Abstract
Phospho-nonsteroidal anti-inflammatory drugs (phospho-NSAIDs) are novel NSAID derivatives with improved anticancer activity and reduced side effects in preclinical models. Here, we studied the metabolism of phospho-NSAIDs by carboxylesterases and assessed the impact of carboxylesterases on the anticancer activity of phospho-NSAIDs in vitro and in vivo. The expression of human liver carboxylesterase (CES1) and intestinal carboxylesterase (CES2) in human embryonic kidney 293 cells resulted in the rapid intracellular hydrolysis of phospho-NSAIDs. Kinetic analysis revealed that CES1 is more active in the hydrolysis of phospho-sulindac, phospho-ibuprofen, phospho-naproxen, phospho-indomethacin, and phospho-tyrosol-indomethacin that possessed a bulky acyl moiety, whereas the phospho-aspirins are preferentially hydrolyzed by CES2. Carboxylesterase expression leads to a significant attenuation of the in vitro cytotoxicity of phospho-NSAIDs, suggesting that the integrity of the drug is critical for anticancer activity. Benzil and bis-p-nitrophenyl phosphate (BNPP), two carboxylesterase inhibitors, abrogated the effect of carboxylesterases and resensitized carboxylesterase-expressing cells to the potent cytotoxic effects of phospho-NSAIDs. In mice, coadministration of phospho-sulindac and BNPP partially protected the former from esterase-mediated hydrolysis, and this combination more effectively inhibited the growth of AGS human gastric xenografts in nude mice (57%) compared with phospho-sulindac alone (28%) (p = 0.037). Our results show that carboxylesterase mediates that metabolic inactivation of phospho-NSAIDs, and the inhibition of carboxylesterases improves the efficacy of phospho-NSAIDs in vitro and in vivo.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are promising agents for the prevention of several types of cancer (Flossmann et al., 2007; Cuzick et al., 2009). However, long-term use of NSAIDs is associated with gastrointestinal and renal toxicities (Singh and Triadafilopoulos, 1999). Considering the limited efficacy of NSAIDs and the prevalence of their side effects, it is questionable whether their clinical benefits outweigh their toxic effects (Cuzick et al., 2009). This prompted us to synthesize novel phospho-derivatives of NSAIDs (Sun and Rigas, 2008; Hua et al., 2009; Zhao et al., 2009; Mackenzie et al., 2010; Huang et al., 2010, 2011; Xie et al., 2011b). Traditionally, modified NSAIDs are considered pharmacologically inactive prodrugs that temporarily mask the acidic moiety as a means to reduce gastrointestinal toxicity (Halen et al., 2009). In the case of phospho-NSAIDs, however, the structural modification leads to both enhanced chempreventive efficacy and reduced gastrointestinal toxicity in preclinical models (Mackenzie et al., 2010; Huang et al., 2011). As an example, phospho-ibuprofen is 16- to 23-fold more potent in inhibiting colon cancer cell growth than ibuprofen (Xie et al., 2011b). Hence, it is intact phospho-NSAIDs, but not the corresponding NSAIDs, that are the pharmacologically potent molecules.
Pharmacokinetic studies in mouse models showed that phospho-NSAIDs given orally are rapidly hydrolyzed to give the parent NSAIDs as the major metabolites in the plasma (Xie et al., 2011a). Phospho-NSAIDs were also shown to be hydrolyzed by esterases in rat and human liver extracts, but the specific enzymes responsible have not been defined. Carboxylesterases are broad-specificity hydrolyases that cleave carboxylic esters or amides into the corresponding carboxylic acid and alcohol or amine, respectively (Redinbo and Potter, 2005). In humans, there are two major carboxylesterases: human liver (CES1) and human intestinal (CES2) isoforms. CES1 and CES2 are important in the detoxification of diverse ester drugs and xenobiotics (Satoh and Hosokawa, 1998; Redinbo and Potter, 2005). CES1 is expressed predominantly in the liver, and it is also detected in monocytes (Markey, 2011) and the lung (Hosokawa, 2008). Expression of CES2 is more widely distributed, with high expression in the small intestine, liver, and kidneys (Satoh and Hosokawa, 1998). It is noteworthy that CES1 and CES2 expression levels are often suppressed in liver and colon tumors compared with the corresponding normal tissues (Guichard et al., 1999; Xie et al., 2002; Tang et al., 2008; Na et al., 2009). Although carboxylesterases generally serve a protective function, they are also responsible for the inactivation of therapeutic drugs (Redinbo and Potter, 2005). Because phospho-NSAIDs consist of an NSAID linked to a spacer and the diethyl phosphate moiety via a carboxylic ester bond, we hypothesized that phospho-NSAIDs could be a target for inactivation in vivo by human carboxylesterases.
Here, we establish that phospho-NSAIDs undergo rapid hydrolysis in cells overexpressing CES1 and CES2, which in turn resulted in a significant reduction in their growth inhibitory effects. Given the impact of carboxylesterases on phospho-NSAID inactivation, we tested the ability of carboxylesterase inhibitors to protect phospho-NSAIDs against carboxylesterase-mediated hydrolysis in vitro and in vivo and evaluated the impact of carboxylesterase inhibition on their anticancer activity.
Materials and Methods
Chemicals.
Phospho-sulindac (OXT-328), phospho-ibuprofen (MDC-917), phospho-aspirin (MDC-46 and MDC-22), phospho-naproxen, phospho-valproic acid, phospho-indomethacin, and phospho-tyrosol-indomethacin were gifts from Medicon Pharmaceuticals, Inc. (Stony Brook, NY) (Supplemental Fig. 1). A549, AGS, HepG2, HEK293, MCF-7, MIA-PaCa-2, Panc-1, and SW480 cells were purchased from the American Type Culture Collection (Manassas, VA). Human CES1 (transcript variant 3; Genbank accession no. NM_001266) and human CES2 (transcript variant 1; Genbank accession no. NM_003869.4) expression plasmids were obtained from Origene (Rockville, MD). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). All other chemicals, unless otherwise stated, were purchased from Sigma-Aldrich (St Louis, MO).
Cell Culture.
HEK293 cells were cultured in RPMI media supplemented with 10% fetal bovine serum and 50 U/ml penicillin-streptomycin (Cellgro, Manassas, VA). Cells were split in a 1:4 ratio every 48 h. Antibiotics were not added during transient transfection. A549, AGS, HepG2, MCF-7, MIA-PaCa-2, Panc-1, and SW480 cells were cultured as recommended by the American Type Culture Collection. All experiments were performed with cells between passages 1 and 10.
HPLC Analysis.
The HPLC system consisted of a Waters Alliance 2695 Separations Module equipped with a Waters 2998 photo-diode array detector (220 nm) (Waters, Milford, MA) and a Thermo BDS Hypersil C18 column (150 × 4.6 mm; particle size 3 μm) (Thermo Fisher Scientific, Waltham, MA). The mobile phase consisted of a gradient between buffer A [formic acid, acetonitrile, and H2O (95:4.9:0.1 v/v/v)] and 100% acetonitrile.
In Situ Hydrolysis of Phospho-NASIDs by CES1 and CES2.
HEK293 cells were seeded into poly-l-lysine-coated 24-well plates at a density of 2.0 × 105 cells/per well the day before transfection. HEK293 cells were transfected with the CES1 or CES2 plasmids or the empty pCMV-XL6 vector with Lipofectamine 2000 according to the manufacturer's instructions. In brief, the transfection complexes were formed in Opti-MEM (Invitrogen) and then added to cells after incubation for 20 min. Overexpression of CES1 and CES2 was confirmed by quantitative reverse transcription-polymerase chain reaction and the hydrolysis of the model substrate p-nitrophenyl acetate. Hydrolysis assays were performed 22 to 24 h after transfection. The media were aspirated and replaced with complete RPMI media containing 100 μM phospho-NSAIDs. In inhibition studies, cells were preincubated with benzil or BNPP for 30 min before the addition of phospho-NSAIDs. The cells were washed once with complete media and collected in 200 μl of lysis solution (50% ethanol). Extraction was performed by sonication for 5 min followed by the addition of 400 μl of ethanol. The samples were centrifuged at 17,000g for 10 min and analyzed by HPLC. The protein pellet was redissolved in 0.1 N NaOH, and the protein content was determined by the Bradford assay (Bradford, 1976).
Enzyme Preparations and In Vitro Carboxylesterase Activity Assay.
HEK293 cells in 100-mm plates were transfected with the CES1 or CES2 plasmids or the empty pCMV-XL6 vector with Lipofectamine 2000. After 48 h, cells were harvested in 2 ml of phosphate-buffered saline and homogenized by sonication. Cellular extracts were stored in −80°C without the addition of protease inhibitors. For in vitro assay, cell extracts (2–10 μl) from the control and CES1- and CES2-expressing cells were diluted with prewarmed 100 mM phosphate buffer, pH 7.4, at 37°C in a total volume of 100 μl. The reaction was then initiated by the addition of phospho-NSAIDs. Apparent enzyme kinetic parameters were estimated in terms of product formation, using various (5–8) concentrations of the substrate. CES1- and CES2-expressing cell lysates demonstrated significant hydrolytic activity toward 4-nitrophenyl acetate with specific activity of 5.2 and 1.2 μmol/min/mg, respectively. Reaction velocity and Vmax values were normalized to per mg CES based on the rates of 4-nitrophenyl acetate hydrolysis by purified CES1 and CES2 (40.7 and 44.8 μmol/min/mg, respectively) (Williams et al., 2011).
Growth Inhibition Assays.
HEK293 cells in 100-mm plates were transfected with the CES1 or CES2 plasmids or the empty pCMV-XL6 vector with Lipofectamine 2000. After 24 h, the transfected cells were subcultivated into 96-well plates at 35,000 cells per well. After overnight incubation, various concentrations of phospho-NSAIDs were added, after which the cells were further incubated for another 24 h. At the end of the incubation, cell viability was determined by a modified colorimetric assay using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT). In brief, the culture medium was removed and replaced with 100 μl of complete medium containing 0.5 mg/ml MTT. After 4-h incubation at 37°C, 100 μl of a solution containing 10% SDS and 0.01 N HCl was added. The plate was incubated and gently mixed until MTT formazan crystals were dissolved. Absorbance at 570 nm was measured on a microplate reader and IC50 was calculated after the subtraction of blank values.
In Situ Hydrolysis of Phospho-NASIDs by Human Cancer Cells.
A549, AGS, HepG2, MCF-7, MIA-PaCa-2, Panc-1, or SW480 cells were seeded into 24-well plates at a density of 2.0 × 105 cells/per well and cultured for 72 h. The media were then aspirated and replaced with complete RPMI media containing 100 μM phospho-NSAIDs. The cells were washed with complete media and collected in 200 μl of lysis solution (50% ethanol). Extraction was performed by sonication for 5 min followed by addition of 400 μl of ethanol. The samples were centrifuged at 17,000g for 10 min and analyzed by HPLC.
Immunoblotting.
Cell lysates were resolved in SDS/electrophoresis gel and transferred to nitrocellulose membrane. Carboxylesterase 1 and carboxylesterase 2 antibodies were purchased from Abcam Inc. (Cambridge, MA). β-Actin antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
In Vivo Studies.
This animal study was approved by the Institutional Animal Care and Use Committee at Stony Brook University. Female nude mice (7–8 weeks old) were purchased from Harlan (Indianapolis, IN). At 9 to 10 weeks, the animals were inoculated subcutaneously in their right flanks, each with 5 × 106 AGS gastric cancer cells suspended in 100 μl of phosphate-buffered saline/BD Matrigel matrix (50:50, v/v). When the average tumor size reached 125 ± 26 mm3, the animals were divided into four groups (n = 6), which were given the following treatments: 1) vehicle (corn oil, orally; 10% Tween, intraperitoneally), 2) BNPP (100 mg/kg/day i.p.), 3) phospho-sulindac (200 mg/kg/day p.o.), and 4) phospho-sulindac (200 mg/kg/day p.o.) and BNPP (100 mg/kg/day i.p.). Tumors were measured twice a week with a digital microcaliper, and tumor volumes were calculated by using the following formula: tumor volume = [length × width × (length + width/2) × 0.56]. After treatment for 3 weeks, the animals were sacrificed, and their tumors were removed. Levels of phospho-sulindac and its metabolites in the tumors were determined by HPLC. Pharmacokinetic studies were performed with BALB/c wild-type mice. Mice were sacrificed at 1 h after drug administration, and the blood was collected and precipitated with acetonitrile. The liver was also collected to analyze drug levels.
Data Analysis.
Data are shown as mean ± S.E.M. Raw data from the kinetics studies and cell growth assays were analyzed by using Prism 5 (GraphPad Software Inc., San Diego, CA). Km and Vmax were derived from a nonlinear regression fit of the Michaelis-Menten model. Statistical differences were determined by using analysis of variance and Student's t test. Differences were considered significant when p ≤ 0.05.
Results
Phospho-NSAIDs Are Hydrolyzed In Situ by CES1- and CES2-Expressing Cells.
To evaluate the effect of human carboxylesterase expression on the metabolism of phospho-NSAIDs, we incubated phospho-NSAIDs (100 μM) with the control and CES1- and CES2-expressing cells and identified the resulting intracellular metabolites by HPLC. In control cells, intracellular phospho-NSAIDs remained primarily intact (>95%) after treatment for 1 h (Table 1). CES1-expressing cells, in contrast, hydrolyzed all phospho-NSAIDs to their corresponding parent NSAIDs to a greater extent compared with the control cells. Phospho-ibuprofen and phospho-naproxen were almost completely hydrolyzed into ibuprofen and naproxen, respectively, in CES1-expressing cells. CES1 overexpression also resulted in significant hydrolysis of other phopho-NSAIDs in the following order: phospho-indomethacin > phospho-aspirin (MDC-46) > phospho-tyrosol-indomethacin > phospho-sulindac > phopho-aspirin (MDC-22). In our analyses, valproic acid could not be detected because of its low extinction coefficient, even in the far UV range. However, the level of intact phospho-valproic acid was more than 100-fold lower in CES1-expressing cells compared with the control cells, suggesting that phospho-valproic acid was significantly hydrolyzed. In addition, we observed a significant decreased in total drug levels (p < 0.01) in CES1-expressing cells (except MDC-46 and MDC-22). This probably reflected the rapid efflux of parent NSAIDs after their liberation from phospho-NSAIDs.
TABLE 1.
In situ hydrolysis of NSAIDs in control and CES1- and CES2-expressing cells
The control and CES1- and CES2-expressing HEK293 cells were incubated with 100 μM phospho-NSAIDs for 1 h, and the intracellular levels of intact drug and metabolites were measured by HPLC. Data are shown as mean ± S.E.M.
| Intracellular Level |
|||
|---|---|---|---|
| HEK293-Control | HEK293-CES1 | HEK293-CES2 | |
| nmol/mg protein | |||
| Phospho-sulindac | |||
| Phospho-sulindac | 25.5 ± 0.2 | 14.6 ± 1.2 | 23.6 ± 4.0 |
| Sulindac | 0.04 ± 0.02 | 4.21 ± 0.82 | 0.30 ± 0.05 |
| Phospho-ibuprofen | |||
| Phospho-ibuprofen | 95.7 ± 12.9 | 1.54 ± 0.87 | 92.6 ± 26.2 |
| Ibuprofen | N.D. | 11.7 ± 5.0 | 1.37 ± 0.94 |
| Phospho-aspirin (MDC-46) | |||
| Intact forms | 20.9 ± 3.9 | 7.02 ± 4.07 | 0.43 ± 0.09 |
| Salicylic acid | N.D. | 12.1 ± 1.9 | 17.9 ± 2.5 |
| Phospho-aspirin (MDC-22) | |||
| Intact forms | 15.2 ± 1.2 | 4.65 ± 0.60 | 0.51 ± 0.01 |
| Salicylic acid | N.D. | 1.20 ± 0.43 | 16.4 ± 2.3 |
| Phospho-naproxen | |||
| Phospho-naproxen | 49.1 ± 12.2 | 0.63 ± 0.62 | 30.8 ± 5.9 |
| Naproxen | 1.27 ± 0.24 | 6.44 ± 3.54 | 6.98 ± 1.80 |
| Phospho-indomethacin | |||
| Phospho-indomethacin | 51.1 ± 13.6 | 2.18 ± 0.92 | 42.1 ± 5.9 |
| Indomethacin | 0.16 ± 0.04 | 5.18 ± 1.47 | 0.39 ± 0.01 |
| Phospho-tyrosol-indomethacin | |||
| Phospho-tyrosol-indomethacin | 67.9 ± 4.0 | 13.0 ± 3.5 | 33.2 ± 4.1 |
| Indomethacin | 1.33 ± 0.13 | 6.55 ± 1.57 | 12.1 ± 1.3 |
| Phospho-valproic acid | |||
| Phospho-valproic acid | 55.6 ± 7.3 | 0.31 ± 0.3 | 5.95 ± 0.59 |
| Valproic acid | N.D. | N.D. | N.D. |
N.D., not detected.
Like CES1, CES2-expressing cells were also significantly more active in the hydrolysis of phospho-NSAIDs compared with the control cells. CES2 almost completely hydrolyzed the phospho-aspirins (MDC-46 and MDC-22) at the end of the incubation period. In addition, phospho-tyrosol-indomethacin and phospho-naproxen were effectively hydrolyzed by CES2. On the other hand, the hydrolysis of phospho-sulindac, phospho-ibuprofen, and phospho-indomethacin by CES2 was much reduced compared with CES1. CES2 probably hydrolyzed phospho-valproic acid, given the lower levels of the intact drug in CES2-expressing cells. These results indicated that the human carboxylesterases CES1 and CES2 were capable of rapid hydrolysis of the evaluated phospho-NSAIDs.
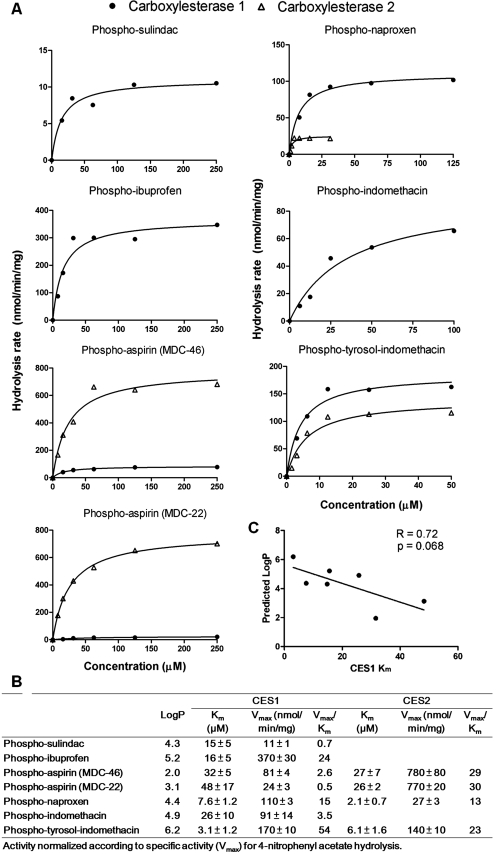
Kinetic Analyses of Phospho-NSAIDs Hydrolysis by CES1 and CES2.
Kinetic studies were then performed with cell lysates from CES1- and CES2-expressing HEK293 cells to determine the parameters Km and Vmax. The kinetic parameters for phospho-NSAIDs hydrolysis by CES1 and CES2 are shown in Fig. 1. Phospho-tyrosol-indomethacin and phospho-naproxen exhibited the lowest Km values for CES1, whereas the highest Km values were observed with the phospho-aspirins (MDC-46 and MDC-22). We observed that the Km values of phospho-NSAIDs toward CES1 decreased with increased hydrophobicity, although this association did not reach statistical significance (p = 0.068). The highest Vmax values were observed with phospho-ibuprofen, followed by phospho-tyrosol-indomethacin, phospho-naproxen, phospho-indomethacin, and phospho-aspirin (MDC-46). Catalytic efficiency (Vmax/Km) of CES1 was the highest for phospho-tyrosol-indomethacin, phospho-ibuprofen, and phospho-naproxen, whereas the hydrolysis of phospho-sulindac and phospho-aspirin (MDC-22) was the least efficient.
Fig. 1.
The kinetics of hydrolysis of phospho-NASIDs by human CES1 and CES2. A, phospho-NSAIDs were incubated with the lysates of CES1-or CES2-expressing cells and the amount of hydrolyzed drug was determined as described under Materials and Methods. The Michaelis-Menten plots with phospho-NSAIDs showed simple hyperbolic profiles. B, a summary of the derived Michaelis-Menten kinetic parameters. C, correlation of CES1 Km values with predicted logP values of phospho-NSAIDs. Predicted logP values were derived by using the MarvinSketch Java applet. An inverse trend was observed between Km and the predicted logP.
We also performed kinetic studies with CES2 lysates on selected substrates, because CES2 showed very poor hydrolytic activity toward some of the phospho-NSAIDs tested. Phospho-naproxen and phospho-tyrosol-indomethacin demonstrated higher affinity (Km) but lower Vmax compared with phospho-aspirins (MDC-46 and MDC-22). Compared with CES1, CES2 showed higher catalytic efficiency (Vmax/Km) toward phospho-aspirin (MDC-46 and MDC-22) hydrolysis. On the other hand, hydrolysis of phospho-naproxen and phospho-tyrosol-indomethacin by CES2 was less efficient compared with CES1. Therefore, with the exception of phospho-aspirin (MDC-46 and MDC-22), CES1 may be the predominant isozyme responsible for the hydrolysis of phospho-NSAIDs in humans.
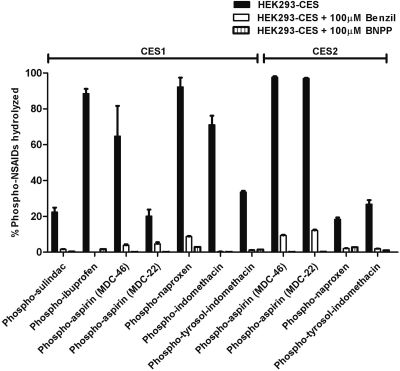
Benzil and BNPP Block the In Situ Hydrolysis of Phospho-NSAIDs by CES1 and CES2.
Benzil was reported to be a potent inhibitor of human carboxylesterases 1 and 2 (Hyatt et al., 2006); BNPP is considered to be a nonspecific esterase inhibitor (Redinbo and Potter, 2005). We, therefore, investigated the potential of benzil and BNPP to inhibit phospho-NSAIDs hydrolysis. Because carboxylesterase inhibition in vivo depends on penetration of the inhibitors into cells, we examined the effect of benzil and BNPP on the hydrolysis of phospho-NSAIDs intracellularly. CES1- and CES2-expressing cells were preincubated with 100 μM benzil or BNPP for 30 min before the addition of phospho-NSAIDs. As shown in Fig. 2, in untreated cells 20 to 90% of the phospho-NSAIDs were hydrolyzed in CES1-expressing cells. However, in the presence of benzil or BNPP, the rate of hydrolysis was significantly diminished. Analysis of the intracellular levels of hydrolyzed phospho-NSAIDs revealed that benzil inhibited CES1-mediated hydrolysis by >90% for all phospho-NSAIDs except phospho-aspirin (MDC-22; 78% inhibition). Benzil also inhibited the CES2-mediated hydrolysis of phospho-aspirin (MDC-46 and MDC-22), phospho-tyrosol-indomethacin, and phospho-naproxen by at least 87%. Likewise, BNPP potently inhibited CES1- and CES2-mediated phospho-NSAIDs hydrolysis. In all cases, preincubation with 100 μM BNPP resulted in potent inhibition of phospho-NSAID hydrolysis, exceeding 90% over the 1-h incubation period. These results suggest that both inhibitors are effective in inhibiting the conversion of phospho-NSAIDs to conventional NSAIDs intracellularly, which is critical for in vivo applications.
Fig. 2.
Carboxylesterase inhibitors, benzil and BNPP, inhibited the intracellular hydrolysis of phospho-NSAIDs. CES1- or CES2-expressing cells were pretreated with 100 μM benzil or BNPP for 30 min, followed by incubation with phospho-NSAIDs for 1 h. The intracellular metabolites were measured by HPLC and expressed as the percentage of phospho-NSAIDs hydrolyzed during the 1-h period.
CES1 and CES2 Overexpression Attenuates the Cytotoxicity of Phospho-NSAIDs.
We have reported previously that phospho-NSAIDs strongly inhibited the growth of cancer cells compared with their parent NSAIDs. It is thus proposed that CES1 and CES2 may be involved in the inactivation of phospho-NSAIDs, leading to reduced bioefficacy. To test this hypothesis, we determined the 24-h IC50 values of phospho-NSAIDs in the control vector-, CES1-, and CES2-expressing cells. Phospho-NSAIDs were highly cytotoxic in the control cells with IC50 values less than 70 μM. Phospho-aspirins (MDC-46 and MDC-22) were the exceptions, with IC50 values of 953 and 507 μM, respectively (Table 2; Fig. 3). Expression of CES1 resulted in higher IC50 values for most phospho-NSAIDs. In particular, the IC50 values for phospho-ibuprofen and phospho-naproxen were dramatically increased by more than 30-fold compared with the control cells, and these two drugs became essentially noncytotoxic below 1 mM in CES1-expressing cells. CES1 expression also resulted in considerably reduced cytotoxicity of phospho-tyrosol-indomethacin (7.8-fold), phospho-indomethacin (3.8-fold), phospho-sulindac (3.3-fold), and phospho-valproic acid (2.9-fold). The rate of hydrolysis may be a key factor in determining the relative IC50 values in control and CES1-expressing cells, because phospho-NSAIDs with the highest efficiency of hydrolysis, including phospho-ibuprofen, phospho-naproxen, and phospho-tyrosol-indomethacin (Fig. 1) also showed the greatest fold-reduction of cytotoxicity in CES1-expressing cells (Table 2). However, CES1 had little impact on the cytotoxicity of phospho-aspirins (MDC-46 and MDC-22), despite significant intracellular hydrolysis (Table 1). It is possible that, for phospho-aspirins, hydrolyzed components, such as aspirin or the linker, could also contribute to their cytotoxicity.
TABLE 2.
IC50 ratios between control and CES1- and CES2-expressing cells
The cells were incubated with phospho-NSAIDs for 24 h, and the IC50 values were determined by the MTT assay.
| Phospho-NASID | IC50 |
CES1/Control | CES2/Control | ||
|---|---|---|---|---|---|
| HEK293-Control | HEK293-CES1 | HEK293-CES2 | |||
| μM | |||||
| Phospho-sulindac | 28 ± 2 | 98 ± 3 | 59 ± 3 | 3.3 | 2.0 |
| Phospho-ibuprofen | 62 ± 6 | 1990 ± 300 | 158 ± 7 | 32 | 2.5 |
| Phospho-aspirin (MDC-46) | 953 ± 34 | 870 ± 27 | 1180 ± 70 | 1.3 | 1.2 |
| Phospho-aspirin (MDC-22) | 507 ± 14 | 464 ± 15 | 896 ± 33 | 0.9 | 1.8 |
| Phospho-naproxen | 69 ± 5 | 2600 ± 370 | 258 ± 15 | 38 | 3.8 |
| Phospho-indomethacin | 45 ± 1 | 170 ± 8 | 41 ± 1 | 3.8 | 0.9 |
| Phospho-tyrosol-indomethacin | 59 ± 3 | 458 ± 96 | 497 ± 101 | 7.8 | 8.5 |
| Phospho-valproic acid | 41 ± 2 | 118 ± 5 | 45 ± 2 | 2.9 | 1.1 |
Fig. 3.
Overexpression of human carboxylesterases attenuated cytotoxicity of phospho-NSAIDs in vitro. Control and CES1- and CES2-expressing HEK293 cells were treated with various concentrations of phospho-NSAIDs for 24 h, and the cell viability was determined by the MTT assay. The corresponding IC50 values are presented in Table 2.
CES2 overexpression, on the other hand, effectively reduced the cytotoxicity of phospho-tyrosol-indomethacin (8.5-fold increase in IC50) and phospho-naproxen (3.8-fold increase in IC50), the two of the phospho-NSAIDs that are susceptible to hydrolysis by CES2. In contrast, CES2 only slightly increased IC50 values for other phospho-NSAIDs (< 2.5-fold), which correlated with lower hydrolytic activity toward phospho-NSAIDs compared with CES1 (Fig. 1; Table 1). Despite being highly effective in the hydrolysis of phospho-aspirin (>90% in 1 h), IC50 values for MDC-46 and MDC-22 were only 1.2- and 1.8-fold higher, respectively, in CES2-expressing cells compared with control cells.
Our results affirmed our proposition that it is the intact phospho-NSAIDs, but not the parent NSAIDs or the phospho-linker, that were responsible for the cytotoxic activities. Moreover, human carboxylesterases are capable of rapid inactivation of phospho-NSAIDs and therefore provide the rationale for devising strategies for the protection of intact phospho-NSAIDs in vivo to enhance bioactivity.
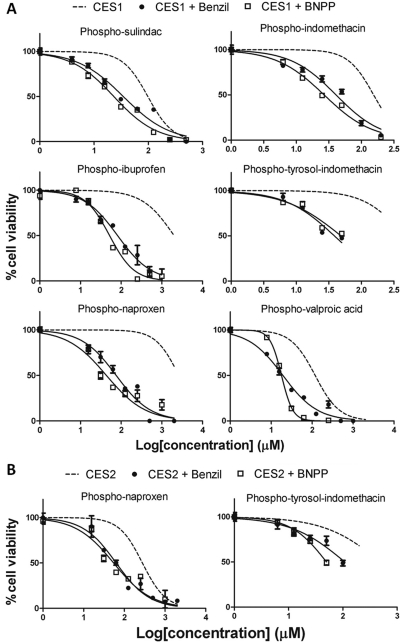
Benzil and BNPP Sensitize CES1- and CES2-Overexpressing Cells to Phospho-NSAID-Induced Cytotoxicity.
Having determined that benzil and BNPP blocked the hydrolysis of phospho-NSAIDs, we next questioned whether these carboxylesterase inhibitors could modulate the sensitivity of CES1 and CES2 expression toward phospho-NSAIDs. Therefore, we determined the 24-h IC50 values by using combinations of selected phospho-NSAIDs with benzil or BNPP (100 μM).
In agreement with Hyatt et al. (2006), treatment with 100 μM benzil alone did not result in any cytotoxicity in the control and CES1- or CES2-expressing cells. Cells were first pretreated with benzil or BNPP for 30 min before the addition of phospho-NSAIDs. As shown in Table 3, cotreatment of benzil with phospho-NSAIDs did not significantly impact the 24-h IC50 values of phospho-NSAIDs in control cells. On the other hand, pretreatment of CES1-expressing cells with benzil resulted in dramatic sensitization of these cells toward phospho-NSAIDs (Fig. 4; Table 3). In the presence of benzil, the 24-h IC50 values of phospho-NSAIDs in the CES1-expressing cells were similar to the control cells. For example, in CES1-expressing cells the IC50 values for phospho-ibuprofen (81.6 μM) and phospho-naproxen (75.4 μM) were decreased more than 20-fold when cotreated with benzil, becoming similar to those of control cells (85.8 and 69.1 μM, respectively) that lack carboxylesterase expression. In all cases, the ratio of IC50 values between control and CES1-expressing cells for all phospho-NSAIDs was between 0.8 and 1.2, suggesting that benzil was able to inhibit intracellular CES1, which in turn led to reduced inactivation of these drugs. Likewise, benzil increased the cytotoxicity of phospho-naproxen and phospho-tyrosol-indomethacin in CES2-expressing cells.
TABLE 3.
Effect of benzil (100 μM) on IC50 values of phospho-NSAIDs
The cells were preincubated with benzil for 30 min, followed by incubation with phospho-NSAIDs for 24 h. The IC50 values were determined by the MTT assay.
| Phospho-NASID | IC50 |
CES1/Control | CES2/Control | ||
|---|---|---|---|---|---|
| HEK293-Control + benzil | HEK293-CES1 + benzil | HEK293-CES2 + benzil | |||
| μM | |||||
| Phospho-sulindac | 35 ± 2 | 29 ± 1 | 0.8 | ||
| Phospho-ibuprofen | 86 ± 6 | 82 ± 5 | 0.9 | ||
| Phospho-naproxen | 69 ± 7 | 75 ± 5 | 63 ± 5 | 1.1 | 0.9 |
| Phospho-indomethacin | 41 ± 2 | 41 ± 2 | 1.0 | ||
| Phospho-tyrosol-indomethacin | 50 ± 3 | 62 ± 5 | 109 ± 8 | 1.2 | 2.2 |
| Phospho-valproic acid | 21 ± 1 | 19 ± 1 | 0.9 | ||
Fig. 4.
Coincubation with benzil and BNPP resensitizes the CES1- and CES2-expressing cells to the cytotoxic effect of phospho-NSAIDs in vitro. CES1-expressing (A) and CES2-expressing (B) HEK293 cells were pretreated for 30 min with benzil or BNPP (100 μM), and various concentrations of phospho-NSAIDs were then added. Cell viability was determined by the MTT assay. The corresponding IC50 values are presented in Tables 3 and 4.
Similar to benzil, BNPP (100 μM) alone did not result in significantly reduced cell viability. Moreover, the effect of BNPP recapitulated that of benzil (Fig. 4; Table 4). It is noteworthy that the IC50 values ratio between control and carboxylesterase-expressing cells ranged from 0.7 to 1.3. Therefore, BNPP cotreatment increased the cytotoxicity of phospho-NSAIDs in the CES1- or CES2-expressing cells to levels similar to control cells, thereby nullifying the impact of carboxylesterase expression. These data are consistent with our hypothesis that the inhibition of intracellular carboxylesterases prevents hydrolysis to conventional NSAIDs and enhances the efficacy of phospho-NSAIDs.
TABLE 4.
Effect of BNPP (100 μM) on IC50 values of phospho-NSAIDs
The cells were preincubated with BNPP for 30 min, followed by incubation with phospho-NSAIDs for 24 h. The IC50 values were determined by the MTT assay.
| Phospho-NSAID | IC50 |
CES1/Control | CES2/Control | ||
|---|---|---|---|---|---|
| HEK293-Control + BNPP | HEK293-CES1 + BNPP | HEK293-CES2 + BNPP | |||
| μM | |||||
| Phospho-sulindac | 27 ± 1 | 25 ± 3 | 0.9 | ||
| Phospho-ibuprofen | 55 ± 4 | 52 ± 3 | 0.9 | ||
| Phospho-naproxen | 56 ± 7 | 43 ± 4 | 54 ± 5 | 0.8 | 1.0 |
| Phospho-indomethacin | 41 ± 2 | 29 ± 6 | 0.7 | ||
| Phospho-tyrosol-indomethacin | 38 ± 3 | 49 ± 4 | 40 ± 2 | 1.3 | 1.0 |
| Phospho-valproic acid | 21 ± 1 | 18 ± 1 | 0.9 | ||
Tumor Cells Have Limited Capacity to Hydrolyze Phospho-NSAIDs.
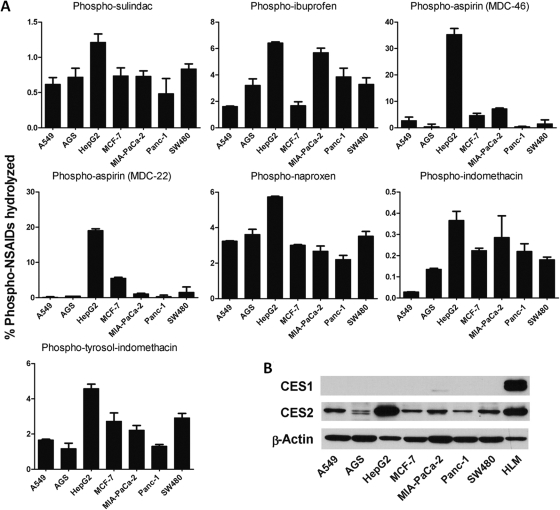
We next evaluated the metabolism of phospho-NSAIDs in several cancer cell lines (Fig. 6). It is noteworthy that we found that cancer cells are inept at hydrolyzing phopho-NSAIDs. Even with extended incubation time (6 h), the percentage of hydrolyzed drug was very low in the cell lines examined. The amount of phospho-sulindac and phospho-indomethacin hydrolyzed was less than 2%, whereas hydrolysis of phospho-ibuprofen, phospho-naproxen, and phospho-tyrosol-indomethacin was less than 8%. Phospho-aspirin (MDC-46 and MDC-22) was significantly hydrolyzed in hepatoma cells (HepG2) by 35 and 19%, respectively, but not in other cell lines. HepG2 cells seemed to have higher carboxylesterase activity than other cell lines, evidenced by the greater percentage of hydrolyzed drug in all of the phospho-NSAIDs.
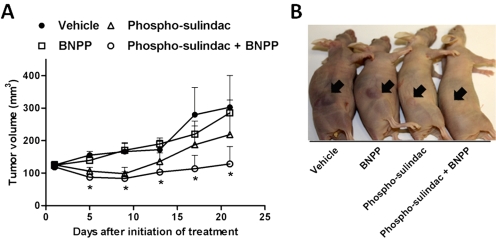
Fig. 6.
Impact of carboxylesterase inhibition on the growth inhibitory effect of phospho-sulindac on gastric xenografts grown in nude mice. AGS human gastric carcinoma cells were implanted subcutaneously into nude mice. Phospho-sulindac (200 mg/kg/day p.o.), BNPP (100 mg/kg/day i.p.), or a combination were administered to mice when the tumors reached an average size of 125 mm3. A, tumor volume growth over time. The values are mean ± S.E.M. (n = 6 per group). B, representative photographs of tumor sizes from the control and treatment groups.
We also examined the expression of CES1 and CES2 in these cells. As shown in Fig. 5, all cancer cell lines had little expression of CES1. On the other hand, HepG2 cells had comparable CES2 expression to human liver microsomes, whereas CES2 levels were much lower than in other cell lines examined. A lack of CES1 expression may explain the poor hydrolytic activity of cancer cells toward phospho-NSAIDs; whereas high CES2 expression in HepG2 cells was consistent with significant hydrolysis of phospho-aspirins (MDC-46 and MDC-22) that are good substrates of CES2. These results indicate that cancer cells have limited carboxylesterase activity and phospho-NSAIDs may be relatively stable once delivered to the tumor sites.
Fig. 5.
Tumor cells in culture have limited capacity to hydrolyze phospho-NSAIDs. A, the human cancer cells were incubated with 100 μM of the individual phospho-NSAIDs for 6 h. The intracellular metabolites were measured by HPLC and expressed as the percentage of phospho-NSAIDs hydrolyzed during the 1-h period. B, Western blots of CES1 and CES2 expression in human cancer cell lines. Human liver microsomes (HLM) were used as a positive control for CES1 and CES2.
BNPP Coadministration Protected Phospho-Sulindac in Mice.
In mice treated with phospho-sulindac alone, no intact drug could be detected in the blood 1 h postadministration. In BNPP-pretreated mice, on the other hand, intact phospho-sulindac (3.2 μM; 23.2% of total drug) could be detected in the blood 1 h after its administration. In addition, the levels of intact phospho-sulindac were 42% higher in the liver of mice pretreated with BNPP. Coadministration with benzil (500 mg/kg), however, did not result in the detection of intact phospho-sulindac in vivo (data not shown). Thus, BNPP, but not benzil, effectively inhibits the hydrolysis of phospho-sulindac in vivo.
BNPP Coadministration Enhanced the Antitumor Efficacy of Phospho-Sulindac in Vivo.
Finally, we investigated the antitumor efficacy of the combination of phospho-sulindac and BNPP in a gastric xenograft model in mice. As shown in Fig. 6, the combination of BNPP and phospho-sulindac suppressed tumor growth, which became statistically significant beginning 4 days after the initiation of treatment (p < 0.05). Phospho-sulindac also suppressed tumor growth, but the inhibition did not reach statistical significance. BNPP, on the other hand, did not affect tumor growth. At the end of the study, the combination of phospho-sulindac and BNPP reduced tumor volume by 57%, whereas phospho-sulindac alone reduced tumor volume by 28% (p < 0.037). We determined the levels of phospho-sulindac and its metabolites in the AGS xenografts (Supplemental Table 1). We found that the level of intact phospho-sulindac was ∼4-fold higher (22 nmol/g, 44% total metabolites) when cotreated with BNPP, compared with phospho-sulindac alone (5.8 nmol/g, 7.7% total metabolites). Thus, inhibition of carboxylesterases improved the delivery of the intact phospho-sulindac, leading to greater efficacy in vivo.
Discussion
Our data established that 1) the strong growth inhibitory effect of phospho-NSAIDs on cancer cells in vitro and in vivo critically depends on the presence of intact, nonhydrolyzed drug, and 2) the hydrolysis of phospho-NSAIDs is mediated by human carboxylesterase 1 and 2, leading to rapid inactivation and a dramatic reduction in their cytotoxicity in vitro, an effect that could be reversed through cotreatment with carboxylesterase inhibitors. Given the profound impact of carboxylesterases on the pharmacological activity of phospho-NSAIDs, this provides a strong rationale for the protection of intact phospho-NSAIDs to enhance their anticancer activity.
Phospho-NSAIDs are novel anticancer drugs that exhibited in vitro and in vivo strong growth inhibition of cancer cells that originate from human colon cancer (Hua et al., 2009; Mackenzie et al., 2010; Xie et al., 2011b), breast cancer (Sun et al., 2011), and pancreatic and lung cancers (Zhao et al., 2009). The potency of phospho-NSAIDs in vitro is 15- to >40-fold stronger than that of the corresponding conventional NSAIDs. By overexpressing CES1 and CES2 in cultured cells, we found that phospho-NSAIDs undergo significant intracellular hydrolysis (20 to >90%) to give the parent NSAIDs (Table 1). Consequently, we observed a strong attenuation (up to 38-fold) of in vitro cytotoxicity of phospho-NSAIDs, in particular, for phospho-ibuprofen and phospho-naproxen. Hence, it is the intact phospho-NSAIDs, rather than the hydrolyzed products, that are responsible for the potent anticancer activity. These data reaffirmed our hypothesis that phospho-NSAIDs are pharmacologically distinct identities to the conventional NSAIDs from which they are derived. Conventional NSAIDs are thought to suppress cancer development and progression via inhibition of cyclooxygenase (COX)-2 (Satoh and Hosokawa, 1998). Paradoxically, modification of NSAIDs at the carboxylic group to generate phospho-NASIDs abrogates their ability to inhibit COX-1 and COX-2 (Mackenzie et al., 2010). Phospho-NSAIDs probably exert their anticancer activity via COX-independent mechanisms, such as the induction of reactive oxygen species (Sun et al., 2011), suppression of polyamine levels (Hua et al., 2009; Huang et al., 2010), and modulation of the thioredoxin system (Hua et al., 2009).
Human CES1 and CES2 are highly expressed in tissues involved in xenobiotic metabolism, including hepatocytes, intestinal mucosa, and proximal tubules in the kidneys (Holmes et al., 2010). Our data indicate that CES1 is the major isoform involved in the hydrolysis of all of the phospho-NSAIDs tested in this study, whereas CES2 is active toward phospho-aspirin, phospho-tyrosol-indomethacin, and phospho-naproxen.
The efficiency of catalysis by carboxylesterases depends on several factors, such as the molecular size and hydrophobicity of the substrate, and relative sizes of the acyl and alcohol substituents (Oboh and Lamango, 2008; Na et al., 2009). The active sites of CES1 and CES2 are lined with aromatic amino acid residues, thus favoring the binding of hydrophobic substrates (Xie et al., 2002). In agreement with Wadkins et al. (2001), we observed that phospho-NSAIDs with higher logP values tend to have lower Km values toward CES1 (Fig. 1). Human CES1 and CES2 exhibited partially overlapping and complementary catalytic activities. In general, CES1 preferentially catalyzes hydrolysis of esters with a larger acyl moiety and a small alcohol group; whereas CES2 prefers substrates with a bulky alcohol group and a smaller acyl domain (Hosokawa, 2008). Because of the relatively larger size of the parent NSAIDs as the acyl moiety compared with the alcohol group (phospho-head), it is not surprising that CES1 is the major enzyme involved in their hydrolysis (Table 2). Indeed, phospho-aspirins, with a relatively small acyl group (aspirin), are the only phospho-NSAIDs that are preferentially hydrolyzed by CES2. CES1 is the major carboxylesterase in the liver; whereas CES2 is highly expressed in the small intestine. Thus, the liver is expected to be a major site of phospho-NSAID hydrolysis in vivo.
The rapid hydrolysis of phospho-NSAIDs by carboxylesterases constitutes a major challenge for the delivery of intact drug to the tumors in vivo. In previous studies in mice, we could detect intact phospho-ibuprofen and phospho-sulindac only at high doses (1200 mg/kg) (Xie et al., 2011b). It is noteworthy that tumor tissues often have impaired carboxylesterase activity. Expression of CES1 and CES2 was found to be lower in colon carcinomas compared with the adjacent normal tissues (Xie et al., 2002; Tang et al., 2008); liver tumors exhibited a remarkably suppressed expression of CES1 (4.6-fold) compared with normal liver tissues (Lim et al., 2002; Na et al., 2009). Data from our analyses have also shown that tumor cells in culture lack the ability to hydrolyze phospho-NSAIDs, and others (Huttlin et al., 2009) have also found that the intestinal tumors from the Apc(Min/+) mice have reduced carboxylesterase activity compared with the intestinal epithelium. Accordingly, the temporary blockade of carboxylesterase activity may be a promising strategy for the delivery of intact phospho-NSAIDs to tumor tissues, where they are less susceptible to esterase hydrolysis.
In this study, we evaluated the ability of two carboxylesterase inhibitors, BNPP and benzil, to inhibit carboxylesterase-mediated phospho-NSAIDs hydrolysis. Both BNPP and benzil were highly effective in blocking the intracellular hydrolysis of phospho-NSAIDs in vitro, and they sensitized CES1- and CES2-expressing cells to the potent growth inhibitory effects of intact phospho-NSAIDs. In mice, coadministration of BNPP with phospho-sulindac resulted in the detection of intact drug (23.2%) in the blood, whereas no intact drug could be detected when phospho-sulindac was given alone. The coadministration of carboxylesterase inhibitors also led to enhanced efficacy in human gastric xenografts in mice, with the combination of phospho-sulindac and BNPP completely arresting tumor growth during the study period. Evaluation of such a treatment regime with phospho-NSAIDs merits future studies.
In summary, our data indicate that the preservation of the intact phospho-NASIDs is critical for their anticancer activity, and the inhibition of carboxylesterase-mediated hydrolysis may be a novel strategy for enhancing their efficacy.
Supplementary Material
This work was supported by the National Institutes of Health National Cancer Institute [Grants R01-CA09242308, R01-CA139454, R01-CA154172]; and the Department of Defense [Grant W81XWH1010873].
B.R. has an equity position in Medicon Pharmaceuticals, Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- NSAID
- nonsteroidal anti-inflammatory drug
- CES1
- carboxylesterase 1
- CES2
- carboxylesterase 2
- COX
- cyclooxygenase
- BNPP
- bis-p-nitrophenyl phosphate
- MTT
- 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- HEK
- human embryonic kidney
- HPLC
- high-performance liquid chromatography.
Authorship Contributions
Participated in research design: Wong, Cheng, Xie, and Rigas.
Conducted experiments: Wong, Cheng, Xie, and Zhu.
Contributed new reagents or analytic tools: Zhou, Constantinides, and Rigas.
Performed data analysis: Wong, Cheng, Xie, and Rigas.
Wrote or contributed to the writing of the manuscript: Wong, Cheng, and Rigas.
References
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, et al. (2009) Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 10:501–507 [DOI] [PubMed] [Google Scholar]
- Flossmann E, Rothwell PM, and British Doctors Aspirin Trial and the UK-TIA Aspirin Trial (2007) Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 369:1603–1613 [DOI] [PubMed] [Google Scholar]
- Guichard S, Terret C, Hennebelle I, Lochon I, Chevreau P, Frétigny E, Selves J, Chatelut E, Bugat R, Canal P. (1999) CPT-11 converting carboxylesterase and topoisomerase activities in tumour and normal colon and liver tissues. Br J Cancer 80:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halen PK, Murumkar PR, Giridhar R, Yadav MR. (2009) Prodrug designing of NSAIDs. Mini Rev Med Chem 9:124–139 [DOI] [PubMed] [Google Scholar]
- Holmes RS, Cox LA, VandeBerg JL. (2010) Mammalian carboxylesterase 3: comparative genomics and proteomics. Genetica 138:695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa M. (2008) Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules 13:412–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua A, Mackenzie GG, Rigas B. (2009) The differential cell signaling effects of two positional isomers of the anticancer NO-donating aspirin. Int J Oncol 35:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Mackenzie G, Ouyang N, Sun Y, Xie G, Johnson F, Komninou D, Rigas B. (2011) The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br J Pharmacol 162:1521–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Zhu C, Sun Y, Xie G, Mackenzie GG, Qiao G, Komninou D, Rigas B. (2010) Phospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: a redox/polyamine-dependent effect. Carcinogenesis 31:1982–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Chen X, Barrett-Wilt GA, Hegeman AD, Halberg RB, Harms AC, Newton MA, Dove WF, Sussman MR. (2009) Discovery and validation of colonic tumor-associated proteins via metabolic labeling and stable isotopic dilution. Proc Natl Acad Sci U S A 106:17235–17240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt JL, Tsurkan L, Wierdl M, Edwards CC, Danks MK, Potter PM. (2006) Intracellular inhibition of carboxylesterases by benzil: modulation of CPT-11 cytotoxicity. Mol Cancer Ther 5:2281–2288 [DOI] [PubMed] [Google Scholar]
- Lim SO, Park SJ, Kim W, Park SG, Kim HJ, Kim YI, Sohn TS, Noh JH, Jung G. (2002) Proteome analysis of hepatocellular carcinoma. Biochem Biophys Res Commun 291:1031–1037 [DOI] [PubMed] [Google Scholar]
- Mackenzie GG, Sun Y, Huang L, Xie G, Ouyang N, Gupta RC, Johnson F, Komninou D, Kopelovich L, Rigas B. (2010) Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology 139:1320–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey GM. (2011) Carboxylesterase 1 (Ces1): from monocyte marker to major player. J Clin Pathol 64:107–109 [DOI] [PubMed] [Google Scholar]
- Na K, Lee EY, Lee HJ, Kim KY, Lee H, Jeong SK, Jeong AS, Cho SY, Kim SA, Song SY, et al. (2009) Human plasma carboxylesterase 1, a novel serologic biomarker candidate for hepatocellular carcinoma. Proteomics 9:3989–3999 [DOI] [PubMed] [Google Scholar]
- Oboh OT, Lamango NS. (2008) Liver prenylated methylated protein methyl esterase is the same enzyme as Sus scrofa carboxylesterase. J Biochem Mol Toxicol 22:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbo MR, Potter PM. (2005) Mammalian carboxylesterases: from drug targets to protein therapeutics. Drug Discov Today 10:313–325 [DOI] [PubMed] [Google Scholar]
- Satoh T, Hosokawa M. (1998) The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol 38:257–288 [DOI] [PubMed] [Google Scholar]
- Singh G, Triadafilopoulos G. (1999) Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol Suppl 56:18–24 [PubMed] [Google Scholar]
- Sun Y, Huang L, Mackenzie GG, Rigas B. (2011) Oxidative stress mediates through apoptosis the anticancer effect of phospho-nonsteroidal anti-inflammatory drugs: implications for the role of oxidative stress in the action of anticancer agents. J Pharmacol Exp Ther 338:775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Rigas B. (2008) The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res 68:8269–8277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Wu H, Wu Z, Wang G, Wang Z, Zhu D. (2008) Carboxylesterase 2 is downregulated in colorectal cancer following progression of the disease. Cancer Invest 26:178–181 [DOI] [PubMed] [Google Scholar]
- Wadkins RM, Morton CL, Weeks JK, Oliver L, Wierdl M, Danks MK, Potter PM. (2001) Structural constraints affect the metabolism of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (CPT-11) by carboxylesterases. Mol Pharmacol 60:355–362 [DOI] [PubMed] [Google Scholar]
- Xie G, Nie T, Mackenzie GG, Sun Y, Huang L, Ouyang N, Alston N, Zhu C, Murray OT, Constantinides PP, et al. (2011a) The metabolism and pharmacokinetics of phospho-sulindac (OXT-328) and the effect of difluoromethylornithine. Br J Pharmacol http://dx.doi.org/10.1111/j.1476–5381.2011.01705.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Sun Y, Nie T, Mackenzie GG, Huang L, Kopelovich L, Komninou D, Rigas B. (2011b) Phospho-ibuprofen (MDC-917) is a novel agent against colon cancer: efficacy, metabolism, and pharmacokinetics in mouse models. J Pharmacol Exp Ther 337:876–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Yang D, Liu L, Xue B, Yan B. (2002) Human and rodent carboxylesterases: immunorelatedness, overlapping substrate specificity, differential sensitivity to serine enzyme inhibitors, and tumor-related expression. Drug Metab Dispos 30:541–547 [DOI] [PubMed] [Google Scholar]
- Williams ET, Bacon JA, Bender DM, Lowinger JJ, Guo WK, Ehsani ME, Wang X, Wang H, Qian YW, Ruterbories KJ, et al. (2011) Characterization of the expression and activity of carboxylesterases 1 and 2 from the beagle dog, cynomolgus monkey, and human. Drug Metab Dispos 39:2305–2313 [DOI] [PubMed] [Google Scholar]
- Zhao W, Mackenzie GG, Murray OT, Zhang Z, Rigas B. (2009) Phosphoaspirin (MDC-43), a novel benzyl ester of aspirin, inhibits the growth of human cancer cell lines more potently than aspirin: a redox-dependent effect. Carcinogenesis 30:512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.