Abstract
Oxidative stress is a major mechanism of a variety of renal diseases. Tocopherols and tocotrienols are well known antioxidants. This study aimed to determine whether γ-tocotrienol (GT3) protects against mitochondrial dysfunction and renal proximal tubular cell (RPTC) injury caused by oxidants. Primary cultures of RPTCs were injured by using tert-butyl hydroperoxide (TBHP) in the absence and presence of GT3 or α-tocopherol (AT). Reactive oxygen species (ROS) production increased 300% in TBHP-injured RPTCs. State 3 respiration, oligomycin-sensitive respiration, and respiratory control ratio (RCR) decreased 50, 63, and 47%, respectively. The number of RPTCs with polarized mitochondria decreased 54%. F0F1-ATPase activity and ATP content decreased 31 and 65%, respectively. Cell lysis increased from 3% in controls to 26 and 52% at 4 and 24 h, respectively, after TBHP exposure. GT3 blocked ROS production, ameliorated decreases in state 3 and oligomycin-sensitive respirations and F0F1-ATPase activity, and maintained RCR and mitochondrial membrane potential (ΔΨm) in injured RPTCs. GT3 maintained ATP content, blocked RPTC lysis at 4 h, and reduced it to 13% at 24 h after injury. Treatment with equivalent concentrations of AT did not block ROS production and cell lysis and moderately improved mitochondrial respiration and coupling. This is the first report demonstrating the protective effects of GT3 against RPTC injury by: 1) decreasing production of ROS, 2) improving mitochondrial respiration, coupling, ΔΨm, and F0F1-ATPase function, 3) maintaining ATP levels, and 4) preventing RPTC lysis. Our data suggest that GT3 is superior to AT in protecting RPTCs against oxidant injury and may prove therapeutically valuable for preventing renal injury associated with oxidative stress.
Introduction
Vitamin E is composed of naturally occurring α-, β-, γ-, and δ-tocopherols and α-, β-, γ- and δ-tocotrienols that are synthesized exclusively by photosynthetic organisms. Tocopherols are commonly found in high concentrations in a wide variety of foods, whereas tocotrienols are relatively rare and found only in a few fruit and vegetable oils, including palm oil (Cottrell, 1991). Vitamin E isomers are well known for their antioxidant properties that prevent oxidative damage to polyunsaturated fatty acids and other events driven by free radicals (Brigelius-Flohé and Traber, 1999). Most studies have been conducted with α-tocopherol (AT), the most commonly used vitamin E supplement and the most abundant vitamin E component in human and animal tissues (Bichay and Roy, 1986; Singh et al., 2006; Boerma et al., 2008). Recently, it has been demonstrated that tocotrienols regulate a large numbers of genes that tocopherols do not control (Berbée et al., 2011) and have additional therapeutic properties including suppression of tumor growth (Goh et al., 1994), antithrombotic, neuroprotective, and cardioprotective activities, and novel hypocholesterolemic effects in humans (Theriault et al., 1999). Results from different reports show that γ- and δ-tocotrienols are more potent anticancer agents than α- and β-tocotrienols (Aggarwal et al., 2010).
The γ-tocotrienol (GT3) has significant antiproliferative, proapoptotic, and anti-invasive activities in different types of neoplastic cells at concentrations that have little or no effect on normal cell growth and function (Sun et al., 2009). Although the exact mechanisms mediating GT3 actions are unknown, postulated mechanisms include the inhibition of prosurvival signaling downstream of the tyrosine kinase receptor mediated by c-Src and phosphoinositide-3 kinase. The antiproliferative effects of GT3 may result, at least in part, from its inhibitory effects on mitogenic signaling from the epidermal growth factor receptor, resulting in the reduction of Akt and nuclear factor κB activities (Sen et al., 2000; Takahashi and Loo, 2004; Sylvester et al., 2005).
In addition to their antioxidant properties, the γ- and δ-tocotrienols are potent suppressors of HMG-CoA reductase activity in hepatocytes (Song and DeBose-Boyd, 2006). These tocotrienols mimic sterols and produce a dose-dependent decrease in HMG-CoA reductase activity by accelerating degradation of HMG-CoA reductase protein (Song and DeBose-Boyd, 2006). In contrast, α- and γ-tocopherols do not show antiproliferative activity even at higher concentrations (McIntyre et al., 2000) and have no measurable effect on HMG-CoA reductase degradation, cholesterol levels, (Pearce et al., 1992), and tumor growth (Goh et al., 1994; Khor et al., 1995). Therefore, it has been postulated that the suppression of HMG-CoA reductase activity is a mechanism mediating the hypocholesterolemic, cardioprotective, and tumor-suppressive actions of γ- and δ-tocotrienols (Elson, 1995; Aggarwal et al., 2010).
Recent data have also shown potent radioprotective effects of tocotrienols, GT3 in particular. GT3 ameliorates intestinal injury, enhances recovery of the intestine after irradiation, decreases vascular oxidative stress, and improves survival in animals subjected to total body irradiation (Felemovicius et al., 1995; Kulkarni et al., 2010; Berbée et al., 2011). GT3-conferred protection and reduction of mortality are associated with accelerated recovery of hematopoietic progenitor and white blood cells, neutrophils, monocytes, platelets, and reticulocytes after lethal radiation doses in mice (Ghosh et al., 2009; Kulkarni et al., 2010). It is noteworthy that the radioprotective action of GT3 against vascular injury is mediated through a HMG-CoA reductase-dependent mechanism (Berbée et al., 2011). Chronic treatment of rats with a tocotrienol-rich fraction from palm oil decreases K2Cr2O7-induced kidney injury by decreasing morphological damage to renal proximal tubules and improving renal proximal tubular function, glomerular filtration rate, and cellular redox status (Khan et al., 2010). However, it is unknown which component of the tocotrienol-rich fraction is responsible for renal protection against K2Cr2O7-induced toxicity. Chronic supplementation with GT3, one of the components of the tocotrienol-rich fraction, protects the heart against ischemia and reperfusion (Lekli et al., 2010). Furthermore, GT3 protects astrocytes from H2O2-induced oxidative stress and apoptosis (Mazlan et al., 2006).
Oxidative stress plays a pivotal role in the pathogenesis of many diseases. It also has been implicated in the mechanisms of acute kidney injury caused by ischemia/reperfusion and exposure to drugs and environmental toxicants, chronic kidney disease, and diabetic nephropathy (Abid et al., 2005; Cachofeiro et al., 2008; Koyner et al., 2008). Clinical presentation and pathophysiology of the acute kidney injury are caused, in major part, by the injury and death of renal proximal tubules. Renal proximal tubular cells (RPTCs) are rich in mitochondria and highly dependent on mitochondrial oxidative metabolism for ATP synthesis. RPTCs are the primary target for oxidative stress within the kidney and have a high capacity for generating free radicals when their mitochondrial function is impaired. Mitochondria have been identified as one of the subcellular targets of proapoptotic actions of GT3 in cancer cells (Takahashi and Loo, 2004), but the mitochondrial targets of GT3 are not known. Furthermore, it is unknown whether GT3 plays a role in the regulation of mitochondrial functions in nonmalignant cells and, if it does, whether it has deleterious or protective actions in this organelle. Therefore, the first goal of the present study was to determine whether GT3 protects against mitochondrial dysfunction and cell injury in RPTCs subjected to oxidative stress. The second goal was to examine mitochondrial functions targeted by GT3 in oxidant-injured RPTCs.
Materials and Methods
Animals and Materials.
Female New Zealand White rabbits (2.0–2.5 kg) were purchased from Myrtle's Rabbitry (Thompson Station, TN). All animal procedures involved in this study were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. The cell culture media (a 50:50 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 nutrient mix without phenol red, pyruvate, and glucose) were purchased from Mediatech (Herndon, VA). 5-(and-6)-Carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) was supplied by Invitrogen (Carlsbad, CA), and N-methyl-N-TMS-trifluoroacetamide was purchased from Restek (Bellefonte, CA). GT3 was supplied by Yasoo Health (Johnson City, TN) and was dissolved in dimethyl sulfoxide. The sources of the other reagents have been described previously (Nowak and Schnellmann, 1996; Nowak et al., 2004).
Isolation and Culture of Renal Proximal Tubular Cells.
Renal proximal tubules were isolated from rabbit kidneys by the iron-oxide perfusion method and cultured in 35-mm culture dishes in improved conditions as described previously (Nowak and Schnellmann, 1996). The culture medium was a 50:50 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 nutrient mix without phenol red, pyruvate, and glucose, supplemented with 15 mM NaHCO3, 15 mM HEPES, and 6 mM lactate (pH 7.4, 295 mOsmol/kg). Human transferrin (5 μg/ml), selenium (5 ng/ml), hydrocortisone (50 nM), bovine insulin (10 nM), and l-ascorbic acid-2-phosphate (50 μM) were added to the media immediately before daily media change.
Oxidant Treatment of RPTC Monolayer.
Confluent monolayers of RPTCs were treated with tert-butyl hydroperoxide (TBHP; 350 μM) for 45 min. Controls were treated with the diluent (dimethyl sulfoxide, 0.1%). After TBHP exposure, the monolayer was washed with fresh, warm (37°C) medium and cultured for an additional 4 or 24 h. To test the effects of GT3 and selected tocopherol, RPTCs were treated with different concentrations of GT3 or AT 24 h before TBHP treatment and immediately after media change after TBHP exposure.
GT3 Uptake.
Confluent RPTC monolayers were treated with 5 μM GT3. At 1, 2, 4, 8, 12, 24, and 36 h after treatment, the media were aspirated, and RPTC monolayers were washed twice with ice-cold phosphate-buffered saline. After the washing, RPTCs were suspended in 1 ml of ice-cold phosphate-buffered saline and spun down at 1000g for 2 min. The pellet was suspended in 0.5 ml of 95% ice-cold methanol, sonicated, and extracted with 0.5 ml of hexane by using vigorous vortexing. The upper layer (hexane) was collected, another 0.5 ml of hexane was added to the suspension of RPTCs in methanol, and the extraction procedure was repeated. Combined hexane extracts were dried under nitrogen. The dried extracts were quantitatively transferred to a deactivated glass micro insert, dried under nitrogen, derivatized by using N-methyl-N-TMS-trifluoroacetamide, and heated at 60°C for 15 min. The derivatized samples were analyzed by using gas chromatography/mass spectrometry by single ion monitoring (Agilent 5975 GC/MSD; Agilent Technologies, Santa Clara, CA). The gas chromatographer was equipped with a 30-m HP-5MS column (0.250 mm, 0.25 μm). Helium was used as the carrier gas with a head pressure of 27 psi, and splitless injection (injector temperature 275°C, transfer line temperature 285°C) column temperature was maintained at 220°C for 2 min followed by a gradient of 25°C/min to 300°C. The mass spectrometer conditions were electron impact, ion source temperature 230°C, and ionization voltage 70eV for single-ion mode with m/z of 482 as the analytical ion and m/z of 223 as the confirming ion. GT3 recovery from RPTC monolayers was also measured and factored into the final data.
Reactive Oxygen/Nitrogen Species Generation.
The carboxyl derivative of fluorescein, carboxy-H2DCFDA, was used to assess oxidant generation in RPTCs as described previously (Nowak et al., 2011). RPTCs were loaded with 5 μM carboxy-H2DCFDA and incubated for 45 min. After incubation, cell suspensions were transferred to 48-well plates, and fluorescence was analyzed by fluorometry. Negative controls (unstained RPTCs) and positive controls (RPTCs treated with 350 μM tert-butyl hydroperoxide for 50 min) were included in each experiment. All results were corrected for autofluorescence and expressed as arbitrary fluorescence units per milligram of cellular protein.
State 3 Respiration.
State 3 respiration in mitochondria energized by electron donors to the respiratory complex I was measured polarographically by using a Clark-type electrode as described previously in the assay buffer containing digitonin (0.1 mg/ml), glutamate (5 mM), and malate (5 mM) (Nowak et al., 2006; Shaik et al., 2008). State 3 respiration was initiated by the addition of 0.4 mM ADP. State 4 respiration was measured after the addition of oligomycin (0.6 μg/ml) to RPTCs respiring at state 3.
Oligomycin-Sensitive Respiration.
Oligomycin-sensitive state 3 respiration was used as a marker of oxidative phosphorylation and F0F1-ATPase function. Oligomycin-sensitive state 3 respiration was calculated as the difference between state 3 respiration and oligomycin-insensitive state 3 respiration, which was measured in the presence of oligomycin (0.6 μg/ml).
Intracellular ATP Content.
Intracellular ATP content in RPTCs was measured by the luciferase method in freshly prepared RPTC lysates by using an ATP Bioluminescence Assay Kit HS II (Roche Diagnostics, Mannheim, Germany) and the manufacturer's protocol as described previously (Nowak, 2002).
Mitochondrial Membrane Potential.
Mitochondrial membrane potential (ΔΨm) was assessed as described previously (Nowak, 2002) using JC-1, a cationic dye that exhibits potential-dependent accumulation and formation of red fluorescent J-aggregates in mitochondria. Each set of samples included a positive control for mitochondrial depolarization (RPTCs treated with 2 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone) and hyperpolarization (RPTCs treated with 0.6 μg/ml oligomycin). Fluorescence was determined by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA) using excitation by a 488-nm argon-ion laser. The JC-1 monomer (green) and the J-aggregates (red) were detected separately in FL1 (emission, 525 nm) and FL2 (emission, 590 nm) channels, respectively. ΔΨm is presented as the percentage of cells with depolarized mitochondria.
Isolation of RPTC Mitochondria.
RPTCs were homogenized and mitochondria were isolated as described previously (Nowak et al., 2004, 2006, 2011; Shaik et al., 2008). Purity of mitochondrial fractions was tested by measuring protein levels of the endoplasmic reticulum marker calreticulin, the lysosomal protein LAMP-1, and the peroxisomal marker catalase. Mitochondria resulting from this isolation were free of lysosomal and endoplasmic reticular contaminations and contained minor amount of peroxisomes that sedimented at g force similar to that used to sediment mitochondria (Shaik et al., 2008; Nowak et al., 2011). The final mitochondrial pellet was resuspended in the assay buffer used for measuring the activity of F0F1-ATPase.
F0F1-ATPase Activity.
ATPase activity of the ATP synthase was determined in freshly isolated mitochondria by measuring the release of Pi from ATP as described previously (Nowak, 2002; Liu et al., 2004). Each sample was run in the absence and presence of oligomycin (10 μg/ml), and the oligomycin-sensitive ATPase activity of F0F1-ATPase was calculated.
Lactate Dehydrogenase Release.
Release of lactate dehydrogenase (LDH) from RPTCs into the culture medium was used as a marker of RPTC plasma membrane permeabilization and cell lysis. LDH activity in culture media and RPTC lysates was determined spectrophotometrically by following NADH (0.3 mM) oxidation (at 37°C) in the presence of 1.8 mM pyruvate as a substrate. The activity of LDH in homogenates obtained by sonication of RPTCs suspended in the culture medium was used as 100%. The activity of LDH in culture media collected from culture dishes was expressed as a percentage of total LDH activity obtained from the same culture dish.
All results were normalized to cellular protein, which was measured by the bicinchoninic acid assay using bovine serum albumin as the standard.
Statistical Analysis.
Data are presented as means ± S.E. and were analyzed for significance by analysis of variance. Multiple means were compared by using Fisher's protected least significance difference test with a level of significance of p < 0.05. Renal proximal tubular cells isolated from an individual rabbit represented one experiment (n = 1) consisting of data obtained from 2 to 10 culture plates.
Results
γ-Tocotrienol Uptake in RPTCs.
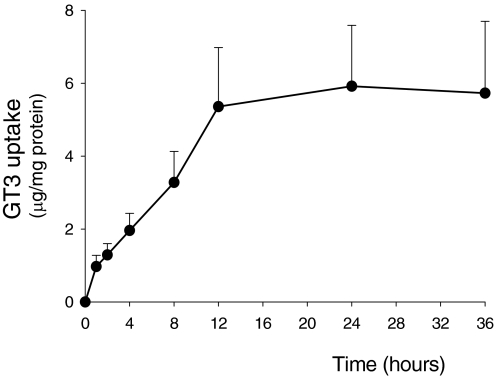
GT3 was readily taken up by RPTC monolayers. Figure 1 presents the time-dependent cellular uptake of GT3 at the concentration that was the most effective in other experiments (5 μM). The uptake of GT3 by RPTCs increased in a linear fashion for 12 h of the exposure, reached a steady state at 24 h, and remained at this level at 36 h of the exposure (Fig. 1). Reaching the steady state was not caused by depletion of GT3 from the culture media. Approximately 50% of GT3 added to the culture dish was taken by RPTC monolayer (approximately 1 × 106 cells) within 24 h of the exposure (2.0 ± 0.43 μg of GT3 taken up versus 4.1 μg of GT3 added to the media). Because 4.1 μg of GT3 is equivalent to 6 × 1015 molecules of GT3, our results show that a single cell took up 2.9 × 109 molecules of GT3 within 24 h of the exposure to the compound. These results also show that GT3 uptake by RPTC does not occur through simple diffusion (Fig. 1).
Fig. 1.
The time-dependent uptake of 5 μM GT3 in noninjured RPTCs. Exposure time is indicated along the abscissa. Results are the average ± S.E. of three independent experiments (RPTC isolations). Each experiment was run in duplicate.
γ-Tocotrienol Blocks ROS Production in Oxidant-Injured RPTC.
TBHP, a model oxidant, was used to produce oxidative stress in RPTCs. Reactive oxygen/nitrogen species production increased to 300% of controls after TBHP-induced injury (Fig. 2A). Pretreatment with 1 and 2 μM GT3 reduced TBHP-induced ROS production to 215 and 226% of controls, respectively, but did not block ROS generation in injured RPTCs (Fig. 2A). Pretreatment of RPTC with either 5, 10, or 20 μM GT3 blocked TBHP-induced ROS generation (Fig. 2A; data not shown). In contrast, pretreatment with 5 and 10 μM AT did not block ROS generation after TBHP exposure in RPTCs (Fig. 2B).
Fig. 2.
The effect of increasing concentrations of GT3 (A) and AT (B) on oxidant production after TBHP exposure (0.35 mM, 45 min) in RPTCs. RPTC cultures were preincubated with GT3 for 24 h before TBHP treatment. The data are expressed as percentage of control. Oxidant generation in controls was 63,967 ± 8499 arbitrary fluorescence units/mg protein. Results are the average ± S.E. of four independent experiments (RPTC isolations). *, P < 0.01 and #, P < 0.05, values significantly different from controls.
These data show that GT3 prevents TBHP-induced ROS production in a dose-dependent manner and that 5 to 20 μM GT3 is the most effective in blocking ROS production. In contrast, at the same concentrations, AT is not an effective antioxidant in TBHP-injured RPTCs.
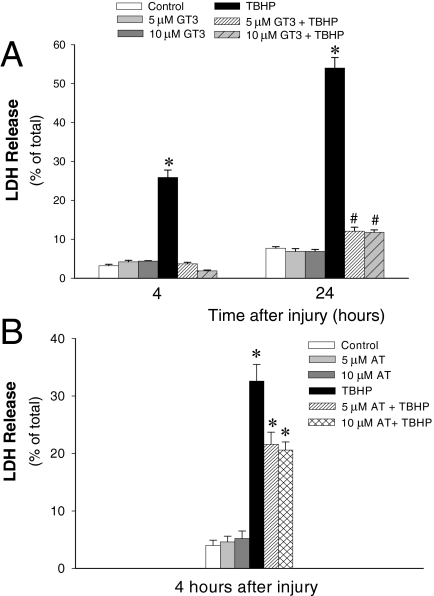
γ-Tocotrienol Prevents Oxidant-Induced RPTC Lysis and Death.
LDH release is a measure of RPTC lysis and a marker of cell death by oncosis. LDH release was 3.2 ± 0.4% in controls, suggesting that approximately 3% of RPTCs treated with the diluent (dimethyl sulfoxide) had undergone lysis during the duration of the experiment, which most likely reflects cell turnover (Fig. 3A; 0% is equivalent to no cell lysis; 100% is equivalent to lysis of all cells). GT3 treatment had no effect on LDH release and morphology in noninjured RPTCs up to the concentration of 20 μM (Fig. 3A; data not shown). However, the exposure of RPTCs to 50 μM GT3 for 24 h caused changes in mitochondrial morphology without producing apparent changes in cell viability and mitochondrial respiration (data not shown). Furthermore, pretreatment with 50 μM GT3 did not protect against the damage in oxidant-injured RPTC monolayers (data not shown).
Fig. 3.
The effect of GT3 (A) and AT (B) on LDH release 4 and 24 h after TBHP exposure (0.35 mM, 45 min). GT3 or AT were present in RPTC cultures for 24 h before and 4 or 24 h after TBHP treatment. Results are the average ± S.E. of 3 to 10 independent experiments (RPTC isolations). *, P < 0.01 and #, P < 0.05, values significantly different from controls.
LDH release increased to 26.0 ± 1.9 and 54.0 ± 2.7% at 4 and 24 h after TBHP-induced injury, respectively, demonstrating that more than 50% of cells were lethally injured and lysed within 24 h after the exposure to oxidant (Fig. 3A). Pretreatment with 5 and 10 μM GT3 blocked the increase in LDH release at 4 h after TBHP exposure (Fig. 3A). Furthermore, GT3 reduced LDH release from 54.0 ± 2.7 to 11.8 ± 0.6% at 24 h after TBHP-induced injury (Fig. 3A). No increase in apoptosis was found in TBHP-treated RPTCs (3.6 ± 0.8 versus 5.3 ± 0.7% in TBHP-injured and control RPTCs, respectively) or RPTCs treated with GT3 and TBHP (data not shown). In contrast, pretreatment with 5 and 10 μM AT decreased cell lysis to 22 and 21%, respectively, at 4 h after TBHP exposure (Fig. 3B). These data show that GT3 blocks RPTC lysis at 4 h after oxidant-induced injury and markedly decreases RPTC lysis at a later time point after injury, whereas α-tocopherol is only partially protective at the same concentrations.
γ-Tocotrienol Improves Mitochondrial Respiration in RPTCs Injured by Oxidant.
State 3 respiration was used to assess the effects of GT3 and AT on the respiratory capacity of mitochondria in injured RPTCs. At 4 h after TBHP exposure, state 3 respiration in TBHP-injured RPTCs decreased to 50% of controls, which demonstrates significant impairment of mitochondrial electron transport chain and respiratory capacity (Fig. 4). Pretreatment of RPTCs with 1 and 2 μM GT3 did not improve state 3 respiration in TBHP-injured RPTCs, whereas pretreatment with 5 and 10 μM GT3 blocked TBHP-induced decreases in state 3 respiration (Fig. 4A). Likewise, pretreatment with 5 and 10 μM AT prevented the decreases in state 3 respiration in oxidant-injured RPTCs (Fig. 4B). These data show that both GT3 and AT protect the respiratory capacity of mitochondria in RPTCs after oxidant-induced injury.
Fig. 4.
The effect of GT3 (A) and AT (B) on state 3 respiration 4 h after TBHP exposure (0.35 mM, 45 min) in RPTCs. RPTC cultures were preincubated with GT3 or AT for 24 h before and 4 h after TBHP treatment. Results are the average ± S.E. of six independent experiments (RPTC isolations). *, P < 0.01 and #, P < 0.05, values significantly different from controls.
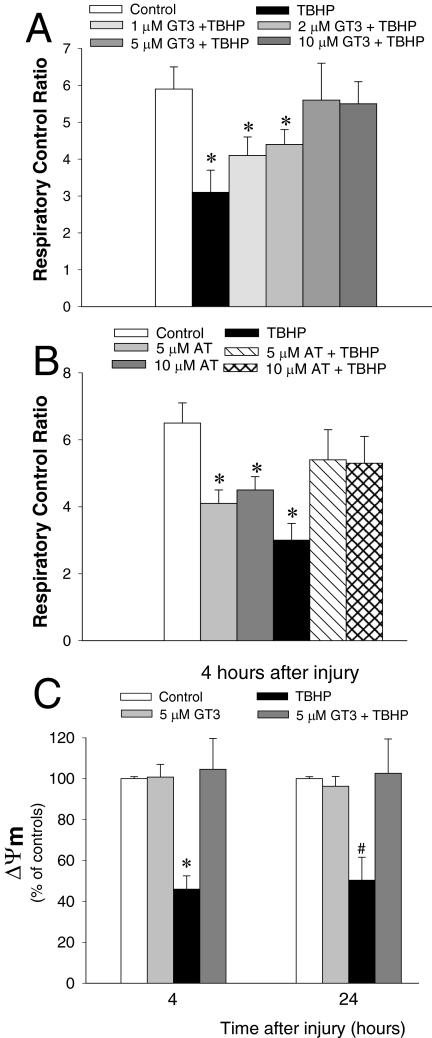
γ-Tocotrienol Prevents Mitochondrial Uncoupling and Loss of Mitochondrial Membrane Potential in Oxidant-Injured RPTCs.
The respiratory control ratio (RCR) is used as a measure of coupling of mitochondrial respiration and oxidative phosphorylation. RCR in noninjured RPTCs was 5.9 ± 0.60 (Fig. 5A), demonstrating tight coupling of mitochondrial respiration and oxidative phosphorylation. RCR decreased by 47% after the exposure of RPTCs to TBHP (Fig. 5A). Pretreatment with 1 and 2 μM GT3 improved RCR in TBHP-injured RPTCs. Pretreatment of TBHP-injured RPTCs with 5 and 10 μM GT3 restored RCR in TBHP-injured RPTCs (Fig. 5A). Pretreatment with 5 and 10 μM AT resulted in RCR in oxidant-injured RPTCs equivalent to 82% of controls (Fig. 5B). These data show that GT3 prevents and AT decreases mitochondrial uncoupling induced by an oxidant in RPTCs.
Fig. 5.
A and B, the effect of GT3 (A) and AT (B) on the respiratory control ratio in RPTCs 4 h after TBHP exposure (0.35 mM, 45 min). C, percentage of RPTCs with depolarized mitochondria 4 h after TBHP exposure. The results are expressed as the percentage of cells with depolarized mitochondria (J-aggregate/JC-1 monomer ratio <0.5 ± 0.2) compared with controls (J-aggregate/JC-1 monomer ratio = 1.2 ± 0.3). GT3 and AT were present in RPTC cultures for 24 h before and 4 h after TBHP treatment. Results are the average ± S.E. of four to six independent experiments (RPTC isolations). *, P < 0.01 and #, P < 0.05, values significantly different from controls.
ΔΨm is created by mitochondrial respiration using energy released from the oxidation of substrates to support the translocation of protons from the matrix across the inner mitochondrial membrane. The percentage of RPTCs with depolarized mitochondria was used to assess changes in ΔΨm (Fig. 5C). Treatment of noninjured RPTCs with 5 μM GT3 had no effect on ΔΨm. Exposure to TBHP induced mitochondrial depolarization in 56% of RPTCs (Fig. 5C). Treatment of RPTCs with 5 μM GT3 before TBHP injury restored the number of RPTCs with polarized mitochondria to control levels at both 4 and 24 h after oxidant exposure (Fig. 5C). These data show that GT3 prevents the loss of mitochondrial membrane potential in RPTCs injured by an oxidant.
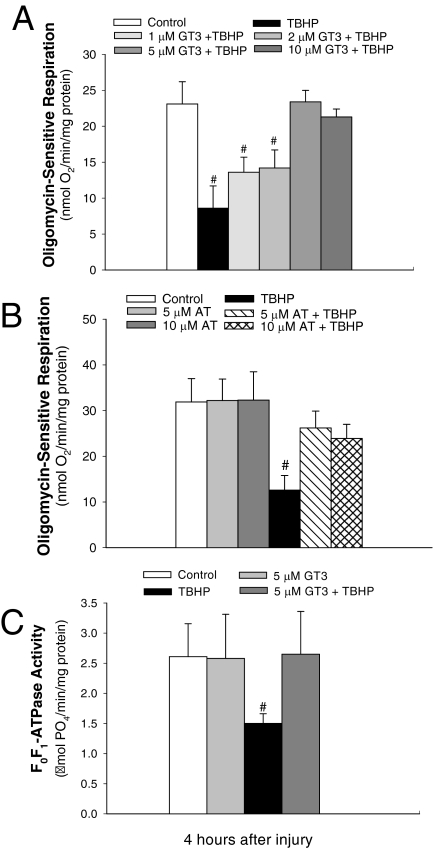
γ-Tocotrienol Maintains the Function and Activity of F0F1-ATPase in Oxidant-Injured RPTCs.
Oligomycin is a specific inhibitor of F0F1-ATPase and thus blocks oxidative phosphorylation and ATP synthesis. Oligomycin-sensitive respiration represents the amount of oxygen consumed by oxidative phosphorylation and is used as an indirect measure of oxidative phosphorylation and the function of F0F1-ATPase in RPTCs. Oligomycin-sensitive respiration in RPTCs decreased by 63% at 4 h after TBHP treatment (Fig. 6, A and B). Pretreatment of RPTCs with 1 and 2 μM GT3 before TBHP injury reduced the decreases in oligomycin-sensitive respiration. Pretreatment with 5 and 10 μM GT3 restored oligomycin-sensitive respiration in TBHP-injured RPTCs (Fig. 6A). Pretreatments with 5 and 10 μM AT resulted in the oligomycin-sensitive respiration in oxidant-injured RPTCs equivalent to 82 and 75% of controls (Fig. 6C).
Fig. 6.
A and B, the effect of GT3 (A) and AT (B) on oligomycin-sensitive respiration 4 h after TBHP exposure (0.35 mM, 45 min) in RPTCs. C, the effect of GT3 on the activity of F0F1-ATPase in isolated RPTC mitochondria 4 h after TBHP exposure (0.35 mM, 45 min). GT3 and AT were present in RPTC cultures for 24 h before and 4 h after TBHP treatment. Results are the average ± S.E. of four independent experiments (RPTC isolations). #, P < 0.05, values significantly different from controls.
Exposure of RPTCs to TBHP decreased F0F1-ATPase activity by 42% 4 h after the exposure (Fig. 6C). GT3 had no effect on F0F1-ATPase activity in noninjured RPTCs. Pretreatment of RPTCs with 5 μM GT3 blocked TBHP-induced decreases in F0F1-ATPase activity (Fig. 6C). These data show that GT3 blocks oxidant-induced decreases in the activity and function of F0F1-ATPase and suggest that GT3 prevents the decreases in oxidative phosphorylation in RPTCs injured by oxidant. Although AT improved the function of F0F1-ATPase, its protective actions were not as potent as those of GT3.
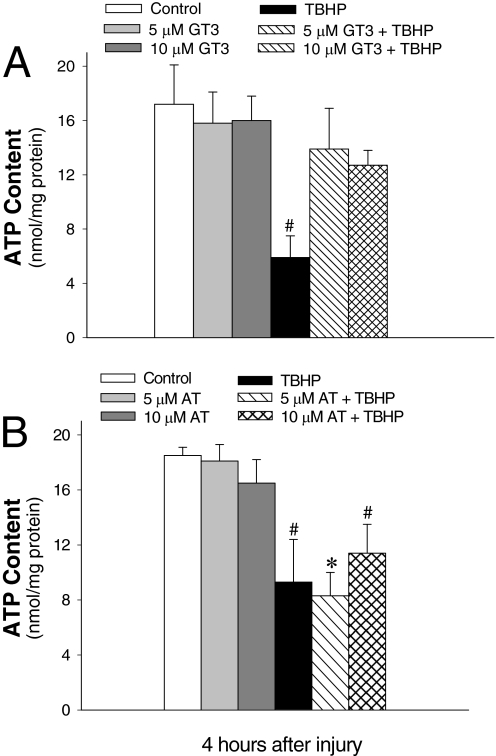
γ-Tocotrienol Maintains ATP Content in Oxidant-Injured RPTCs.
ATP content was used to evaluate the energy status of RPTCs after TBHP-induced injury. ATP content in TBHP-treated RPTCs decreased by 65% (Fig. 7). Treatment with GT3 and AT had no effect on ATP content in noninjured RPTCs (Fig. 7). Pretreatment of RPTCs with GT3 before TBHP exposure maintained ATP content in injured RPTCs (Fig. 7A). However, the same concentrations of AT were unable to protect against ATP decreases in TBHP-injured RPTCs (Fig. 7B). These data show that GT3 maintains ATP content and energy status of oxidant-injured RPTCs and GT3 is superior to AT in protecting against oxidant-induced loss of ATP in renal proximal tubules.
Fig. 7.
The effect of GT3 (A) and AT (B) on ATP content in RPTCs 4 h after TBHP exposure (0.35 mM, 45 min). GT3 and AT were present in RPTC cultures for 24 h before and 4 h after TBHP treatment. Results are the average ± S.E. of four independent experiments (RPTC isolations). *, P < 0.01 and #, P < 0.05, values significantly different from controls.
Discussion
Renal proximal tubules are the major target of toxicant and ischemic injury in the kidney. Because mitochondria play a crucial role in ATP generation and survival of RPTCs, mitochondrial dysfunction caused by toxicant exposure or ischemia leads to RPTC injury and death and plays a major role in the development of acute kidney injury (Abid et al., 2005; Cachofeiro et al., 2008; Koyner et al., 2008). Oxidative stress is believed to be an important mechanism of toxicant- and ischemia-induced injury to RPTCs (Abid et al., 2005; Cachofeiro et al., 2008; Koyner et al., 2008). Therefore, we hypothesized that GT3, a potent antioxidant, may limit or prevent mitochondrial dysfunction, energy deficits, and cell death caused by oxidant exposure. Our data demonstrate that GT3 is taken up by RPTCs by a process that is saturated within 24 h, which suggests that GT3 is not taken up by simple diffusion. The saturation of GT3 uptake is not caused by depletion of GT3 from the cell culture media. At this point of our study, it is unknown whether GT3 crosses the plasma membrane and is transported into the cytoplasm. However, protective effects of GT3 on several subcellular targets suggest that GT3 penetrates RPTC plasma membrane. Our data show that GT3 blocks ROS production, protects against mitochondrial dysfunction, and maintains energy status in oxidant-injured RPTCs. Protective actions of GT3 involve improving mitochondrial respiration, maintaining ΔΨm and coupling, protecting F0F1-ATPase function and activity, and maintaining ATP levels in RPTCs. The protective actions of GT3 result in greatly decreased cell lysis and improved viability of RPTCs subjected to injury. GT3 does not delay but blocks cell injury and death. Assessment of ROS generation, LDH release, and examination of RPTC morphology at later time points after TBHP exposure show maintained RPTC viability, which demonstrates that GT3 offers protection against cell injury and death and not a delay of these events. Thus, this is a first report demonstrating that GT3 acts as a powerful protectant in renal proximal tubular cells.
Our data show that low micromolar concentrations (2–5 μM) of GT3 are sufficient to block free radical burst in oxidant-treated RPTCs. These concentrations are similar to protective concentrations of GT3 in neuronal cells (10 μM) (Then et al., 2009). Reduced production of ROS by GT3 is associated with protected mitochondrial functions and increased viability and survival of TBHP-injured RPTCs. It is noteworthy that the protective effect of GT3 was present only at lower micromolar concentrations of GT3 (<20 μM). Microscopic examination of noninjured RPTC cultures treated with higher concentrations (50 μM) of GT3 revealed no morphological changes. However, pretreatment with 50 μM GT3 was not effective in improving viability and protecting against morphological damage in oxidant-injured RPTC monolayers (data not shown). Mitochondrial superoxide generated as a by-product of oxidative phosphorylation is a major source of intracellular free radicals and significantly contributes to oxidative stress and damage in the cell. Mitochondrial superoxide production depends on the proton-motive force and coupling of mitochondria. Oxidative phosphorylation and proton translocation through F0F1-ATPase are not completely coupled, and protons can leak across the inner mitochondrial membrane independently of F0F1-ATPase. Inhibition of F0F1-ATPase decreases oxidative phosphorylation but increases the proton accumulation and proton-motive force across the inner membrane because protons cannot return to the matrix through the proton channel in F0F1-ATPase. Elevation of proton gradient eventually leads to dysfunction of respiratory complexes and increased superoxide production caused by more electrons escaping the electron transfer chain.
We hypothesize that GT3 exerts its protective effects through several different mechanisms at the level of the mitochondrial membrane. 1) GT3 may decrease mitochondrial damage through maintaining F0F1-ATPase function and proton gradient across the inner mitochondrial membrane. This action would prevent the build-up of protons across the inner membrane caused by decreased return of protons through the F0 portion of ATP synthase and the subsequent proton leak. Mitochondrial coupling (RCR) and ΔΨm were reduced by TBHP 4 h after the exposure, indicating a decreased ability of mitochondria to produce ATP. TBHP reduced F0F1-ATPase activity, thus further limiting mitochondrial capacity to synthesize ATP. GT3 maintained mitochondrial coupling and ΔΨm, and we speculate that GT3 may maintain mitochondrial coupling and oxidative phosphorylation through its actions on F0F1-ATPase. 2) The burst of free radicals during oxidant exposure can trigger an opening of the mitochondrial permeability transition (MPT) pore, resulting in mitochondrial depolarization, decreased proton-motive force and ATP synthesis, and reduced ATP levels in RPTCs. Our data show that GT3 inhibits oxidant-induced MPT and maintains ΔΨm, suggesting that it may act on the MPT pore, prevent its opening and mitochondrial depolarization, and block RPTC lysis and death. 3) State 3 respiration (maximum mitochondrial respiration) is decreased 50% by oxidant exposure, indicating disruption of integrity of the respiratory chain and decreased electron transfer through the chain. This may be caused by free radical-induced modifications and damage of the chain's components. By acting as an antioxidant, GT3 protects the components of the respiratory chain and improves the mitochondrial flow of electrons and state 3 respiration. It is noteworthy that state 3 respiration was not completely restored by GT3 in TBHP-injured RPTCs, whereas GT3 fully restored F0F1-ATPase function and ΔΨm. This suggests that the latter mitochondrial functions may be primary targets for GT3-offered protection and the damage to the respiratory chain is more severe and/or caused by multiple mechanisms, some of which may not be targets for GT3.
In contrast, AT, which is the best known member of the vitamin E family, was not as effective as GT3 in protecting against the oxidative damage in RPTCs. At the same micromolar concentrations, AT did not block ROS generation in injured RPTCs, which suggests that the antioxidant effect may require higher concentrations of AT and GT3 is a superior antioxidant. Although AT presence improved state 3 respiration in injured cells to a similar degree as GT3, GT3 was superior in maintaining the coupling of mitochondria (RCR) and the function of F0F1-ATPase (measured as oligomycin-sensitive respiration). As a result, AT was ineffective in protecting against decreases in ATP content in injured RPTCs, whereas GT3 treatment resulted in full protection against ATP loss. Further, at the same concentrations, AT offered only a mild protection against oxidant-induced cell lysis, whereas GT3 completely blocked RPTC lysis.
Concentrations of tocotrienols in human tissues are lower than those of tocopherols but tocotrienols have superior antioxidant activity in some tissues (Kamat and Devasagayam, 1995; Aggarwal et al., 2010). Unlike tocopherols, tocotrienols block cell proliferation, survival, angiogenesis, and inflammation and induce apoptosis through the mitochondrial pathway in neoplastic cells, but protect against cell death and damage in cardiac and neuronal cells (Kumar et al., 2006; Aggarwal et al., 2010). Protective actions of tocotrienols against neuronal cell death and brain damage have been attributed mainly to their antioxidant properties and the ability to reduce oxidative stress accompanying different disorders (Mazlan et al., 2006; Sen et al., 2009). Tocotrienols are more effective than tocopherols in protecting against oxidative damage to mitochondrial proteins caused by exposure to free radicals (Kamat and Devasagayam, 1995). In addition, tocotrienols are several-fold more effective than tocopherols in inhibiting proliferation of neoplastic cells and inducing apoptosis (McIntyre et al., 2000). Finally, GT3 is the most effective of all tocols in protecting the heart against ischemia/reperfusion-induced damage (Das et al., 2008; Lekli et al., 2010). These differences have been attributed to preferential tissue accumulation of tocotrienols. GT3 is readily taken up and accumulated in RPTCs. It remains to be determined whether GT3 mediates its protective actions in RPTCs through blocking oxidative stress and/or by other mechanisms.
Reports suggest that, at low concentrations, tocotrienols may protect some tissues, including neuronal tissue, by antioxidant-independent mechanisms involving up-regulation of the phosphoinositide-3 kinase-Akt-mammalian target of rapamycin pathway and down-regulation of the c-Src-mediated pathway (Sen et al., 2000; Aggarwal et al., 2010). In addition, it was demonstrated that the interaction of mitogen-activated protein kinases with caveolin 1/3 and proteasome stabilization play a role in GT3-mediated cardioprotection by altering the availability of prosurvival and antisurvival proteins (Das et al., 2008). However, at concentrations higher than 30 μM, tocotrienols become toxic in many systems, including neuronal and cancer cells (Kumar et al., 2006; Then et al., 2009). Data suggest that the toxic effect is caused by the inability of some cell types to detoxify quinone metabolites of GT3, which have pro-oxidant activity (Kumar et al., 2006).
Evidence suggests that the protective effects of GT3 are mediated, in part, through down-regulating protein levels and the activity of HMG-CoA reductase (Pearce et al., 1992; Qureshi and Qureshi, 1993; Khor et al., 1995; Song and DeBose-Boyd, 2006; Berbée et al., 2011). The inhibitory effect of GT3 on HMG-CoA reductase activity is more pronounced at lower concentrations of GT3 and δ-tocotrienol (Khor et al., 1995). Other tocols have no measurable effect on HMG-CoA reductase degradation (Song and DeBose-Boyd, 2006) and can attenuate down-regulation of this enzyme by GT3 (Qureshi et al., 1995). GT3 is a potent radioprotectant and reduces animal mortality and lipid peroxidation caused by total body irradiation (Kumar et al., 2006; Ghosh et al., 2009). A recent report has shown that GT3-conferred protection against vascular radiation injury is mediated through HMG-CoA reductase-dependent mechanism, and mevalonate, the product of HMG-CoA reductase, attenuates radioprotective and antioxidant effects of GT3 in the vascular/endothelial system (Berbée et al., 2011). It is not known whether HMG-CoA reductase is down-regulated by GT3 in RPTCs or is involved in the antioxidant and/or protective effects of GT3 in this cell type. HMG-CoA reductase is a key regulatory enzyme in the biosynthesis of ubiquinone, the mobile electron carrier shuttling electrons between complexes I, II, and III of the respiratory chain. Insufficient amounts of ubiquinone may lead to the escape of electrons from the respiratory chain and increased formation of superoxide. It is not clear whether protective actions of GT3 against mitochondrial dysfunction in RPTCs depend on the down-regulation of HMG-CoA reductase activity.
In conclusion, this is the first report demonstrating that GT3 is taken up by RPTCs, reduces mitochondrial dysfunction, restores ATP levels, blocks free radical burst, and prevents RPTC lysis and death after oxidant-induced injury. Mitochondria are a major target of protective actions of GT3 in injured RPTCs. GT3 improves mitochondrial respiration, coupling, and ΔΨm and maintains oxidative phosphorylation and ATP levels in injured RPTCs. In contrast, at the same concentrations, α-tocopherol is ineffective in blocking ROS production, maintaining ATP content, and protecting against cell lysis in oxidant-injured RPTCs.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK059558] (to G.N.) and the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant AI67798] (to M.H.-J.). The University of Arkansas for Medical Sciences Translational Research Institute, supported by the National Institutes of Health National Center for Research Resources [Grant UL1-RR029884], provided partial funding for the Flow Cytometry Core at the University of Arkansas for Medical Sciences.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- AT
- α-tocopherol
- GT3
- γ-tocotrienol
- RPTC
- renal proximal tubular cell
- TBHP
- tert-butyl hydroperoxide
- ROS
- reactive oxygen species
- LDH
- lactate dehydrogenase
- RCR
- respiratory control ratio
- carboxy-H2DCFDA
- 5-(and-6)-carboxy-2′,7′-dichlorodihydro-fluorescein diacetate
- ΔΨm
- mitochondrial membrane potential
- MTP
- mitochondrial permeability transition.
Authorship Contributions
Participated in research design: Nowak, Hauer-Jensen, and Compadre.
Conducted experiments: Nowak, Bakajsova, Hayes, and Compadre.
Contributed new reagents or analytic tools: Hauer-Jensen and Compadre.
Performed data analysis: Nowak, Bakajsova, Hayes, and Compadre.
Wrote or contributed to the writing of the manuscript: Nowak, Bakajsova, Hayes, Hauer-Jensen, and Compadre.
References
- Abid MR, Razzaque MS, Taguchi T. (2005) Oxidant stress in renal pathophysiology. Contrib Nephrol 148:135–153 [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Prasad S, Kannappan R. (2010) Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol 80:1613–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbée M, Fu Q, Garg S, Kulkarni S, Kumar KS, Hauer-Jensen M. (2011) Pentoxifylline enhances the radioprotective properties of γ-tocotrienol: differential effects on the hematopoietic, gastrointestinal and vascular systems. Radiat Res 175:297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichay TJ, Roy RM. (1986) Modification of survival and hematopoiesis in mice by tocopherol injection following irradiation. Strahlenther Onkol 162:391–399 [PubMed] [Google Scholar]
- Boerma M, Roberto KA, Hauer-Jensen M. (2008) Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and α-tocopherol. Int J Radiat Oncol Biol Phys 72:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohé R, Traber MG. (1999) Vitamin E: function and metabolism. FASEB J 13:1145–1155 [PubMed] [Google Scholar]
- Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. (2008) Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl (111):S4–S9 [DOI] [PubMed] [Google Scholar]
- Cottrell RC. (1991) Introduction: nutritional aspects of palm oil. Am J Clin Nutr 53 (Suppl 4):989S–1009S [DOI] [PubMed] [Google Scholar]
- Das S, Lekli I, Das M, Szabo G, Varadi J, Juhasz B, Bak I, Nesaretam K, Tosaki A, Powell SR, et al. (2008) Cardioprotection with palm oil tocotrienols: comparision of different isomers. Am J Physiol Heart Circ Physiol 294:H970–H978 [DOI] [PubMed] [Google Scholar]
- Elson CE. (1995) Suppression of mevalonate pathway activities by dietary isoprenoids: protective roles in cancer and cardiovascular disease. J Nutr 125:1666S–1672S [DOI] [PubMed] [Google Scholar]
- Felemovicius I, Bonsack ME, Baptista ML, Delaney JP. (1995) Intestinal radioprotection by vitamin E (α-tocopherol). Ann Surg 222:504–508; discussion 508–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao TC, Hauer-Jensen M, Kumar KS. (2009) γ-Tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol 85:598–606 [DOI] [PubMed] [Google Scholar]
- Goh SH, Hew NF, Norhanom AW, Yadav M. (1994) Inhibition of tumour promotion by various palm-oil tocotrienols. Int J Cancer 57:529–531 [DOI] [PubMed] [Google Scholar]
- Kamat JP, Devasagayam TP. (1995) Tocotrienols from palm oil as potent inhibitors of lipid peroxidation and protein oxidation in rat brain mitochondria. Neurosci Lett 195:179–182 [DOI] [PubMed] [Google Scholar]
- Khan MR, Siddiqui S, Parveen K, Javed S, Diwakar S, Siddiqui WA. (2010) Nephroprotective action of tocotrienol-rich fraction (TRF) from palm oil against potassium dichromate (K2Cr2O7)-induced acute renal injury in rats. Chem Biol Interact 186:228–238 [DOI] [PubMed] [Google Scholar]
- Khor HT, Chieng DY, Ong KK. (1995) Tocotrienols inhibit liver HMG CoA reductase activity in the guinea pig. Nutr Res 15:537–544 [Google Scholar]
- Koyner JL, Sher Ali R, Murray PT. (2008) Antioxidants. Do they have a place in the prevention or therapy of acute kidney injury? Nephron Exp Nephrol 109:e109–e117 [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, Gambles K, Toles R, Kao TC, Hauer-Jensen M, et al. (2010) γ-Tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res 173:738–747 [DOI] [PubMed] [Google Scholar]
- Kumar KS, Raghavan M, Hieber K, Ege C, Mog S, Parra N, Hildabrand A, Singh V, Srinivasan V, Toles R, et al. (2006) Preferential radiation sensitization of prostate cancer in nude mice by nutraceutical antioxidant γ-tocotrienol. Life Sci 78:2099–2104 [DOI] [PubMed] [Google Scholar]
- Lekli I, Ray D, Mukherjee S, Gurusamy N, Ahsan MK, Juhasz B, Bak I, Tosaki A, Gherghiceanu M, Popescu LM, et al. (2010) Co-ordinated autophagy with resveratrol and γ-tocotrienol confers synergetic cardioprotection. J Cell Mol Med 14:2506–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Godwin ML, Nowak G. (2004) Protein kinase C-α inhibits the repair of oxidative phosphorylation after S-(1,2-dichlorovinyl)-l-cysteine injury in renal cells. Am J Physiol Renal Physiol 287:F64–F73 [DOI] [PubMed] [Google Scholar]
- Mazlan M, Sue Mian T, Mat Top G, Zurinah Wan Ngah W. (2006) Comparative effects of α-tocopherol and γ-tocotrienol against hydrogen peroxide induced apoptosis on primary-cultured astrocytes. J Neurol Sci 243:5–12 [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester PW. (2000) Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids 35:171–180 [DOI] [PubMed] [Google Scholar]
- Nowak G. (2002) Protein kinase C-α and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem 277:43377–43388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Bakajsova D, Clifton GL. (2004) Protein kinase C-ε modulates mitochondrial function and active Na+ transport after oxidant injury in renal cells. Am J Physiol Renal Physiol 286:F307–F316 [DOI] [PubMed] [Google Scholar]
- Nowak G, Bakajsova D, Samarel AM. (2011) Protein kinase C-ε activation induces mitochondrial dysfunction and fragmentation in renal proximal tubules. Am J Physiol Renal Physiol 301:F197–F208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Clifton GL, Godwin ML, Bakajsova D. (2006) Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol 291:F840–F55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Schnellmann RG. (1996) l-Ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol Cell Physiol 271:C2072–C2080 [DOI] [PubMed] [Google Scholar]
- Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ. (1992) Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem 35:3595–3606 [DOI] [PubMed] [Google Scholar]
- Qureshi AA, Bradlow BA, Brace L, Manganello J, Peterson DM, Pearce BC, Wright JJ, Gapor A, Elson CE. (1995) Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids 30:1171–1177 [DOI] [PubMed] [Google Scholar]
- Qureshi N, Qureshi AA. (1993) Tocotrienols: novel hypocholesterolemic agents with antioxidant properties, in Vitamin E in Health and Disease (Packer L, Fuchs J. eds) pp 247–268, Marcel Dekker, Inc., New York [Google Scholar]
- Sen CK, Gordillo GM, Khanna S, Roy S. (2009) Micromanaging vascular biology: tiny microRNAs play big band. J Vasc Res 46:527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Roy S, Packer L. (2000) Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J Biol Chem 275:13049–13055 [DOI] [PubMed] [Google Scholar]
- Shaik ZP, Fifer EK, Nowak G. (2008) Akt activation improves oxidative phosphorylation in renal proximal tubular cells following nephrotoxicant injury. Am J Physiol Renal Physiol 294:F423–F432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Shafran RL, Jackson WE, 3rd, Seed TM, Kumar KS. (2006) Induction of cytokines by radioprotective tocopherol analogs. Exp Mol Pathol 81:55–61 [DOI] [PubMed] [Google Scholar]
- Song BL, DeBose-Boyd RA. (2006) Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase stimulated by δ- and γ-tocotrienols. J Biol Chem 281:25054–25061 [DOI] [PubMed] [Google Scholar]
- Sun W, Xu W, Liu H, Liu J, Wang Q, Zhou J, Dong F, Chen B. (2009) γ-Tocotrienol induces mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J Nutr Biochem 20:276–284 [DOI] [PubMed] [Google Scholar]
- Sylvester PW, Shah SJ, Samant GV. (2005) Intracellular signaling mechanisms mediating the antiproliferative and apoptotic effects of γ-tocotrienol in neoplastic mammary epithelial cells. J Plant Physiol 162:803–810 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Loo G. (2004) Disruption of mitochondria during tocotrienol-induced apoptosis in MDA-MB-231 human breast cancer cells. Biochem Pharmacol 67:315–324 [DOI] [PubMed] [Google Scholar]
- Then SM, Mazlan M, Mat Top G, Wan Ngah WZ. (2009) Is vitamin E toxic to neuron cells? Cell Mol Neurobiol 29:485–496 [DOI] [PubMed] [Google Scholar]
- Theriault A, Chao JT, Wang Q, Gapor A, Adeli K. (1999) Tocotrienol: a review of its therapeutic potential. Clin Biochem 32:309–319 [DOI] [PubMed] [Google Scholar]