Abstract
Preclinical studies have demonstrated that repeated methamphetamine (METH) injections (referred to herein as a “binge” treatment) cause persistent dopaminergic deficits. A few studies have also examined the persistent neurochemical impact of METH self-administration in rats, but with variable results. These latter studies are important because: 1) they have relevance to the study of METH abuse; and 2) the effects of noncontingent METH treatment do not necessarily predict effects of contingent exposure. Accordingly, the present study investigated the impact of METH self-administration on dopaminergic neuronal function. Results revealed that self-administration of METH, given according to a regimen that produces brain METH levels comparable with those reported postmortem in human METH abusers (0.06 mg/infusion; 8-h sessions for 7 days), decreased striatal dopamine transporter (DAT) uptake and/or immunoreactivity as assessed 8 or 30 days after the last self-administration session. Increasing the METH dose per infusion did not exacerbate these deficits. These deficits were similar in magnitude to decreases in DAT densities reported in imaging studies of abstinent METH abusers. It is noteworthy that METH self-administration mitigated the persistent deficits in dopaminergic neuronal function, as well as the increases in glial fibrillary acidic protein immunoreactivity, caused by a subsequent binge METH exposure. This protection was independent of alterations in METH pharmacokinetics, but may have been attributable (at least in part) to a pretreatment-induced attenuation of binge-induced hyperthermia. Taken together, these results may provide insight into the neurochemical deficits reported in human METH abusers.
Introduction
Methamphetamine (METH) is a widely abused psychostimulant that can cause persistent alterations in monoaminergic neuronal function. For example, imaging studies indicate that METH abusers display a 15 to 25% decrease in caudate dopamine (DA) transporter (DAT) density (Volkow et al., 2001; Chang et al., 2007) that can persist for years (McCann et al., 1998), as well as variable effects on caudate vesicular monoamine transporter-2 (VMAT-2) density (Johanson et al., 2006; Boileau et al., 2008). Postmortem studies of METH abusers also indicate variable changes in caudate VMAT-2 immunoreactivity or binding, as well as decreased tyrosine hydroxylase (TH) and DAT immunoreactivity (Wilson et al., 1996; Kitamura et al., 2007). However, the magnitude of alterations in DAT in the postmortem studies is generally greater than in the imaging studies cited above, perhaps owing to the presence of METH in the brains of the former. Alternatively, abusers assessed postmortem may have been exposed to greater METH levels than those assessed in imaging studies (Melega et al., 2007). Differences in the amount or presence of METH in the brain may contribute to differences in findings between postmortem studies and imaging studies.
As in humans, METH can cause persistent dopaminergic deficits in rodent models. For example, administration of a “binge” METH regimen (e.g., 4–6 injections; 7.5–15 mg/kg/injection; 2- to 6-h intervals): 1) decreases DAT and VMAT-2 binding, immunoreactivity, and function; and 2) decreases DA content and TH immunoreactivity (Brown et al., 2000; Cappon et al., 2000; Eyerman and Yamamoto, 2007). Binge METH treatment increases glial fibrillary acidic protein (GFAP) expression as well (Hadlock et al., 2010).
It is noteworthy that although the binge model has provided insight into both METH-induced dopaminergic deficits and DA-related degenerative disorders its associated neurochemical alterations are frequently greater in magnitude than those reported in human imaging studies (Chang et al., 2007; Brennan et al., 2010). These and other data demonstrate the importance of evaluating the impact of METH self-administration (versus binge administration) in that the effects of noncontingent METH treatment do not necessarily predict the effects of contingent exposure (Brennan et al., 2010; Frankel et al., 2011). Accordingly, the present study investigated the impact of METH self-administration on dopaminergic neuronal function.
Of relevance are preclinical findings that pretreatment with intermittent low-dose or multiple escalating-dose METH injections attenuates the dopaminergic deficits caused by a subsequent binge treatment (McFadden et al., 2011 and references therein). Likewise, a binge pretreatment protects against both acute and persistent dopaminergic deficits caused by a second binge treatment (Thomas and Kuhn, 2005; Hanson et al., 2009). These studies are important, because most individuals who abuse METH receive multiple exposures to the drug. However, little is known regarding mechanisms underlying these “neuroprotective” changes.
As noted above, studies have focused on the effects of METH self-administration in the rodent model. Some investigators (Schwendt et al., 2009; Krasnova et al., 2010), but not all (Stefanski et al., 2002; Shepard et al., 2006; Brennan et al., 2010), reported persistent dopaminergic deficits caused by self-administration. For example, Schwendt et al. (2009) reported relatively small “neuroadaptations” that were specific to the DAT. In contrast, Krasnova et al. (2010) reported larger deficits in several monoaminergic markers, including the DAT. Given these disparate findings, and again noting that the effects of noncontingent METH treatment do not necessarily predict the effects of contingent exposure (Brennan et al., 2010; Frankel et al., 2011), one purpose of the present study was to further investigate the neurochemical impact of METH self-administration. A second purpose was to investigate whether repeated self-exposure to METH, like effects observed after intermittent low-dose or multiple escalating-dose injections, influences the expression of dopaminergic deficits caused by a subsequent binge METH treatment. Finally, another purpose of this study was to investigate possible mechanisms underlying the changes in dopaminergic deficits after a subsequent binge treatment of METH. The results of the current study may provide important insight into the neurochemical deficits reported in human METH abusers.
Materials and Methods
Animals.
Male Sprague-Dawley rats (275–300 g; Charles River Breeding Laboratories, Portage, MI) were housed four rats per cage (35 × 30 × 16 cm). After surgery, each rat was individually housed in a transparent plastic cage (45 × 23 × 21 cm). Water was available in their home cages ad libitum. During food training, rats were food-restricted such that no rat dropped below 90% of its starting body weight. Rats were maintained under the same 14:10-h light/dark cycle in the animal facility and in the operant chambers. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Drugs.
(±)-METH hydrochloride (Research Triangle Institute, Research Triangle Park, NC) was dissolved in 0.9% sterile saline, with the dose described as the free base form. Equithesin (3 ml/kg) or ketamine (90 mg/kg; Hospira Inc., Lake Forest, IL) and xylazine (7 mg/kg; Sigma-Aldrich, St. Louis, MO) was used to anesthetize animals. The antibiotic cefazolin (10 mg/ml; Schein Pharmaceutical, Florham Park, NJ) was dissolved in heparinized saline (63.33 U/ml; Sigma-Aldrich). Flunixin meglumine (1.1 mg/kg; MWI Veterinary Supply, Meridian, ID) was used for postsurgery analgesia.
Apparatus.
Food training and self-administration occurred in an operant chamber (30.5 × 25.5 × 30.5 cm; Coulbourn Instruments, Allentown, PA) located within a sound-attenuating cubical (79 × 53 × 53 cm; Coulbourn Instruments). Each chamber was equipped with a food pellet hopper, two retractable levers, and house light (Coulbourn Instruments).
Food Training.
Before surgery, each rat was trained to press for a 45-mg food pellet during four overnight 14-h sessions. During the first 2 h of each session, a food pellet was dispensed every 90 s or after an active lever press, whichever came first. Each time the pellet was dispensed the levers were retracted for 20 s. For the remaining 12 h, food pellets were dispensed based on a fixed ratio 1 schedule of reinforcement. Upon each active lever press, both levers were retracted for 20 s, and a food pellet was dispensed. Inactive lever presses were recorded, but had no programmable consequence. On each day the active lever was counterbalanced among all of the rats such that half of the rats had the right lever as the active lever, and the other half had the left lever as active. For each rat, the active lever was changed from day to day such that each lever was reinforced for 2 days of training.
Catheters and Surgery.
The catheter was constructed in the laboratory as described previously (Frankel et al., 2011). Rats were anesthetized, and an indwelling catheter was implanted. The outlet of the catheter was implanted subcutaneously in the back, and the free end of the Silastic tubing was inserted 25 mm into the right jugular vein and secured to the surrounding tissue with sutures. Each rat received flunixin meglumine (subcutaneously) on the day of the surgery and the day after the surgery. Immediately after surgery and daily thereafter, each rat was infused with 0.1 ml of the antibiotic cefazolin followed by 0.05 ml of heparinized saline and heparinized glycerol through the catheter. Catheter patency was checked by infusing 0.03 ml (20 mg/ml) of xylazine.
Self-Administration.
Rats underwent 7 days of self-administration (8 h/session; fixed ratio 1) during the light cycle in a room maintained at 29 ± 1°C to promote lever pressing (Cornish et al., 2008). For each active lever press, an infusion pump (Coulbourn Instruments) connected to a liquid swivel (Coulbourn Instruments) suspended outside of the operant chamber delivered 10 μl of METH (0.06, 0.12, or 0.24 mg/infusion) or saline per infusion over a 5-s duration through a polyethylene tube located within a spring leash (Coulbourn Instruments) tethered to the rat. During this period, both levers were retracted. After the infusion, the levers remained retracted for an additional 20 s. The active lever was counterbalanced within each group, but remained the same for each rat from day to day. Pressing the inactive lever resulted in no programmed consequences although it was recorded. Rectal temperatures were measured by using a digital thermometer (Physiotemp Instruments, Clifton, NJ) approximately 30 min after the end of each session and 30 and 90 after each METH injection.
Tissue Preparation.
Tissue preparation was conducted as described previously (Hanson et al., 2009). After decapitation, the striata were quickly dissected out, and the right striatum was homogenized in ice-cold sucrose buffer (0.32 M sucrose, 3.8 mM NaH2PO4, and 12.7 mM Na2HPO4). The left striatum was quickly frozen on dry ice.
Plasmalemmal and Vesicular [3H]DA Uptake Assays.
[3H]DA uptake assays were conducted according to Johnson-Davis et al. (2004). For plasmalemmal uptake of [3H]DA, striatal synaptosomes were prepared accordingly and resuspended in ice-cold Krebs' buffer (126 nM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 16 mM sodium phosphate, 1.4 mM MgSO4, 11 mM dextrose, and 1 mM ascorbic acid, pH 7.4). Assay tubes containing 1.5 mg of striatal tissue and 1 μM pargyline were incubated (3 min, 37°C) with [7,8-3H]DA (0.5 nM final concentration; PerkinElmer Life and Analytical Sciences, Waltham, MA). Nonspecific values were ascertained in the presence of 10 μM cocaine. Samples were filtered by using a filtering manifold (Brandel, Inc., Gaithersburg, MD) through Whatman GF/B filters (Whatman, Maidstone, UK) soaked previously in 0.05% polyethylenimine and washed three times with 3 ml of ice-cold 0.32 M sucrose.
For vesicular [3H]DA uptake, synaptic vesicles were isolated according to Johnson-Davis et al. (2004) and resuspended in assay buffer (25 mM HEPES, 100 mM potassium tartrate, 1.7 mM ascorbic acid, 0.05 mM EGTA, 0.1 mM EDTA, and 1.8 mM ATP-Mg2+; pH 7.5). Vesicles were incubated (3 min, 30°C) in the presence of [7,8-3H]DA (30 nM final concentration; PerkinElmer Life and Analytical Sciences). Nonspecific values were found by measuring vesicular [3H]DA uptake at 4°C in the absence of ATP. Samples were filtered through GF/F filters (Whatman) previously soaked in 0.5% polyethylenimine and washed three times with cold wash buffer. The radioactivity trapped in filters was counted by using a liquid scintillation counter. Protein concentrations were determined by using the Bradford Protein Assay.
Western Blotting.
Western blotting was conducted according to Hadlock et al. (2009). Equal quantities of protein (8 μg) were loaded into each well of a 4 to 12% NuPAGE Novex Bis-Tris Midi gradient gel (Invitrogen, Carlsbad, CA) and electrophoresed by using a XCell4 Surelock Midi-Cell (Invitrogen). Membranes were blocked for 30 min with Starting Block Blocking Buffer (Thermo Fisher Scientific, Waltham, MA) and incubated for 1 h at room temperature with a DAT antibody (a generous gift from Dr. Roxanne Vaughan, University of North Dakota, Grand Forks, ND), GFAP (556329; BD Biosciences, San Jose, CA), or TH (MAB418; Millipore Corporation, Billerica, MA). The polyvinylidene difluoride membrane was then washed five times in Tris-buffered saline with Tween (250 mM NaCl, 50 mM Tris, pH 7.4, and 0.05% Tween 20). The membranes were then incubated for 1 h with a horseradish peroxidase-conjugated secondary antibody (BioSource International, Camarillo, CA). After five washes in Tris-buffered saline with Tween, the bands were visualized by using Western Lightning Chemiluminescence Reagents Plus (PerkinElmer Life and Analytical Sciences) and quantified by densitometry using a FluorChem SP Imaging System (Alpha Innotech, San Leandro, CA). Protein concentrations were determined by using the Bradford Protein Assay. Immunoreactivity was normalized from arbitrary units to percentage of saline control.
DA Content.
The anterior portion of the left striatum was sonicated for 3 to 5 s in 1 ml of tissue buffer (0.1 M phosphate/citrate buffer, pH 2.5, containing 15–20% methanol) and prepared according to Haughey et al., (2000). Fifty microliters was injected onto a partisphere C-18 reverse-phase analytical column (5-μm spheres; 250 × 4.6 mm; Whatman, Clifton, NJ). Mobile phase consisting of 0.05 M sodium phosphate, 0.03 M citrate buffer, 0.1 M EDTA, 0.035% sodium octylsulfate, and 15 to 20% methanol (pH 2.8; flow rate 0.75 ml/min) was used. DA was detected by using an ampherometric electrochemical detector with the working electrode potential set at +0.73 V relative to an Ag+/AgCl reference electrode.
METH Concentrations.
Brain METH concentrations were measured by liquid chromatography-tandem mass spectrometry as described previously (Truong et al., 2005). The whole brains (except for the striatum, hippocampus, and frontal cortex) were weighed and homogenized separately in 10 ml of water. A Vibra Cell homogenizer (Sonics, Newton, CT) was used for the homogenization. A 0.5-ml volume of the homogenate was used for the analysis. An Agilent liquid chromatograph (Agilent Technologies, Santa Clara, CA) coupled to a ThermoQuest Finnigan TSQ 7000 tandem mass spectrometer (Thermo Fisher Scientific) was used for the analysis. Electrospray ionization was used. The limit of quantitation was 1 ng/ml in the homogenates.
Statistical Analysis.
All samples within a given experiment were processed concurrently. Statistical analysis was conducted in SAS 9.2 (SAS Institute, Cary, NC). Statistical analyses among groups were conducted by using a t test, one-way analysis of variance (neurochemical data/self-administration temperature data), or repeated-measures analysis of variance (lever pressing/binge temperature data) followed by Newman-Keuls post hoc analyses. Violations of the sphericity assumption resulted in the use of a Huynh-Feldt correction. Neurochemical analyses were normalized to the percentage of saline self-administering rats. The data represent means ± S.E.M. of 3 to 21 rats per group.
Results
For analysis of the data presented in Fig. 1, rats self-administering METH (0.06 mg/infusion) were divided into low- and high-pressing rats (Fig. 1A) based largely on the criteria set by Brennan et al. (2010). Specifically, rats were considered high pressers (HP) if: 1) they pressed an average of more than 10 active lever presses per day; and 2) the ratio of active/inactive lever presses was ≥2:1. Rats that did not achieve these criteria were considered low pressers (LP). Rats that met HP criteria discriminated between the active and inactive levers at a ratio as much as 9:1 beginning on day 4 and also pressed the active lever significantly more than the saline self-administering animals beginning on day 4. It is noteworthy that because of a low number of rats meeting the LP criteria in this and other experiments (e.g., those presented in Figs. 2–6), only data from HP rats are provided.
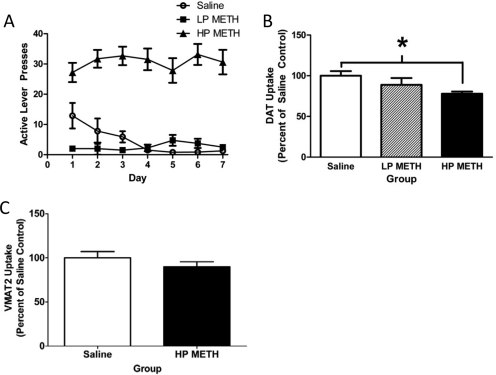
Fig. 1.
METH self-administration leads to persistent changes in DAT function. A, rats self-administered METH (0.06 mg/infusion; n = 25) or saline (10 μl/infusion; n = 10) for 7 days (8 h/day). B, rats were treated as in A and sacrificed 8 days after the final self-administration session, and DAT function was assessed. C, rats were treated as in A and sacrificed 8 days after the final self-administration session, and VMAT2 function was assessed. Mean saline values were 1.60 fmol/μg protein (B) and 80.7 fmol/μg protein (C). Values represent the mean of 10 saline, 4 LP METH, and 21 HP METH (B) or 11 saline and 14 HP METH animals (C). *, p < 0.05 versus saline self-administering rats.
Fig. 2.
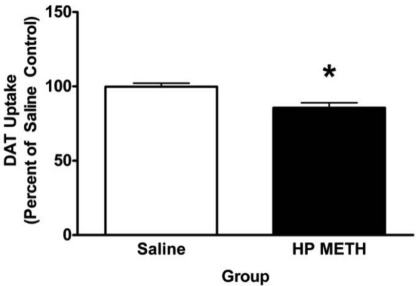
METH self-administration leads to changes in DAT function assessed 30 days after the last self-administration session. Rats self-administered METH (0.06 mg/infusion) or saline (10 μl/infusion) for 7 days (8 h/day). Mean saline values were 0.96 fmol/μg protein. Values represent the mean of three saline and nine HP METH animals. *, p < 0.05 versus saline self-administering rats.
Fig. 3.
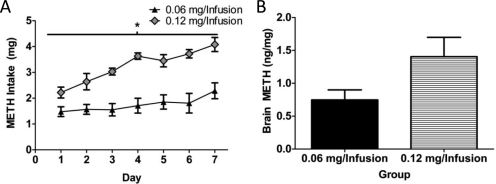
METH intake and brain levels in self-administering rats. A, rats self-administered METH (0.06 or 0.12 mg/infusion; n = 8 and 4, respectively) for 7 days (8 h/day). B, rats were sacrificed 1 h after the final self-administration session (n = 6 rats at 0.06 mg/infusion and n = 5 rats at 0.12 mg/infusion), and brain concentrations of METH were assessed. *, p < 0.05 versus 0.06 mg of METH/infusion.
Fig. 4.
METH self-administration leads to persistent changes in DAT function. A, rats self-administered METH (0.12 mg/infusion; n = 6) or saline (10 μl/infusion; n = 6) for 7 days (8 h/day) and were sacrificed 8 days after the final self-administration session. B, DAT transporter function was assessed in these animals. Mean saline values were 2.68 fmol/μg. *, p < 0.05 versus saline self-administration.
Fig. 5.
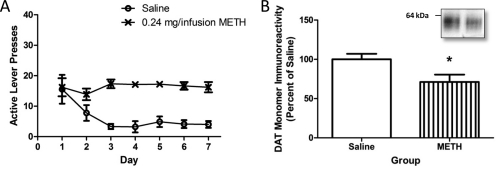
METH self-administration leads to persistent changes in DAT immunoreactivity. A, rats self-administered METH (0.24 mg/infusion; n = 9) or saline (10 μl/infusion; n = 8) for 7 days (8 h/day) and were sacrificed 8 days after the last session. B, DAT immunoreactivity was assessed in these animals. *, p < 0.05 versus saline self-administration. Inset, representative blot of saline self-administering rat (left) and 0.24 mg of METH/infusion rat (right).
Fig. 6.
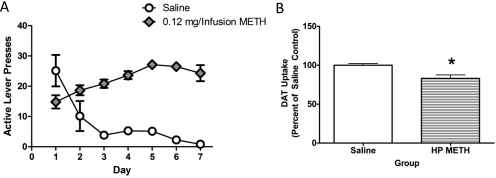
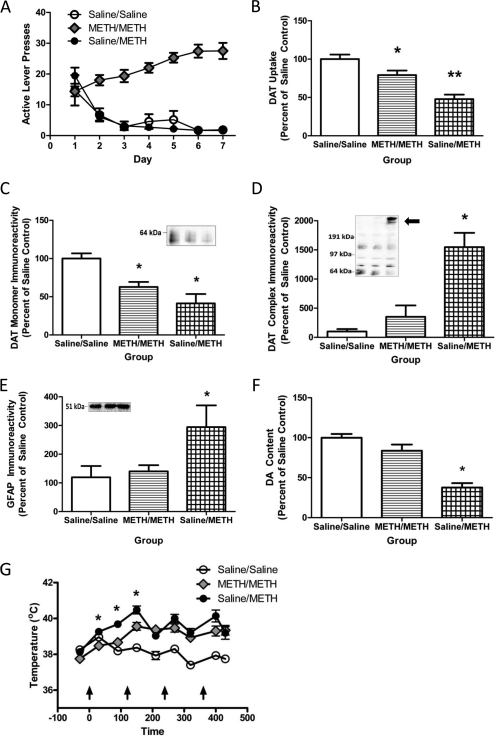
METH self-administration mitigates the effects of a subsequent binge METH treatment. A, rats self-administered METH (0.12 mg/infusion) or saline (10 μl /infusion) for 7 days (8 h/day). B, 24 h after the beginning of the final self-administration session, rats received METH (4 × 7.5 mg/kg s.c./injection; 2-h intervals) or saline (1 ml/kg s.c./injection; 2-h intervals) and were sacrificed 7 days later, and DAT function was assessed. C to E, DAT monomer (C), DAT complex (D), and GFAP (E) immunoreactivity in striatal synaptosomes were assessed in these animals. Insets, representative blot of samples from saline self-administering/saline-challenged rats (left), METH self-administering/METH-challenged (center), and saline self-administering/METH-challenged rats (right). F, DA content was assessed in the anterior portion of the left striatum. G, temperatures were taken every 30 and 90 min after each injection of METH or saline. Average saline values for DAT uptake were 2.85 fmol/μg protein. Average of 7 saline self-administering/saline-challenged, 14 METH self-administering/METH-challenged, and 11 saline self-administering/METH-challenged rats was used. *, p < 0.05 versus all other groups.
Results presented in Fig. 1B revealed that 7 days of METH self-administration (0.06 mg/infusion; 8-h sessions) decreased DAT function in HP rats compared with saline-administering controls, with no differences between LP and saline-administering rats (F2,32 = 7.87; p < 0.01), as assessed 8 days after the final self-administration session. It is noteworthy that the HP rats did not escalate the number of daily active lever presses over the course of the 7-day METH exposure (F6,114 = 0.83, ns). This regimen likewise decreased in DAT immunoreactivity in HP but not LP rats (saline, 100 ± 2.63%; LP METH, 85.88 ± 12.14%; HP METH, 79.54 ± 4.05%; F2,31 = 3.91; p < 0.05). In contrast, METH self-administration was without effect on striatal GFAP immunoreactivity (saline, 100 ± 25.7%; LP METH, 128.7 ± 45.2%; HP METH, 88.8 ± 16.9%; F2,31 = 0.45, ns), TH immunoreactivity (saline, 100.0 ± 5.46%; LP METH, 100.21 ± 11.95%; HP METH, 94.26 ± 3.98%; F2,9 = 0.41, ns), or DA content (saline, 224.3 ± 20.3 pg/μg; LP METH, 187.9 ± 32.8 pg/μg; HP METH, 235.9 ± 11.9 pg/μg; F2,22 = 0.88, ns). The total quantities of METH received over the entire 7 days of self-administration in LP and HP rats were 2.39 ± 0.27 and 14.07 ± 1.16 mg, respectively (t23 = 4.33; p < 0.05). METH self-administration also increased core body temperatures (saline, 37.68 ± 0.07°C; LP METH, 38.08 ± 0.12°C; HP METH, 38.6 ± 0.05°C; F2,32 = 50.1; p < 0.05).
In a separate experiment, the impact of this METH regimen [7 days of METH self-administration (0.06 mg/infusion)] on VMAT-2 activity was assessed. Results revealed that METH self-administration did not alter cytoplasmic VMAT-2 activity in HP rats as assessed 8 days after the last self-administration session (t23 = 1.12, ns; Fig. 1C). In this experiment, all rats met the HP criteria. The rats received a total of 12.37 ± 1.53 mg of METH over the entire 7-day course of self-administration and had core body temperatures of 38.39 ± 0.08°C versus 37.61 ± 0.06°C in saline controls (t23 = 7.76; p < 0.05).
To determine whether the alterations in DAT activity persist, rats were allowed to self-administer METH as described for Fig. 1, and then were sacrificed 30 days later. Results presented in Fig. 2 demonstrate that the decreases in DAT activity in HP rats persisted for 30 days (t10 = 2.29; p < 0.05). In contrast, METH self-administration (0.06 mg/infusion) did not alter striatal DAT immunoreactivity (saline, 100 ± 2.62%; METH HP, 89.93 ± 5.32%; t10 = 1.05, ns), DA content (saline, 248.9 ± 6.52 pg/μg; HP, 223.8 ± 13.2 pg/μg; t10 = 1.05, ns), TH immunoreactivity (saline, 100 ± 6.33%; HP, 89.22 ± 5.46%; t10 = 1.05, ns), or GFAP immunoreactivity (saline, 100 ± 21.55%; HP, 138.2 ± 20.24%; t10 = 1.01, ns) as assessed 30 days after the final drug exposure. Rats in this experiment received a total of 11.45 ± 1.83 mg of METH over the entire 7-day course of self-administration and attained core body temperatures of 38.27 ± 0.09°C versus 37.65 ± 0.09°C for saline controls (t10 = 3.66; p < 0.05).
To better characterize our self-administration paradigm, we examined the effects of increasing the dose/infusion of METH. Results presented in Fig. 3A demonstrate that increasing the dose of METH received per infusion from 0.06 to 0.12 mg/10 μl infusion led to a greater escalation in daily METH intake (F6,60 = 3.02; p < 0.05; Fig. 3A). This resulted in greater total METH intake over the course of the 7 days of self-administration (0.06 mg/infusion HP, 12.26 ± 1.65 mg; 0.12 mg/infusion HP, 22.77 ± 0.70 mg; t10 = 4.29; p < 0.05). However, both METH self-administration groups had similar increased average end-of-session body temperatures compared with saline control rats (saline, 37.69 ± 0.09°C; 0.06 mg/infusion HP, 38.37 ± 0.14°C; 0.12 mg/infusion HP, 38.48 ± 0.07°C; F2,13 = 22.11; p < 0.05). A separate group of rats was sacrificed 1 h after the last session, and brain METH levels were assessed. Doubling the METH infusion dose nearly doubled METH levels within the brain (t9 = 2.09; p = 0.07; Fig. 3B).
Results presented in Fig. 4 confirm that, as in Fig. 3A, increasing the dose of METH received per infusion to 0.12 mg/infusion led to an escalation in daily active lever presses (F6,30 = 8.65; p < 0.05; Fig. 4A). However, this did not enhance the magnitude of decrease in DAT uptake (t10 = 3.36; p < 0.05; Fig. 4B) compared with using 0.06 mg/infusion as assessed 8 days after the last 8-h session (see Fig. 1B). This METH regimen increased core body temperature (METH, 38.48 ± 0.07°C; saline, 37.69 ± 0.08°C; t10 = 6.92; p < 0.05), and rats infused a total of 21.24 ± 0.83 mg of METH over the 7-day course of treatment. This paradigm decreased DAT immunoreactivity (saline, 100 ± 1.70%; METH HP, 82.8 ± 5.92%; t8 = 2.79; p < 0.05), but did not alter DA content (saline, 356.58 ± 14.76 pg/μg; HP, 379.13 ± 30.19 pg/μg; t10 = 0.6418, ns), TH immunoreactivity (saline, 100 ± 11.43%; HP, 99.65 ± 7.75%; t8 = 0.03, ns), or GFAP immunoreactivity (saline, 100 ± 28.59%; HP, 76.95 ± 9.51%; t8 = 0.76, ns) as assessed 8 days after the final METH exposure.
Figure 5A demonstrates that increasing the infusion dose of METH still further to 0.24 mg/infusion did not result in escalation in daily METH active lever pressing (F6,48 = 0.72, ns). Although increasing the dose resulted in greater total METH intake compared with the 0.12 mg/infusion group because of the greater amount of METH/infusion (0.24 mg/infusion total METH, 32.56 ± 1.89 mg) and increased core body temperature (saline, 37.80 ± 0.07°C; METH, 38.64 ± 0.07°C; t16 = 8.45; p < 0.05), decreases in DAT monomer immunoreactivity were similar to those resulting from lower infusion doses (saline, 100.00 ± 7.19%; METH, 71.19 ± 9.28%; t15 = 2.40; p < 0.05; Fig. 5B; compare with Figs. 1B and 3B). DAT uptake was not assessed in this experiment. This regimen did not alter GFAP (saline, 100.0 ± 15.03%; METH, 90.1 ± 12.9%; t16 = 0.50, ns) or TH immunoreactivity (saline, 100.0 ± 5.11; METH, 95.85 ± 5.49%; t16 = 0.59, ns). However, this regimen decreased striatal DA content (saline, 227.74 ± 5.62 pg/μg protein; METH, 165.00 ± 17.00 pg/μg protein; t16 = 3.50; p < 0.05).
To better model the human condition wherein drug escalation increases over time (Cho, 1990; Kitamura et al., 2006), the 0.12 mg/infusion dose of METH was selected for further study because it caused an escalation in daily intake from days 1 to 7 (Figs. 3A, 4A, and 6A). Results presented in Fig. 6B demonstrate that 7 days of METH self-administration (0.12 mg/infusion; 8-h sessions) attenuated the decrease in DAT uptake caused by a subsequent binge METH treatment (four injections, 7.5 mg/kg/injection, 2-h intervals, initiated 24 h after the beginning of the final METH self-administration session), as assessed 7 days after the binge exposure (F2,29 = 15.45; p < 0.05). METH self-administration also attenuated the binge-induced decrease DAT monomer immunoreactivity (Fig. 6C; F2,29 = 8.06; p < 0.05), increase in DAT complex formation (Fig. 6D; F2,29 = 12.97; p < 0.05), increase in GFAP immunoreactivity (Fig. 6E; F2,29 = 3.82; p < 0.05), and decrease in DA content (F2,28 = 4.859; p < 0.05; Fig. 6F) in these animals. Decreased striatal TH immunoreactivity in the saline self-administering and METH-challenged rats was also found, but did not reach significance (saline/saline, 100.00 ± 6.51%; METH/METH, 100.50 ± 5.85%; saline/METH, 82.88 ± 5.58%; F2,29 = 2.80; p = 0.08).
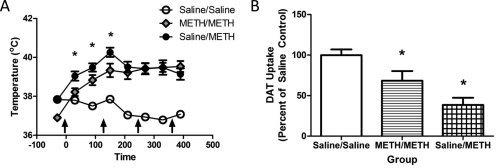
It is noteworthy that the results presented in Fig. 6G demonstrate that METH self-administration attenuated the hyperthermic response to a subsequent binge METH treatment, as assessed during the first 2.5 h of the binge regimen (F16,224 = 7.70, p < 0.05). In this experiment, no attempt was made to manipulate core body temperature. However, because it is well established that attenuation of hyperthermia can attenuate the monoaminergic deficits caused by a binge METH treatment (see Discussion), an additional experiment was conducted wherein rats were placed in a warm environment during the binge treatment to promote METH-induced hyperthermia. In this experiment, lever-pressing behavior was similar to that presented in Fig. 6A, with rats pressing for a total of 21.68 ± 1.86 mg of METH over the 7 days of treatment. Results revealed that despite treatment in the warm environment (i.e., a common practice used to promote METH-induced hyperthermia; see, for example Hadlock et al., 2010; McFadden et al., 2011) METH self-administration still attenuated the hyperthermic response caused by the binge treatment during the first 2.5 h of the binge (Fig. 7A; F14,112 = 14.44; p < 0.05). As in Fig. 6A, prior exposure to METH via self-administration attenuated the deficits in DAT uptake caused by the binge treatment (Fig. 7B; F2,16 = 11.15; p < 0.05), and attenuated DA depletions in the striatum (F2, 16 = 16.04; p < 0.05; saline/saline, 150.10 ± 9.28 pg/μg; METH/METH, 104.60 ± 17.97 pg/μg; saline/METH, 39.90 ± 14.36 pg/μg).
Fig. 7.
METH self-administration mitigates the effects of a subsequent binge METH treatment when rats are kept in a warm environment to promote hyperthermia. Rats self-administered METH (0.12 mg/infusion) or saline (10 μl /infusion) for 7 days (8 h/day). A, 24 h after the beginning of the final self-administration session, rats received METH (4 × 7.5 mg/kg s.c./injection; 2-h intervals) or saline (1 ml/kg s.c./injection; 2-h intervals), and core temperatures were assessed 30 and 90 min after each injection. B, animals were sacrificed 7 days later, and DAT function was assessed. Average of eight saline self-administering/saline-challenged, six METH self-administering/METH-challenged, and five saline self-administering/METH-challenged rats was used. *, p < 0.05 versus all other groups.
Brain concentrations of METH were assessed in saline and METH (0.12 mg/infusion) self-administering animals challenged with a binge of METH that were sacrificed 1 h after the last injection. Prior self-administration of METH led to no significant changes concentrations of METH compared with rats who self-administered saline before the binge (saline/METH, 7.77 ± 0.62 ng/mg wet weight; METH/METH, 9.09 ± 0.97 ng/mg wet weight; t18 = 1.08, ns). Furthermore, no significant differences were seen in brain amphetamine concentrations (saline/METH, 2.21 ± 0.18 ng/mg wet weight; METH/METH, 2.59 ± 0.15 ng/mg wet weight; t18 = 1.63, ns). METH self-administering animals pressed for a total of 21.78 ± 1.24 mg of METH during the 7 days of self-administration and had pressing behavior similar to that in Fig. 5A. Although METH self-administering rats were kept in a warm environment during the binge of METH, similar attenuation in hyperthermia was seen in the animals that self-administered METH compared with saline before the METH binge at the 30-, 150-, and 270-min time points (F14,168 = 11.79; p < 0.05).
Discussion
Previous studies indicated that METH self-administration in rats provides a clinically relevant exposure pattern of human METH abuse (Yahyavi-Firouz-Abadi and See, 2009). Results of the current study extend these findings by revealing that METH self-administration decreases striatal DAT immunoreactivity and/or function. Specifically, this study expands on the current preclinical METH self-administration literature by being the first to demonstrate that: 1) changes in DAT function persist for at least 30 days; 2) increasing the amount of METH per infusion from 0.06 to 0.24 mg per infusion did not lead to greater deficits in DAT immunoreactivity and/or function; 3) the deficits in dopaminergic parameters and the concentrations of METH within the brain 1 h after self-administration of 0.06 to 0.12 mg per infusion are comparable with those of human METH abusers; and 4) the self-administration of METH attenuates the dopaminergic deficits induced by a subsequent binge METH treatment. Attenuation of hyperthermia, but not alterations in pharmacokinetics, probably contributes to this latter phenomenon.
As reported by Brennan et al. (2010) and Frankel et al. (2011), the present study provides evidence that the effects of noncontingent METH treatment do not necessarily predict the effects of contingent exposure. For example, the magnitudes of the deficits in DAT induced by METH self-administration were less than that often reported after a binge METH treatment (Baucum et al., 2004; Eyerman and Yamamoto, 2007; Hadlock et al., 2010). Likewise, the decreases in DA content caused by self-administration were less than those caused by a binge treatment (Johnson-Davis et al., 2004; Cadet et al., 2009; Brennan et al., 2010) and were evident only after administration of the 0.24 mg/infusion dose. Furthermore, and in contrast to effects of binge METH treatment (Hadlock et al., 2010), no increases in GFAP immunoreactivity, a marker of astrocytic activation and neuronal damage, were found after METH self-administration. This is consistent with previous reports that indicate self-administration produces adaptations that differ from the effects of binge METH treatment (Schwendt et al., 2009; Brennan et al., 2010).
It is noteworthy that increasing the dose of METH per infusion did not linearly increase the magnitude of DAT-associated deficits. Specifically, all three doses tested (e.g., 0.06, 0.12, and 0.24 mg per infusion) similarly affected DAT function and/or immunoreactivity, as well as METH-induced hyperthermia. Because hyperthermia has been shown to contribute to the persistent effects of METH on the DAT (Bowyer, 1995) as discussed below, the similarity in core body temperature may contribute to the lack of dose dependence in DAT function across the dosing range tested. Alternatively and as discussed below, the self-administration regimen per se may have engendered a tolerance phenomenon (defined herein as the ability to protect against a subsequent METH challenge) such that the last treatment session could not effect larger alterations in the dopaminergic parameters under study.
The findings of the current study are generally consistent with the neuroadaptations reported in Schwendt et al. (2009) after METH self-administration. In particular, METH exposure persistently decreased DAT, without altering GFAP, TH, or VMAT-2 immunoreactivity in the striatum. Similar to the findings of Schwendt et al. (2009) and Brennan et al. (2010), no significant decreases in DA content were seen 8 days after self-administration in animals when 0.12 or 0.06 mg per infusion was administered. In contrast, decreases in DA content were observed after self-administration of the higher dose (0.24 mg/infusion) of METH. The present findings also stand in contrast to previous self-administration studies that did not demonstrate changes in DAT protein or mRNA (Stefanski et al., 2002; Shepard et al., 2006) or reported large dopaminergic deficits resembling those after binge METH treatment (Krasnova et al., 2010). Differences in the dosing and duration of the self-administration sessions may contribute to these differences [e.g., Shepard et al. (2006) and Stefanski et al. (2002) used lower doses, and Krasnova et al. (2010) used 15-h self-administration sessions].
Although the effects of METH self-administration demonstrated in this article differ from those described preclinically after a binge-like treatment, some consequences are very similar to those reported in METH abusers. As one example, METH concentrations within the brain of rats self-administering 0.12 mg/infusion were similar to 8 of 14 subjects reported by Kalasinsky et al. (2001), whereas METH concentrations after the binge of METH resulted in higher concentrations than 12 of 14 subjects. This result must be interpreted cautiously because metabolism differences exist between the species. Furthermore, there is a large amount of variability in total METH intake in both humans and rats and duration of METH use in human abusers that may lead to differences between the two species.
Other similarities between the present findings and reports involving imaging of abstinent METH abusers are the magnitude of changes in DAT within the striatum. In both self-administration and imaging studies of abstinent human METH abusers, decreases in DAT densities by approximately 15 to 28% occurred (Chang et al., 2007). Similar to human imaging studies of DAT densities, the magnitude of the changes DAT uptake decreased during protracted abstinence (Volkow et al., 2001). However, these changes were less than those reported in postmortem studies, perhaps because of the absence of METH in the body at the time of the assays. In agreement with human imaging studies or postmortem transporter binding, small, but nonsignificant, decreases in VMAT uptake were also observed (Wilson et al., 1996; Johanson et al., 2006; but see Boileau et al., 2008).
It is noteworthy that prior METH self-administration attenuates the persistent dopaminergic deficits caused by a subsequent binge METH exposure. This protection resembles effects of escalating-dose and other pretreatment exposures (Stephans and Yamamoto, 1996; Johnson-Davis et al., 2004; Thomas and Kuhn, 2005; Cadet et al., 2009). In particular, METH self-administration attenuated the decreases in DAT activity, DA content, and TH immunoreactivity caused by a subsequent binge METH regimen. METH self-administration also attenuated the increase in DAT complex formation, a phenomenon that our laboratory has demonstrated is an indicator of, and perhaps contributor to, the persistent DA deficits caused by the binge treatment (Hadlock et al., 2009, 2010). Furthermore, GFAP immunoreactivity caused by a binge METH treatment was attenuated by prior METH self-administration. These novel findings are of potential clinical relevance in that the resistance to binge-induced dopaminergic deficits caused by the repeated METH exposures may provide a model to explain why human METH abusers do not display greater dopaminergic deficits, even after using large quantities of METH.
Previous studies involving escalating-dose treatments have suggested the role of hyperthermia in attenuating the persistent monoaminergic deficits caused by a subsequent binge METH treatment (Johnson-Davis et al., 2004; O'Neil et al., 2006). This is likely because increases in core body temperatures such as those facilitated by METH can exacerbate the formation of reactive oxygen and nitrogen species that enhances toxicity (Bowyer, 1995; Krasnova and Cadet, 2009; Yamamoto et al., 2010). Likewise, the present study permits speculation that the attenuation of hyperthermia afforded by prior METH self-administration contributed to its ability to attenuate deficits caused by a subsequent binge treatment.
In contrast to previous studies involving escalating-dose pretreatment (Schmidt et al., 1985), alterations in pharmacokinetics did not seem to underlie the attenuated METH-induced dopaminergic deficits caused by the subsequent binge exposure. Future research will examine other possible mechanisms underlying this apparent neuroprotection; these mechanisms potentially include changes in glutamate or DA release and/or DA receptor activation because each has been implicated in the persistent neurotoxic effects of METH (O'Neil et al., 2006; for review, see Fleckenstein et al., 2007; Krasnova and Cadet, 2009; Yamamoto et al., 2010).
In summary, the present article demonstrates that METH self-administration (0.12 mg/infusion) led to an escalation of pressing and brain METH levels similar to human users (Cho, 1990; Kalasinsky et al., 2001). This resulted in persistent changes in DAT within the striatum that were comparable in magnitude to imaging studies of human METH abusers, but smaller than the changes after binge exposures to METH in rats. The findings of the first experiment suggest that these changes involved DAT, but neither VMAT-2 function nor GFAP and TH immunoreactivity, thus suggesting that changes may represent neuroadaptations involving dopaminergic neurons rather than a loss of dopaminergic nerve terminals. In addition, these changes in DAT were persistent and did not seem dose-dependent because increasing the amount of METH/infusion resulted in similar magnitude of DAT alterations. Finally, a novel finding of the present study is that the self-administration of METH attenuated many of the persistent dopaminergic deficits caused by subsequent investigator-administered high doses of METH. This protection was independent of alterations in METH pharmacokinetics, but probably attributable (at least in part) to an attenuation of METH-induced hyperthermia. These data permit speculation that the lack of dose-dependent response was caused by a “tolerance” phenomenon engendered by the self-administration paradigm. Overall, METH self-administration in rats may provide insight into the persistent dopaminergic alterations associated with METH abuse.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA09407, DA019447, DA013367, DA00869, DA11389, DA04222, DA00378].
This work was presented in part previously: McFadden LM, Vieira-Brock PL, Stout KA, Nielson SM, Wilkins DG, Hanson GR, and Fleckenstein AE (2010) Contingent methamphetamine administration decreases dopamine and vesicular monoamine-2 transporter function, at the Experimental Biology Annual Meeting; 2010 April 24–28; Anaheim, CA; American Society for Investigative Pathology, Bethesda, MD. McFadden LM, Hanson GR, and Fleckenstein AE (2010) Methamphetamine self-administration leads to acute changes to dopaminergic markers, at the Society for Neuroscience Conference; 2010 Nov 13–17; San Diego, CA; Society for Neuroscience, Washington, DC. McFadden LM, Hanson GR, and Fleckenstein AE (2010) Methamphetamine self-administration leads to persistent dopaminergic deficits, at the Society for Neuroscience Conference; 2010 Nov 13–17; San Diego, CA; Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- METH
- methamphetamine
- DA
- dopamine
- DAT
- DA transporter
- VMAT-2
- vesicular monoamine-2 transporter
- LP
- low presser
- HP
- high presser
- GFAP
- glial fibrillary acidic protein
- TH
- tyrosine hydroxylase
- ns
- not significant.
Authorship Contributions
Participated in research design: McFadden, Hanson, and Fleckenstein.
Conducted experiments: McFadden, Hadlock, Allen, Vieira-Brock, Stout, Ellis, Hoonakker, Andrenyak, and Nielsen.
Performed data analysis: McFadden and Wilkins.
Wrote or contributed to the writing of the manuscript: McFadden, Hanson, and Fleckenstein.
References
- Baucum AJ, 2nd, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. (2004) Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J Neurosci 24:3436–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P, Guttman M, Saint-Cyr JA, Wilson AA, Kish SJ. (2008) Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT-2 a stable dopamine neuron biomarker? J Neurosci 28:9850–9856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF. (1995) The role of hyperthermia in amphetamine's interactions with NMDA receptors, nitric oxide, and age to produce neurotoxicity. Ann NY Acad Sci 765:309–310 [DOI] [PubMed] [Google Scholar]
- Brennan KA, Colussi-Mas J, Carati C, Lea RA, Fitzmaurice PS, Schenk S. (2010) Methamphetamine self-administration and the effect of contingency on monoamine and metabolite tissue levels in the rat. Brain Res 1317:137–146 [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. (2000) Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem 74:2221–2223 [DOI] [PubMed] [Google Scholar]
- Cadet JL, McCoy MT, Cai NS, Krasnova IN, Ladenheim B, Beauvais G, Wilson N, Wood W, Becker KG, Hodges AB. (2009) Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PLoS One 4:e7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. (2000) Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res 863:106–111 [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. (2007) Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102 (Suppl 1):16–32 [DOI] [PubMed] [Google Scholar]
- Cho AK. (1990) Ice: a new dosage form of an old drug. Science 249:631–634 [DOI] [PubMed] [Google Scholar]
- Cornish JL, Clemens KJ, Thompson MR, Callaghan PD, Dawson B, McGregor IS. (2008) High ambient temperature increases intravenous methamphetamine self-administration on fixed and progressive ratio schedules in rats. J Psychopharmacol 22:100–110 [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. (2007) A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem 103:1219–1227 [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. (2007) New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol 47:681–698 [DOI] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. (2011) Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol Exp Ther 336:809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Baucum AJ, 2nd, King JL, Horner KA, Cook GA, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. (2009) Mechanisms underlying methamphetamine-induced dopamine transporter complex formation. J Pharmacol Exp Ther 329:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Chu PW, Walters ET, Hanson GR, Fleckenstein AE. (2010) Methamphetamine-induced dopamine transporter complex formation and dopaminergic deficits: the role of D2 receptor activation. J Pharmacol Exp Ther 335:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Birdsall E, Seferian KS, Crosby MA, Keefe KA, Gibb JW, Hanson GR, Fleckenstein AE. (2009) Methamphetamine-induced dopaminergic deficits and refractoriness to subsequent treatment. Eur J Pharmacol 607:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Metzger RR, Hanson GR. (2000) The effects of methamphetamine on serotonin transporter activity: role of dopamine and hyperthermia. J Neurochem 75:1608–1617 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, et al. (2006) Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 185:327–338 [DOI] [PubMed] [Google Scholar]
- Johnson-Davis KL, Truong JG, Fleckenstein AE, Wilkins DG. (2004) Alterations in vesicular dopamine uptake contribute to tolerance to the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther 309:578–586 [DOI] [PubMed] [Google Scholar]
- Kalasinsky KS, Bosy TZ, Schmunk GA, Reiber G, Anthony RM, Furukawa Y, Guttman M, Kish SJ. (2001) Regional distribution of methamphetamine in autopsied brain of chronic human methamphetamine users. Forensic Sci Int 116:163–169 [DOI] [PubMed] [Google Scholar]
- Kitamura O, Tokunaga I, Gotohda T, Kubo S. (2007) Immunohistochemical investigation of dopaminergic terminal markers and caspase-3 activation in the striatum of human methamphetamine users. Int J Legal Med 121:163–168 [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. (2006) Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 186:48–53 [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. (2009) Methamphetamine toxicity and messengers of death. Brain Res Rev 60:379–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. (2010) Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One 5:e8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. (1998) Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 18:8417–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hoonakker AJ, Vieira-Brock PL, Stout KA, Sawada NM, Ellis JD, Allen SC, Walters ET, Nielsen SM, Gibb JW, et al. (2011) Methamphetamine treatment during development attenuates the dopaminergic deficits caused by subsequent high-dose methamphetamine administration. Synapse 65:771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Laćan G. (2007) Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse 61:216–220 [DOI] [PubMed] [Google Scholar]
- O'Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP. (2006) Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse 60:465–473 [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Gehlert DR, Peat MA, Sonsalla PK, Hanson GR, Wamsley JK, Gibb JW. (1985) Studies on the mechanism of tolerance to methamphetamine. Brain Res 343:305–313 [DOI] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. (2009) Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther 331:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. (2006) Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology (Berl) 185:505–513 [DOI] [PubMed] [Google Scholar]
- Stefanski R, Lee SH, Yasar S, Cadet JL, Goldberg SR. (2002) Lack of persistent changes in the dopaminergic system of rats withdrawn from methamphetamine self-administration. Eur J Pharmacol 439:59–68 [DOI] [PubMed] [Google Scholar]
- Stephans S, Yamamoto B. (1996) Methamphetamines pretreatment and the vulnerability of the striatum to methamphetamine neurotoxicity. Neuroscience 72:593–600 [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. (2005) Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem 92:790–797 [DOI] [PubMed] [Google Scholar]
- Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, Hanson GR, Fleckenstein AE. (2005) Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: implications for neurotoxicity. J Pharmacol Exp Ther 314:1087–1092 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, et al. (2001) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703 [DOI] [PubMed] [Google Scholar]
- Yahyavi-Firouz-Abadi N, See RE. (2009) Anti-relapse medications: preclinical models for drug addiction treatment. Pharmacol Ther 124:235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. (2010) Amphetamine toxicities: classical and emerging mechanisms. Ann NY Acad Sci 1187:101–121 [DOI] [PMC free article] [PubMed] [Google Scholar]