase (G3PDH) is the loading control. B, time course of uptake of 2.5 μM tritiated folates and antifolates [■, (6S)5-methylTHF; ▴, MTX; ▾, (6S)5-formylTHF; ♦, folic acid; ●, pemetrexed]. In the inset, influx of 2.5 μM tritiated pemetrexed in OATP2B1- and in mock-transfected cells is represented. All other data were corrected for uptake in mock-transfected cells. Data are the mean ± S.E.M. from three independent experiments.
pH Dependence of OATP2B1-Mediated Pemetrexed and BSP Influx.
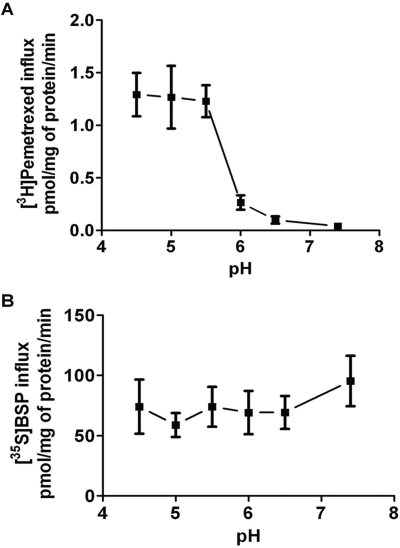
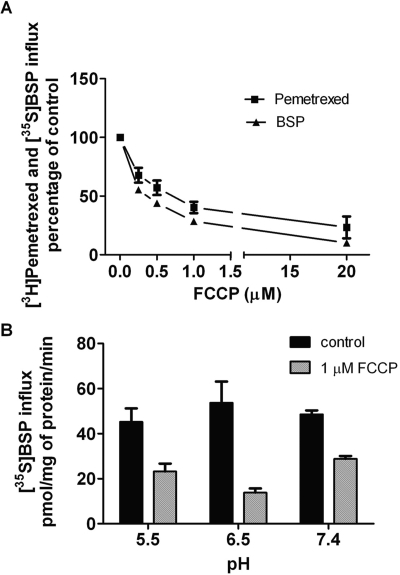
Figure 2A indicates that OATP2B1-mediated pemetrexed influx is highly pH-dependent with the highest activity between pH 4.5 and 5.5, decreasing precipitously when the pH is increased beyond this level and negligible beyond a pH of 6.5. This is in contrast to the pH profile for BSP; influx was unchanged over a pH range of 4.5 to 7.4. The slight apparent increase in influx as the proton concentration was increased from 6.5 to 7.4 was not statistically significant (P = 0.2) (Fig. 2B).
Fig. 2.
pH Dependence of OATP2B1-mediated influx of pemetrexed and BSP. [3H]Pemetrexed (2.5 μM) influx (A) and [35S]BSP (2.5 μM) influx (B) over 4 min as a function of pH in OATP2B1-expressing HeLa R1-11 cells. Data in both panels are corrected for uptake in mock-transfected cells and represent the mean ± S.E.M. from three independent experiments. For the pH values where the influx is optimal, uptake in mock-transfected cells does not exceed 15% that in OATP2B1-transfected cells.
Kinetics of Pemetrexed Influx Mediated by OATP2B1.
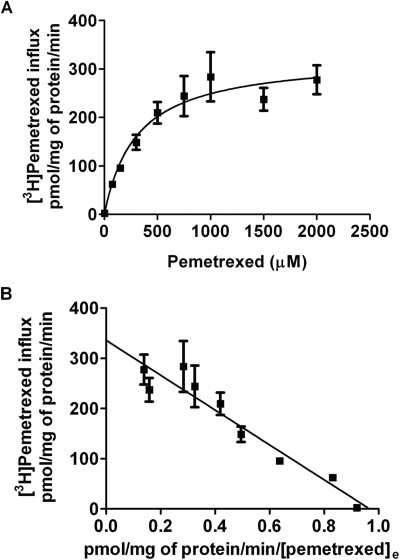
Preliminary studies suggested that the Km for pemetrexed influx was in the 300 μM range. To establish an interval over which pemetrexed uptake was unidirectional under these conditions, the time course of pemetrexed uptake was assessed over 9 min at an extracellular concentration of 300 μM. It can be seen that uptake of pemetrexed as a function of time over this interval was constant with a slope that intercepts the point of origin at zero time, reflecting the influx phase of the uptake process; uptake in mock-transfected cells was trivial under these conditions (Fig. 3A). Influx kinetics was subsequently determined within this interval. As indicated in Fig. 3B, when the MTX concentration was 300 μM, uptake was detected but pemetrexed influx still exceeded that for MTX by a factor of ∼3. Influx of (6S)5-methylTHF, (6S)5-formylTHF, and folic acid was again not perceptible. Influx of pemetrexed as a function of concentration mediated by OATP2B1 then was assessed over 4 min at an extracellular pH of 5.5 and indicated saturability (Fig. 4A). Based upon a nonlinear regression analysis of this data, the influx Km was computed to be 307.1 ± 90.8 μM, and the influx Vmax was calculated as 325.2 ± 28.9 pmol · mg protein−1 · min−1. These kinetics constants are similar to what was obtained with an Eadie-Hofstee analysis as illustrated in Fig. 4B; the influx Km was computed at 348 ± 37 μM, and the influx Vmax was 336 ± 20 pmol · mg protein−1 · min−1.
Fig. 3.
Comparison of OATP2B1-mediated influx among folate substrates at a concentration of 300 μM. A, [3H]pemetrexed influx in OATP2B1- and mock-transfected HeLa R1-11 cells. B, OATP2B1-mediated influx of tritiated folate and antifolate compounds at pH 5.5 [■, pemetrexed; ▴, MTX; ▾, (6S)5-formylTHF; ♦, folic acid; ●, (6S)5-methylTHF]. Data are corrected for uptake in mock-transfected cells and are expressed as the mean ± S.E.M. from three independent experiments.
Fig. 4.
Kinetic analysis of [3H]pemetrexed influx. Initial uptake of [3H]pemetrexed was assessed over 4 min at pH 5.5 in OATP2B1-transfected HeLa R1-11 cells. Data were corrected for uptake in mock-transfected cells. A, [3H]pemetrexed influx as a function of substrate concentration. The line is best-fit to the Michaelis-Menten equation (V = Vmax[S]/(Kt + [S])). B, a Hofstee plot of the data. Results are the mean ± S.E.M. from three independent experiments.
Substrate Specificity.
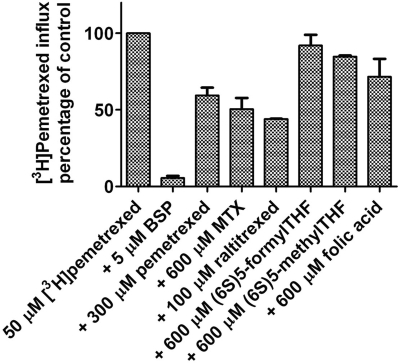
To further assess the specificity of OATP2B1-mediated folate transport, the inhibitory effect of a variety of folates and antifolates on influx of 50 μM [3H]pemetrexed was assessed. Figure 5 illustrates that 5 μM BSP, as expected, completely abolished [3H]pemetrexed influx, consistent with its very high affinity for this transporter. Nonlabeled pemetrexed inhibition of [3H]pemetrexed influx produced an influx Ki of 350 μM, consistent with the measured influx Km. Raltitrexed was the most potent competitor with a Ki of ∼70 μM. The MTX influx Ki was ∼600 μM; (6S)5-methylTHF and (6S)5-formylTHF at a concentration of 600 μM did not produce significant inhibition; 600 μM folic acid produced a small decrease in pemetrexed influx that did not reach statistical significance (P = 0.1).
Fig. 5.
Inhibitory effects of folates/antifolates and BSP on [3H]pemetrexed transport. Influx of 50 μM [3H]pemetrexed was assessed in OATP2B1-transfected cells at pH 5.5 over 4 min in the absence (control) or presence of nonlabeled folates, antifolates, and BSP at the indicated concentrations. Uptake in the mock-transfected cells was subtracted from uptake in the OATP2B1-transfected cells. Data represent the mean ± S.E.M. from three independent experiments. The inhibition constants (Ki) were determined from the formula: v1/v2 = (VmaxS/S + Km (1 + (i/Ki))/(VmaxS/S + Km), where v1 is influx in the presence of inhibitor (i), v2 is influx in absence of inhibitor, and Km and Vmax were derived from the measured [3H]pemetrexed (S) influx kinetics (Fig. 4).
The Effects of FCCP on OATP2B1-Mediated Pemetrexed and BSP Influx.
OATP2B1-mediated pemetrexed and BSP influx was highly sensitive to FCCP. As indicated in Fig. 6A, at pH 5.5 there was a progressive decrease in pemetrexed and BSP influx as the FCCP concentration was increased to 1 μM. At this point, influx of these substrates was decreased by ∼74 and 62%, respectively. At 20 μM FCCP, pemetrexed and BSP influx at pH 5.5 was abolished. It is of particular interest that FCCP suppressed BSP influx over a broad pH range and even when the extracellular pH was 7.4 in the absence of a pH gradient (Fig. 6B). At 20 μM FCCP, influx of BSP at pH 7.4 was abolished (not shown). In these experiments, cells were pretreated with FCCP, but the ionophore was not present at the time of transport determinations, precluding a direct competitive inhibitory effect of FCCP on the transport process. These effects on BSP transport at neutral pH imply FCCP actions beyond effects on the pH gradient (see Discussion).
Fig. 6.
Effects of FCCP on BSP and pemetrexed influx mediated by OATP2B1. OATP2B1- and mock-transfected cells were preincubated at pH 7.4 with FCCP at the indicated concentrations for 20 min, after which buffer was replaced without FCCP before influx determinations were made. Control cells were incubated with the dimethyl sulfoxide vehicle. A, influx of [3H]pemetrexed (300 μM) or [35S]BSP (2.5 μM) was assessed at pH 5.5 over 4 min. Data were corrected for uptake in the mock-transfected cells and is reported as percentage of control uptake (in the absence of inhibitor). Data are expressed as the mean ± S.E.M. from three independent experiments. B, influx of [35S]BSP (2.5 μM) in the presence or absence of 1 μM FCCP was assessed at different pH levels over a period of 4 min. Data were corrected for uptake in the mock-transfected cells and are expressed as the mean ± S.E.M. from three independent experiments.
Properties of OATP2B1-Mediated Transport of Folates in X. laevis Oocytes.
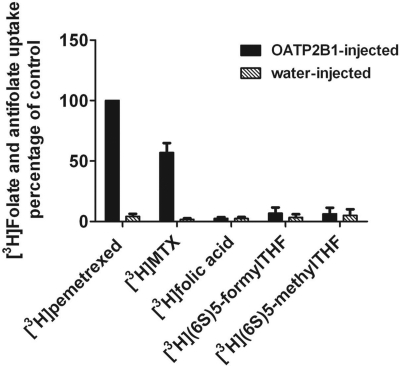
To further confirm the substrate specificity of this transporter, X. laevis oocytes were injected with OATP2B1 cRNA, and 3 days later, uptake of 300 μM tritiated folates was assessed over 30 min at pH 5.5 (Fig. 7). Data were consistent with what was observed in OATP2B1-transfected HeLa R1-11 cells (see Fig. 3B). MTX uptake was approximately half that of pemetrexed, and again there was no significant uptake of folic acid and the reduced folates.
Fig. 7.
Uptake of folates and antifolates in X. laevis oocytes. Uptake of 300 μM [3H]pemetrexed, [3H]MTX, [3H]folic acid, [3H](6S)5-formylTHF, or [3H](6S)5-methylTHF was assessed over 30 min at room temperature 3 to 4 days after oocytes were injected with water or OATP2B1 cRNA. Data are representative of two experiments; uptake was assessed in 10 oocytes.
Electrophysiological Properties of OATP2B1-Mediated Folate Transport.
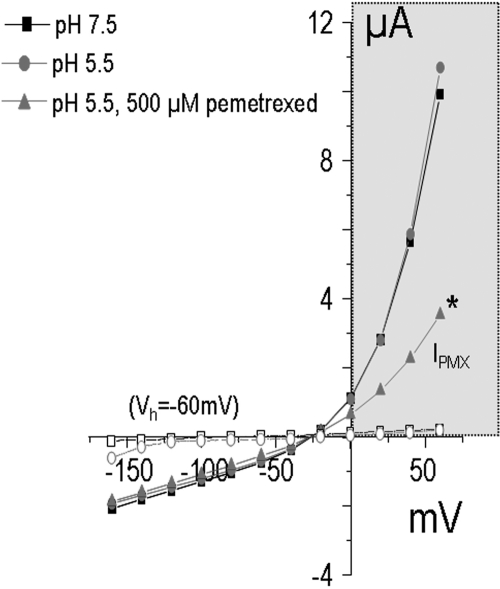
At a transmembrane voltage gradient of −60 mV, substrate-elicited currents could not be detected with either pemetrexed or raltitrexed in X. laevis oocytes that express OATP2B1 (data not shown). Figure 8 illustrates the relationship between current and voltage as a function of pH in the presence and absence of pemetrexed. It can be seen that there was a negative current at pH 7.5 and 5.5 in the absence of pemetrexed at −60 mV. This current decreased as the voltage was increased to −25 mV. The addition of pemetrexed had no effect on the voltage-current relationship at negative membrane potentials. However, as the voltage was increased to above −25 mV and then reversed, there was a comparable increase in a positive current at pH 7.5 and 5.5 in the absence of pemetrexed. However, at these positive membrane potentials, there was a decrease in current when pemetrexed was present in the perfusate (IPMX). It can be seen that there were no currents detected when water-injected oocytes were subjected to the same protocol (Fig. 8, open symbols). Voltages above +20 mV were used to increase the sensitivity of detection of OAT2B1 transport characteristics.
Fig. 8.
Voltage dependence of pemetrexed transport mediated by OATP2B1 in X. laevis oocytes. Oocytes were clamped to −60 mV (Vh), and current was assessed in response to voltage changes between −160 and + 60 mV. The sequence of changes in the composition of the perfusate is indicated in the top left quadrant of the figure (from top to bottom).*, the current (I) changes with the addition of pemetrexed at pH 5.5 (IPMX). Currents in water-injected oocytes are indicated by the open symbols connected by the interrupted lines.
Impact of OATP2B1 on Antifolate Accumulation and Growth Inhibition in HeLa Cells.
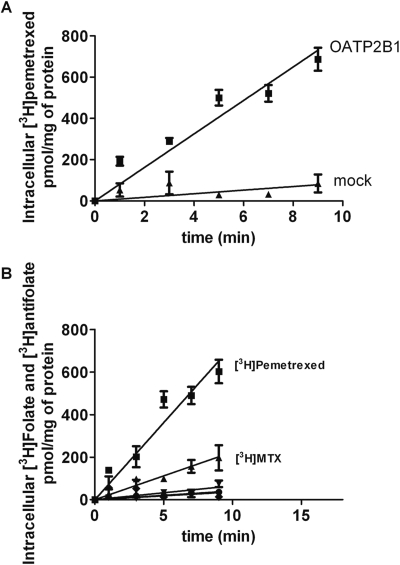
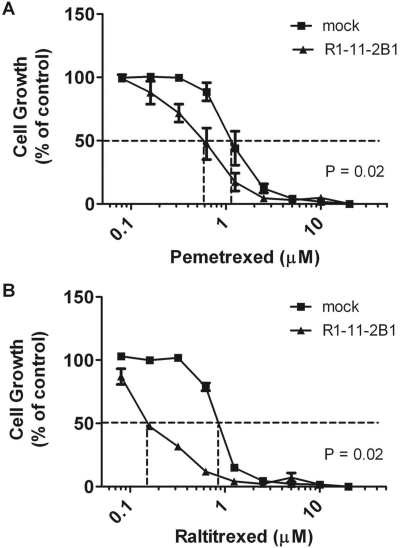
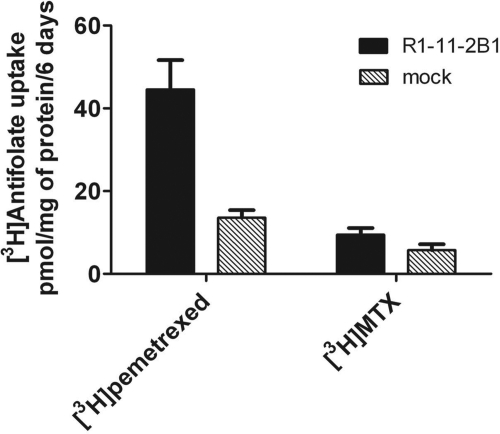
To determine whether OATP2B1-mediated pemetrexed transport into HeLa cells is associated with pharmacological activity, the pemetrexed IC50 (concentration of drug that suppresses growth to 50% the rate in the absence of drug) was assessed in cells stably transfected with OATP2B1 (HeLa R1-11-2B1) growing in RPMI 1640 medium containing 2 μM folic acid. The pH of the medium decreased minimally to 7.2 after 6 days of cell growth. Although expression of OATP2B1 in stably transfected cells was lower than it was in transiently transfected cells (not shown) and transport activity was not measurable at neutral pH, the pemetrexed IC50 was decreased ∼2-fold compared with vector-only transfected cells (Fig. 9A). This was associated with an ∼3-fold increase in the cellular accumulation of pemetrexed compared with the mock-transfected cells (Fig. 10). Based upon its inhibition of pemetrexed influx, raltitrexed was predicted to be a much better substrate for this transporter. This was confirmed by the degree of growth inhibition achieved by this agent. The raltitrexed IC50 was decreased by a factor of ∼5.5 in the OATP2B1-transfected cells (Fig. 9B). There was a 2-fold increase in MTX uptake in the OATP2B1-transfected cells over 6 days compared with mock-transfected cells (Fig. 10), but this did not result in significant growth inhibition (not shown). Stability of the tritiated antifolates was confirmed by high-performance liquid chromatography analyses at the end of the experiments.
Fig. 9.
Growth inhibition mediated by pemetrexed or raltitrexed. HeLa R1-11 cells stably transfected with OATP2B1 and mock-transfected cells were grown in the presence of pemetrexed (A) or raltitrexed (B) at the indicated concentrations for 6 days. Growth in the absence of drug is indicated as 100%. Data are the mean ± S.E.M from three independent experiments. The vertical line intercepts the x axis at the concentration at which growth inhibition is 50% of the level of growth in the absence of drug (IC50).
Fig. 10.
Accumulation of pemetrexed and MTX over 6 days. OATP2B1 stably transfected and mock-transfected cells were exposed to tritiated pemetrexed or MTX at a concentration of 1 μM for 6 days in growth medium. Data are the mean ± S.E.M from three different experiments.
Discussion
The major focus of studies on transport of folates and antifolates in mammalian cells has been on the high-affinity folate-specific transporters RFC and PCFT and the folate receptors. There has also been an interest in the multidrug resistance-associated proteins and the breast cancer resistance protein that export folate/antifolate monoglutamates and, in some cases, the lower polyglutamate forms that affect the net transport of these compounds (Assaraf, 2007). Some members of the superfamily of facilitative solute carriers also transport folates and antifolates at neutral pH, although their physiological and pharmacological roles are unclear (Abe et al., 2001; Takeda et al., 2002; Uwai and Iwamoto, 2010). Among the OATPs, OATP1B1 and OATP1B3 mediate MTX hepatic transport with a Km of 25 to 40 μM (Abe et al., 2001); OATP1A2 mediates transport of MTX across the apical brush-border membrane of distal nephrons with a Km of 450 μM (Badagnani et al., 2006). The extent to which these carriers transport physiological folates is not known. Prominent low-pH transport activities have been observed for several OATPs (Leuthold et al., 2009); of these, only OATP1A2 is known to have MTX transport activity at low pH (Badagnani et al., 2006). The current study establishes OATP2B1 as a low-affinity, but highly selective, low pH antifolate transporter and demonstrates the critical role that pH, substrate, and substrate concentration can play in identifying the spectrum of activities of a transporter.
If OATP2B1 had been screened at neutral pH, no activity would have been detected for any of the folate compounds. At low pH and low concentration (2.5 μM), activity would have been detected only for pemetrexed. Even at a high concentration (300 μM), transport activity was detected only for pemetrexed and MTX. These observations may account, in part, for the reason why folate/antifolate transport activities have not been detected in the past when the functions of this family of transporters were studied, probably at neutral pH and usually at low substrate concentrations. Although raltitrexed is a much better substrate for this transporter, it is not available in radiolabeled form, and because the transport process does not seem to be electrogenic, its transport could not be detected in X. laevis oocytes. However, the impact of OATP2B1 expression on raltitrexed cytotoxic activity strongly suggests that raltitrexed is a preferred substrate among antifolates for OATP2B1.
OATP2B1, similar to other organic anion transporters, mediates the cellular uptake of a wide variety of amphipathic organic compounds, including bile salts, steroid conjugates, thyroid hormone, and numerous drugs, with a broad spectrum of affinities for these substrates (Hagenbuch and Meier, 2004; Rizwan and Burckhardt, 2007). For instance, the OATP2B1-mediated influx Km values for atorvastatin, BSP, and estrone-3-sulfate (E3S) are 0.2, 0.7, and 9 μM, respectively (Kullak-Ublick et al., 2001; Tamai et al., 2001; Grube et al., 2006). It is interesting that although OATP2B1 transports organic anions with very diverse structures (Hagenbuch and Meier, 2004), there is a high degree of substrate specificity among the folates/antifolates with relatively small structural differences. Consistent with the differences observed in the pH sensitivities of pemetrexed and BSP transport, there are also differences in pH sensitivities among other OATP2B1 substrates. Optimal transport of E3S and dehydroepiandrosterone sulfate occurs at low pH with ∼50% activity retained at neutral pH (Kobayashi et al., 2003; Nozawa et al., 2004; Sai et al., 2006). There is a much lower level of activity retained at neutral pH for pravastatin and thyroxin (Kobayashi et al., 2003; Nozawa et al., 2004; Leuthold et al., 2009). On the other hand, tebipenem pivoxil is transported only under neutral or weakly alkaline conditions (Kato et al., 2010).
Pemetrexed influx was detectable only at low pH, and transport activity was markedly decreased when the pH gradient was dissipated by the proton ionophore FCCP, similar to what was reported previously for E3S using a much higher FCCP concentration (Sai et al., 2006). A transmembrane E3S gradient also correlated with the proton gradient in OATP2B1-expressing membrane vesicles (Sai et al., 2006). It is surprising that the results with BSP were markedly different from what was observed with pemetrexed. BSP transport was entirely independent of extracellular pH over a range of 5.5 to 7.4, indicating that influx was independent of the pH gradient. However, not only did FCCP suppress BSP transport at low pH, there was comparable suppression of transport at pH 7.4 in the absence of a pH gradient implying inhibitory effects of this agent beyond alterations in the membrane potential. Because FCCP was not present at the time transport measurements were made, its effects cannot be attributed to direct competitive inhibition of BSP transport. It is more likely that inhibition of BSP transport was related to FCCP effects on the membrane potential and/or mitochondrial function, even at the low concentration used in this study (Benz and McLaughlin, 1983). The lack of pH dependence for BSP is particular puzzling because, among the organic anions, BSP has the highest affinity for OATP2B1 (see above), a property that cannot be attributed to an allosteric interaction between the BSP and proton binding sites or, apparently, proton coupling or bicarbonate exchange. Further insights might be gleaned from studies that characterize transmembrane gradients of BSP as a function of pH under conditions of net transport. In contrast, the marked pH dependence of pemetrexed transport probably is related to the salutary impact of protons on pemetrexed binding to the carrier.
At a transmembrane voltage gradient of −60 mV, substrate-elicited currents could not be detected with either pemetrexed or raltitrexed in X. laevis oocytes that express OATP2B1. These observations are consistent with the absence of a net charge transfer as these antifolates are transported into the cell or a transport process too slow for current to be detected. The presence of a prominent folate substrate-uncoupled conductance mediated by OATP2B1 that was unaffected by the pH gradient was of particular interest, and it was assumed to represent a channel-like phenomenon. The observation that the conductance at negative potentials was reversed and markedly increased at positive membrane potentials (Fig. 8) is consistent with a net negative flow associated with OATP2B1 under these conditions. This current was diminished when pemetrexed was present (IPMX), suggesting that the carrier now had a greater positive charge or that there was an exchange with an anion to decrease the net negative flow into the oocytes.
The substrate specificity and pH sensitivity for OATP2B1 are much different from what were observed for the folate-specific PCFT. Although both have low pH optima, there is substantial residual activity for PCFT at neutral pH so that this transporter delivers folates and antifolates at physiological levels of the former and pharmacological levels of the latter (Qiu et al., 2006; Zhao et al., 2008). However, under optimal conditions for this carrier at low pH, there is a narrow influx Km range (0.5–3.0 μM) for PCFT-mediated transport for most folates and antifolates (Qiu et al., 2006; Zhao et al., 2008). On the other hand, folate/antifolate transport activity for OATP2B1 was not detectable at neutral pH over short intervals; however, there was a wide spectrum of influx Km and Ki values for folates and antifolates at an optimal pH of 5.5. It was not possible to assess the kinetic basis for the loss of pemetrexed transport activity mediated by OATP2B1 as the pH is increased because of the precipitous fall in activity when the pH is increased beyond 5.5. The increase in transport activity at low pH for the organic anion transporters, when studied, is usually associated with a decrease in Km without a change in Vmax (Leuthold et al., 2009). Although in one case, there was a 7-fold increase in influx Vmax for E3S when the pH was decreased from 7.4 to 5.5 without a change in Km (Nozawa et al., 2004). The folate-specific solute carriers differ in their pH sensitivities. For RFC, the decrease in transport activity with a decrease in pH is primarily the result of a decrease in influx Vmax, with only a small increase in influx Km (∼2-fold) (Matherly and Goldman, 2003; Wang et al., 2004). On the other hand, for PCFT, both parameters change with an increase in pH but to different extents with different substrates. The fall in influx Vmax and increase in Km with increasing pH are much smaller for pemetrexed than they are for MTX or folic acid, consistent with the preservation of transport and pharmacological activity of this agent at neutral pH (Qiu et al., 2006; Zhao et al., 2008).
Although OAPT2B1, similar to PCFT, is expressed in the small intestine (Sai et al., 2006) at the apical brush-border membrane (Kobayashi et al., 2003), the lack of detectable transport activity for folic acid, 5-formylTHF, and 5-methylTHF, even at very high substrate levels (300 μM), excludes the possibility that this transporter plays a role in the intestinal absorption of folates that occurs with the administration of high oral folate doses in the treatment of subjects with hereditary folate malabsorption that lack PCFT function (Mahadeo et al., 2010). With regard to the antifolates, MTX is the only compound currently available for oral use, particularly for the treatment of inflammatory/autoimmune diseases. MTX is a very poor substrate for OATP2B1, with a Ki of 600 μM at pH 5.5. The pH at the microclimate of the proximal jejunum is 5.8 to 6.0; at that pH, transport mediated by OATP2B1 is decreased by a factor of 5 compared with its peak activity at pH 5.5. In contrast, the Km for MTX transport mediated by PCFT at the pH of the jejunal surface is 3.4 μM (Qiu et al., 2006). The concentration of MTX achieved in jejunal fluid after an oral dose has been estimated at ∼20 μM (Nakai et al., 2007) or 1/30th of the MTX Ki for OATP2B1 and in the range of the MTX Km for PCFT. Although there is no information on the relative expression of these transporters in intestine and their transport Vmax in vivo, on the basis of the Km/Ki values, it is unlikely that OATP2B1 can have any role at all in the intestinal absorption of MTX.
OATP2B1 might play a role in antifolate pharmacology. Indeed, OATP2B1 is expressed in a number of solid tumors. These include two colon carcinoma cell lines (CX-1 and Caco-2) (Tamai et al., 2000; Hayeshi et al., 2008), with higher levels of OATP2B1 mRNA detected in metastatic prostatic cancer (Wright et al., 2011). In breast cancer, OATP2B1 mRNA levels correlate with tumor grade (Al Sarakbi et al., 2006). In the current study, there was sufficient uptake of this antifolate to produce significant growth inhibition when cells were grown in culture for 6 days over a pH range of 7.2 to 7.4 at concentrations that are relevant pharmacologically. Because influx of pemetrexed mediated by this carrier is highly pH-dependent, with negligible activity at physiological pH, and because the solid tumor microenvironment is acidic (Tannock and Rotin, 1989), OATP2B1 could play a role in the delivery of antifolates to tumors cells. For instance, the maximal plasma concentration (Cmax) for pemetrexed at the current dose of 500 mg/m2 is ∼200 μM (Mita et al., 2006). Whereas RFC and PCFT-mediated transport would be saturated in this concentration range, OATP2B1 would not and, depending upon the Vmax, might be a source of drug delivery into cells. Because OATP2B1-mediated transport is selective for pemetrexed, in contrast to folic acid and the reduced folates, its expression would not augment cellular folate pools that suppress the polyglutamation and activity of this drug (Zhao et al., 2001). Although the radiolabeled form of raltitrexed was not available, its transport and higher affinity for the carrier relative to pemetrexed were confirmed by the much greater growth inhibition produced by this agent in OATP2B1-transfected cells. New generations of antifolates might have even higher affinities for this transporter (Wang et al., 2010). However, whether transport mediated by OATP2B1 could contribute to the pharmacological activity of antifolates in vivo would depend upon several factors: (i) the level of expression in solid tumors, (ii) the pH in the tumor microenvironment, (iii) and the relative levels of expression and activities of the much higher-affinity and potent folate transporters RFC and PCFT.
Acknowledgments
We thank Dr. Hagenbuch for providing the pCMV6-XL4/OATP2B1 constructs, Dr. Alan Wolkoff for providing [35S]BSP, and Dr. Myles Akabas for providing training on transport studies on X. laevis oocytes.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA82621] (to I.D.G.); the National Institutes of Health National Eye Institute [Grant EY017732] (to M.F.R.); the National Institutes of Health National Institute of Diabetes and Kidney and Digestive Diseases [Grants DK083007, DK090728] (to M.F.R.); and the American Heart Association [Scientist Development Grant] (to M.H.C.).
Data in this study contributed to the following dissertation: Visentin M (2011) Organic anion transporting polypeptide 2B1 (OATP2B1) as a new pH-dependent low-affinity folate transporter, Ph.D. thesis, University of Udine Graduation School of Medical Sciences, Udine, Italy.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- MTX
- methotrexate (4-amino-10-methyl-pteroylglutamic acid)
- BSP
- sulfobromophthalein
- RFC
- reduced folate carrier
- FCCP
- (4-(trifluoromethoxy)phenylhydrazone)
- OATP
- organic anion-transporting polypeptide
- PCR
- polymerase chain reaction
- PCFT
- proton-coupled folate transporter
- SLC
- solute carrier family
- (6S)5-formylTHF
- (6S)5-formyltetrahydrofolate
- (6S)5-methylTHF
- (6S)5-methyltetrahydrofolate
- MES
- 2-(N-morpholino)ethanesulfonic acid
- ORF
- open-reading frame
- IPMX
- pemetrexed current
- E3S
- estrone-3-sulfate
- I-V
- current-voltage.
Authorship Contributions
Participated in research design: Visentin, Chang, Romero, Zhao, and Goldman.
Conducted experiments: Visentin and Chang.
Wrote or contributed to the writing of the manuscript: Visentin, Romero, and Goldman.
References
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, et al. (2001) LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 120:1689–1699 [DOI] [PubMed] [Google Scholar]
- Al Sarakbi W, Mokbel R, Salhab M, Jiang WG, Reed MJ, Mokbel K. (2006) The role of STS and OATP-B mRNA expression in predicting the clinical outcome in human breast cancer. Anticancer Res 26:4985–4990 [PubMed] [Google Scholar]
- Assaraf YG. (2007) Molecular basis of antifolate resistance. Cancer Metastasis Rev 26:153–181 [DOI] [PubMed] [Google Scholar]
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, Kawamoto M, Johns SJ, Ferrin TE, Carlson EJ, et al. (2006) Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther 318:521–529 [DOI] [PubMed] [Google Scholar]
- Benz R, McLaughlin S. (1983) The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys J 41:381–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. (2009) Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Molecular Cancer Therapeutics 8:2424–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman ID, Chattopadhyay S, Zhao R, Moran R. (2010) The antifolates: evolution, new agents in the clinic, and how targeting delivery via specific membrane transporters is driving the development of a next generation of folate analogs. Curr Opin Investig Drugs 11:1409–1423 [PubMed] [Google Scholar]
- Grube M, Köck K, Oswald S, Draber K, Meissner K, Eckel L, Böhm M, Felix SB, Vogelgesang S, Jedlitschky G, et al. (2006) Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther 80:607–620 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665 [DOI] [PubMed] [Google Scholar]
- Hayeshi R, Hilgendorf C, Artursson P, Augustijns P, Brodin B, Dehertogh P, Fisher K, Fossati L, Hovenkamp E, Korjamo T, et al. (2008) Comparison of drug transporter gene expression and functionality in Caco-2 cells from 10 different laboratories. Eur J Pharm Sci 35:383–396 [DOI] [PubMed] [Google Scholar]
- Kato K, Shirasaka Y, Kuraoka E, Kikuchi A, Iguchi M, Suzuki H, Shibasaki S, Kurosawa T, Tamai I. (2010) Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: involvement of human OATP family in apical membrane transport. Mol Pharm 7:1747–1756 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2003) Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther 306:703–708 [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. (2001) Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120:525–533 [DOI] [PubMed] [Google Scholar]
- Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. (2009) Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am J Physiol Cell Physiol 296:C570–C582 [DOI] [PubMed] [Google Scholar]
- Mahadeo KM, Min SH, Diop-Bove N, Kronn D, Goldman ID. (2010) Hereditary folate malabsorption: congenital folate malabsorption, in GeneReviews [Internet] (Pagon RA, Bird TD, Dolan CR, Stephens K. eds) University of Washington, Seattle: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=folate-mal [PubMed] [Google Scholar]
- Matherly LH, Goldman DI. (2003) Membrane transport of folates. Vitam Horm 66:403–456 [DOI] [PubMed] [Google Scholar]
- Matherly LH, Hou Z. (2008) Structure and function of the reduced folate carrier a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam Horm 79:145–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita AC, Sweeney CJ, Baker SD, Goetz A, Hammond LA, Patnaik A, Tolcher AW, Villalona-Calero M, Sandler A, Chaudhuri T, et al. (2006) Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol 24:552–562 [DOI] [PubMed] [Google Scholar]
- Nakai Y, Inoue K, Abe N, Hatakeyama M, Ohta KY, Otagiri M, Hayashi Y, Yuasa H. (2007) Functional characterization of human proton-coupled folate transporter/heme carrier protein 1 heterologously expressed in mammalian cells as a folate transporter. J Pharmacol Exp Ther 322:469–476 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2004) Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther 308:438–445 [DOI] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. (2006) Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127:917–928 [DOI] [PubMed] [Google Scholar]
- Rizwan AN, Burckhardt G. (2007) Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res 24:450–470 [DOI] [PubMed] [Google Scholar]
- Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. (1998) Cloning and functional expression of rNBC, an electrogenic Na(+)-HCO3− cotransporter from rat kidney. Am J Physiol 274:F425–F432 [DOI] [PubMed] [Google Scholar]
- Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, Artursson P, Tsuji A. (2006) Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug Metab Dispos 34:1423–1431 [DOI] [PubMed] [Google Scholar]
- Sharif KA, Goldman ID. (2000) Rapid determination of membrane transport parameters in adherent cells. Biotechniques 28:926–928, 930,, 932 [DOI] [PubMed] [Google Scholar]
- Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, Sekine T, Endou H. (2002) Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther 302:666–671 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. (2000) Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun 273:251–260 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nozawa T, Koshida M, Nezu J, Sai Y, Tsuji A. (2001) Functional characterization of human organic anion transporting polypeptide B (OATP-B) in comparison with liver-specific OATP-C. Pharm Res 18:1262–1269 [DOI] [PubMed] [Google Scholar]
- Tannock IF, Rotin D. (1989) Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res 49:4373–4384 [PubMed] [Google Scholar]
- Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. (2009) The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem 284:17846–17857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwai Y, Iwamoto K. (2010) Transport of aminopterin by human organic anion transporters hOAT1 and hOAT3: comparison with methotrexate. Drug Metab Pharmacokinet 25:163–169 [DOI] [PubMed] [Google Scholar]
- Wang L, Cherian C, Desmoulin SK, Polin L, Deng Y, Wu J, Hou Z, White K, Kushner J, Matherly LH, et al. (2010) Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry. J Med Chem 53:1306–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Goldman ID. (2004) Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clin Cancer Res 10:6256–6264 [DOI] [PubMed] [Google Scholar]
- Wright JL, Kwon EM, Ostrander EA, Montgomery RB, Lin DW, Vessella R, Stanford JL, Mostaghel EA. (2011) Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev 20:619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Babani S, Gao F, Liu L, Goldman ID. (2000) The mechanism of transport of the multitargeted antifolate (MTA) and its cross-resistance pattern in cells with markedly impaired transport of methotrexate. Clin Cancer Res 6:3687–3695 [PubMed] [Google Scholar]
- Zhao R, Chattopadhyay S, Hanscom M, Goldman ID. (2004) Antifolate resistance in a HeLa cell line associated with impaired transport independent of the reduced folate carrier. Clin Cancer Res 10:8735–8742 [DOI] [PubMed] [Google Scholar]
- Zhao R, Diop-Bove N, Visentin M, Goldman ID. (2011) Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 31:177–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Gao F, Goldman ID. (2001) Marked suppression of the activity of some, but not all, antifolate compounds by augmentation of folate cofactor pools within tumor cells. Biochem Pharmacol 61:857–865 [DOI] [PubMed] [Google Scholar]
- Zhao R, Matherly LH, Goldman ID. (2009) Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med 11:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. (2008) The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol 74:854–862 [DOI] [PMC free article] [PubMed] [Google Scholar]