Abstract
Collectins contribute to host defense through interactions with glycoconjugates on pathogen surfaces. We have prepared recombinant trimeric neck and carbohydrate recognition domains (NCRDs) of collectins and we now show that the NCRDs of bovine conglutinin and CL-46 (like that of CL-43) have greater intrinsic antiviral activity for influenza A virus (IAV) than the human SP-D NCRD (hSP-D-NCRD). The three serum collectins differ from SP-D by having insertions adjacent to amino acid 325 and substitution of hydrophobic residues for arginine 343. We previously showed that a 3 amino acid (RAK) insertion, as found in CL-43, increases antiviral activity and mannan binding activity of the hSP-D-NCRD, while the substitution of valine at 343, as in conglutinin, more strongly increased these activities. Mannan binding activity of collectins has been considered to predict for ability to bind to high mannose glycans on viruses or other pathogens. We now show, however, that combined mutants containing the RAK insertion and R343V or R343I substitutions have greatly increased mannan binding ability, but lower IAV binding or inhibiting activity than mutants containing R343V or R343I substitutions only. These findings indicate differences in the recognition of glycan structures of mannan and IAV by the NCRDs and emphasize the importance of the flanking sequences in determining the differing interactions of human SP-D and bovine serum collectins with mannose-rich glycoconjugates on IAV and other pathogens. Of interest, we show conservation of some monoclonal antibody binding epitopes between bovine collectin NCRDs and hSP-D, suggesting shared structural motifs.
Keywords: influenza virus, CL-43, CL-46, conglutinin
Introduction
Surfactant protein D (SP-D) is present in lung lining fluids and a variety of other mucosal locations where it participates in binding and inhibiting a wide range of infectious organisms, including bacteria, fungi, and viruses [1]. SP-D is a member of the collectin family of innate defense proteins that contain a structurally important collagen domain and trimeric neck and carbohydrate recognition domains (termed NCRDs from here on) that are involved in calcium dependent binding to specific carbohydrate epitopes on microorganisms or mammalian cells.
We and others have studied the interactions of SP-D with influenza A viruses (IAV). Mice lacking SP-D due to gene-deletion exhibit more severe illness, higher viral loads, and greater inflammatory response when infected with human strains of IAV [2-5]. Inhibition of IAV by SP-D is determined mainly by the presence of high mannose oligosaccharides on the viral hemagglutinin (HA) [6-9]. SP-D also plays an important role in inhibiting inflammatory responses triggered by LPS and bacteria. Of interest, binding of SP-D to the highly conserved core of LPS is mediated by binding to heptoses through a crystallographically-distinct mechanism from its binding to monosaccharides like glucose or mannose [10]. Finally SP-D plays an important role in maintenance of surfactant lipid homeostasis in vivo. SP-D binds specifically to phosphatidylinositol (PI) through recognizing the inositol moiety [11], and this may be responsible for SP-Ds effects on surfactant homeostasis [12, 13].
Our previous studies suggest that the lectin domains of serum collectin NCRDs have intrinsically greater antiviral activity than the corresponding domain of SP-D. For instance, full length chimeric molecules containing the N-terminus and collagen domains of SP-D connected to the NCRDs of conglutinin or human mannose binding lectin (MBL) have significantly greater neutralizing activity than wild type SP-D [14, 15]. Furthermore full length trimers of CL-43, or the CL-43 NCRD have strong antiviral activity [16]. Given that SP-D recognizes high mannose glycans associated with the viral hemagglutinin and the neuraminidase [6], our initial hypothesis was that the same structural adaptations were responsible for the enhanced recognition of mannose-rich oligosaccharides of mannan and IAV by CL-43 [16].
In this paper we compare antiviral properties of NCRD preparations of SP-D and the serum collectins. We report for the first time strong antiviral activity of the bovine serum collectin CL-46 NCRD. To further analyze the increased antiviral activity of bovine serum collectins we prepared novel mutant versions of hSP-D-NCRD in which specific residues found in serum collectins replace those of wild type SP-D. These mutants were then compared for antiviral activity and binding to mannan. Finally we determine interactions of functionally enhancing monoclonal antibodies raised against SP-D with bovine collectin NCRDs.
Materials and Methods
Virus Preparations
IAV was grown in the chorioallantoic fluid of ten day old chicken eggs and purified on a discontinuous sucrose gradient as previously described [17]. The virus was dialyzed against PBS to remove sucrose, aliquoted and stored at -80°C until needed. Philippines 82/H3N2 (Phil82) and Brazil78/H1N1 (Braz78) strains and their bovine serum inhibitor resistant variants, Phil82/BS and Braz78/BS, were kindly provided by Dr. E. Margot Anders (Univ. of Melbourne, Melbourne, Australia) [18]. Post thawing the viral stocks contained ~5×108 plaque forming units/ml.
Collectin Preparations
Dodecamers of wild type recombinant human SP-D were used as a control and was expressed in CHO cells and purified as described [19]. Trimeric NCRD fusion proteins, including the wild type human and rat NCRDs (hereafter, called hSP-D-NCRD and rNCRD, respectively), mutant constructs of the hSP-D-NCRD and rNCRD, and NCRDs of other collectins (apart from that of CL-46) were produced in E. coli as described [20, 21]. All fusion proteins contain an identical N-terminal His-tag that facilitates purification. An internal S-protein binding site permits detection using S-protein horseradish peroxidase, as previously described [21]. All NCRDs migrated as a single major band of the appropriate size for trimers on SDS-PAGE with the expected decrease in mobility on reduction, consistent with the formation of normal intrachain disulfide bonds. All showed retention of some or all of the calcium-dependent carbohydrate binding activities of the native protein. Using the wild type hSP-D-NCRD and R343V mutant we not found evidence of aggregation on storage of the proteins [22]. The endotoxin level of all SP-D preparations was 0.1~0.5 EU/ml (Limulus Lysate Assay, Cambrex, Walkersville, MD). The CL-46 NCRD was prepared in Pichia pistoris as described [23]. Briefly the alpha-helical coiled-coil neck region and the CRD of CL-46 was amplified by PCR and ligated into the pPIC9K-vector (Invitrogen). The pPIC9K derivatives were transformed into XL-10 E. coli, purified, linearized, and transformed into Pichia pastoris (GS115). Clones were double-selected by growth on histidine deficient plates and plates with increasing concentrations of geneticin.
Monoclonal antibodies
mAbs 245-01 and -02 and 246-02 through 246-08 were raised against SP-D by inoculating mice with 10μg/ml of human SP-D as previously described [24]. The 3C3-C-20 mAb was developed by Dr. Jeffrey Whitsett, Cincinnati Children's Hospital and Medical Center, Cincinnati, OH. MAbs 6B2, 7A10, and 7C6 were produced by Dr. Kuroki as described [25].
Binding of mAbs to SP-D or NCRD
SP-D preparations were diluted in coating buffer to a concentration of 2 μg/ml and coated on ELISA plates overnight, followed by washing and addition of mAbs. The final concentration of mAbs used for the ELISA assay was 1 μg/ml. Bound mAbs were detected with horseradish peroxidase (HRP)-conjugated donkey-anti mouse antibodies labeled followed by TMB peroxidase. OD450 values were measured on a POLARstar OPTIMA plate reader (BMG Labtech, Durham NC).
Binding of NCRDs to IAV or mannan
Binding of NCRD fusion proteins to IAV or mannan was measured as described by use of the S protein binding site on the fusion tag of the NCRD. In brief, IAV (Phil82 strain) or mannan was coated onto the surface of ELISA plates and, following washing, NCRDs were added [21]. After incubation and washing, S protein HRP was added and peroxidase activity measured.
Hemagglutination (HA) inhibition assay
HA inhibition was measured by serially diluting collectins or other host defense protein preparations in round bottom 96 well plates (Serocluster U-Vinyl plates; Costar, Cambridge, MA) using PBS containing calcium and magnesium as a diluent [26]. After adding 25 μl of IAV, giving a final concentration of 40 HA units per ml or 4 HA units/well, the IAV/protein mixture was incubated for 15 min. at room temperature, followed by addition of 50 μl of a type O human erythrocyte suspension. The minimum concentration of protein required to fully inhibit the hemagglutinating activity of the viral suspension was determined by noting the highest dilution of protein that still inhibited hemagglutination. Inhibition of HA activity in a given well is demonstrated by absence of formation of an erythrocyte pellet. If no inhibition of HA activity was observed at the highest protein concentration used then the value is expressed as > the maximal protein concentration.
Fluorescent focus assay of IAV infectivity
MDCK cell monolayers were prepared in 96 well plates and grown to confluency. These layers were then infected with diluted IAV preparations for 45 min. at 37°C in PBS and tested for presence of IAV infected cells after 7 hrs. using a monoclonal antibody directed against the influenza A viral nucleoprotein (provided by Dr. Nancy Cox, CDC, Atlanta, GA) as previously described [8, 27]. IAV was pre-incubated for 30 min. at 37°C with collectins or control buffer, followed by addition of these viral samples to the MDCK cells. Where indicated collectins were first incubated with mAbs prior to adding them to IAV.
Results
Antiviral activities of wild type NCRDs of SP-D and serum collectins
In addition to MBL three serum collectins have been identified in bovidae: conglutinin, CL-43 and CL-46. We have previously reported that hSP-D-NCRD has minimal binding to IAV. Using identically prepared trimeric NCRD fusion proteins, which have S-protein binding sites on the N-terminal tags, we were able to directly compare binding activity of the NCRDs of conglutinin and CL-43 to IAV. Both of these bovine collectin NCRDs bound significantly more strongly to IAV than hSP-D-NCRD, with the strongest binding obtained with CL-43 (figure 1A). We next compared neutralizing activity of NCRDs using each at a concentration of 20μg/ml (figure 1B). Results obtained on viral neutralization assays were generally consistent with the binding results. As we have previously shown, the hSP-D-NCRD lacks neutralizing activity at this concentration; however, NCRDs of conglutinin and CL-43 had strong activity. Again CL-43 had significantly stronger activity than conglutinin.
Figure 1. Viral binding and neutralization by NCRDs of human SP-D and bovine serum collectins.
Viral binding was measured by solid phase ELISA using S-protein HRP to detect bound NCRDs (panel A). Both conglutinin and CL-43 bound to the Phil82 strain of IAV to a significantly greater extent than hSP-D-NCRD. Binding of CL-43 was also significantly greater than that of conglutinin. In panel B, viral neutralization was measured by pre-incubating IAV with 20μg/ml of NCRDs of SP-D, conglutinin and CL-43, followed by infection of MDCK cells for six hours with the treated virus and detection of infectious foci using anti-nucleoprotein mABs. Conglutinin and CL-43 NCRDs caused significantly greater inhibition than hSP-D-NCRD. The effect of CL-43 NCRD was also significantly greater than that of conglutinin. Results are mean±SEM of 4 experiments. Panel C shows a dose response curve for viral neutralization by CL-46 NCRD. Infectious foci were reduced significantly by all concentrations of CL-46 NCRD shown (p<0.01 compared to viral control; n=4).
We also tested a preparation of the NCRD of CL-46 that was generated in Pichia pistoris as previously described [23]. Because this preparation lacks a fusion tag, we could not directly compare binding affinity to IAV; however, the NCRD of CL-46 also had substantial neutralizing activity (figure 1C). In addition, the CL-46 NCRD inhibited HA activity of various strains of IAV (Table 1). As for SP-D and CL-43, the activity was dependent on glycosylation of the viral strain and the presence of calcium. The Braz7/BS and Phil82/BS strains were derived from the wild type parental strains by Dr. E. Margot Anders through growth in the presence of bovine serum β inhibitors (subsequently shown to be principally conglutinin) [18, 28]. These strains differ from the parental strains in lacking a single high mannose oligosaccharide positioned close to the sialic acid binding site of the HA, and they are partially or fully resistant to inhibition by SP-D, MBL, conglutinin and CL-43. The PR-8 strain lacks all high mannose attachments on its envelope proteins and was highly resistant to CL-46.
Table 1. Hemagglutination inhibition caused by CL-46 NCRD.
| Viral Strain | Strain Characteristics | HA Inhibitory Concentration (ng/ml) |
|---|---|---|
| Phil82 | Wild type H3N2 HA and neuraminidase | 230±63 |
| Phil82+EDTA | Same as above but assay done in EDTA | >6000 |
| Phil82/BS | Lacks single oligomannose attachment on tip of HA | >1600 |
| Braz78 | Wild type H1N1 HA and neuraminidase | 103±6 |
| Braz78/BS | Lacks single oligomannose attachment near tip of HA | 263±22 |
| PR-8 | Lacks all oligomannose attachments on envelope proteins | >9000 |
Results are mean±SEM of three or more experiments. Where > is shown there was no activity of CL-46 NCRD up to the concentration indicated.
Generation and antiviral testing of NCRD mutants modeled on serum collectins
Amino acid residues 325 and 343 define ridges on either side of the primary calcium coordination (lectin) site of SP-D (Figure 2). These residues have a major impact on binding properties of SP-D [20, 29, 30]. All of the serum collectins have a hydrophobic residue at position 343 (SP-D numbering), and we have previously shown that hSP-D-NCRDs with an R343V or R343I substitution have increased mannan binding activity and greatly increased antiviral activity [29].
Figure 2. Comparison of key residues of CRDs and diagram depicting positions of RAK insertion and R343 in the human SP-D CRD.

Amino acids 321-343 of CRDs of human SP-D and bovine serum collectins are shown in top of figure. Conserved residues are shown in bold. The primary calcium coordinating residues are residues 320-322 (EPN). In bottom of the figure, a diagram based on crystallographic analysis of hSP-D-NCRD is shown. Calcium molecules are depicted in green and a single mannose molecule is depicted in the primary calcium coordination and lectin site.
As shown in figure 2, the bovine serum collectins also all have an insertion adjacent to residue 325, which is predicted to alter the topography around that site [21, 31]. We have shown that placing the RAK insertion found in CL-43 in the analogous site in hSP-D-NCRD modestly increases mannan binding and antiviral activity [21]. Figure 2 shows the location of this insertion in the structure of the NCRD. We, therefore, prepared double mutants containing both the RAK insertion and hydrophobic substitutions R343I or R343V to see if additive increases in antiviral activity could be achieved.
The RAK+R343I and RAK+R343V double mutants had greatly increased mannan binding activity compared to R343I (or R343V), RAK or hSP-D-NCRD (figure 3). The double mutants also showed increased viral binding and antiviral activity compared to hSP-D-NCRD; however, unexpectedly, these activities were reduced compared to the mutants with single site substitutions at residue 343 (figure 4 and Table 3). Figure 4A compares viral binding by R343V and RAK+R343V. The combined mutant RAK+R343V had less HA inhibitory (Table 3) and neutralizing activity (figure 4B) than R343V. Similar results were obtained in comparing the RAK+R343I combined mutant to R343I (table 3 and figure 4C).
Figure 3. Binding of hSP-D-NCRD mutants to mannan.
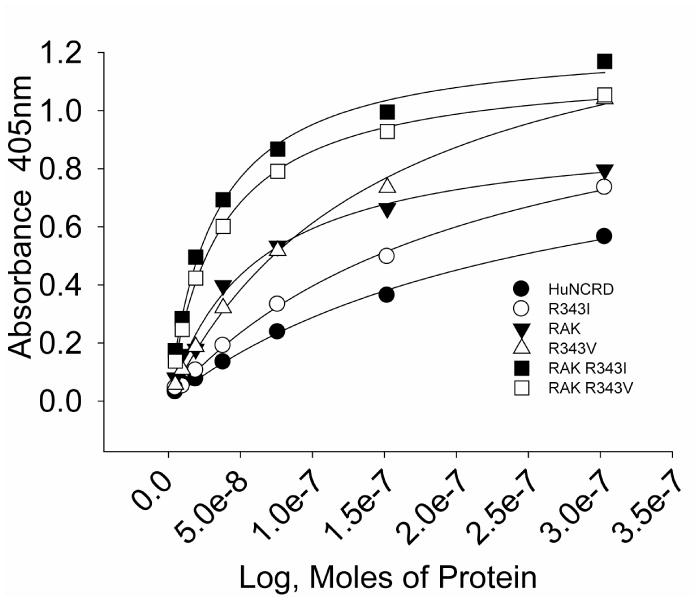
Results were obtained by ELISA using plates coated with mannan. Binding was detected using S protein-HRP. All of the NCRDs shown have S protein binding sites. Results represent 3 or more experiments and binding of RAK R343I and RAK R343V was significantly higher than all other NCRDs except at the highest concentration of R343V.
Figure 4. Viral binding and neutralizing activity of hSP-D-NCRD mutants.

Panel A shows binding of the indicated NCRDs to ELISA plates coated with IAV (Phil82 strain). Panels B and C show viral neutralization as assessed by fluorescent focus assay. Results are mean±SEM of three or more experiments.
* indicates where R343V or R343I caused significantly greater neutralization than the other NCRDs tested.
** indicates where binding of R343V to IAV was significantly greater than binding of RAK+R343V
Table 3. HA inhibitory activity of wild type NCRDs alone or after crosslinking with mAbs.
| NCRD | HA Inhibiting Concentration (μg/ml) | |||
|---|---|---|---|---|
| Control | +246-04 | +246-08 | 6B2 | |
| CL-46a | 0.62±0.08 b | 0.23±0.02 | 0.11±0.008* | 0.02±0.001* |
| CL-43 | 2.1±0.18 | 2.07±0.18 | 1.6±0.2 | 0.425±.004* |
| Conglutinin | 13.7±0.38 | 12.8±0.95 | 12.9±0.48 | |
| hSP-D-NCRD | >15 | 14±1.2* | 7.4±0.9* | 8±0.5* |
| R343V | 0.55±0.13a | 0.19±0.03* | 0.07±0.02* | 0.047±0* |
| RAK+R343V | 7.66±0.33 | |||
| R343I | 0.92±0.08 | 0.29±0.05* | 0.15±0.03* | |
| RAK+R343I | 4.0±0 | 0.23±0.02* | 0.08±0.012* | |
This NCRD was prepared in Pichia pistoris by a different methodology from the other NCRDs so results are not directly comparable.
Results shown are mean±SEM of 4 or more experiments and are expressed as the μg/ml needed to inhibit 40 hemagglutination units of the Phil82 strain of IAV. MAbs 7A10 and 7C6 had no effect on activity of serum collectin NCRDs (data not shown).
Values were not determined for spaces left blank..
indicates where amounts of NCRD needed for inhibition were signifi less than control (p<0.05) in the presence of mAb
Conservation of functionally enhancing mAb binding epitopes between NCRDs of bovine serum collectins and hSP-D-NCRD
Dr. Holmskov has developed a panel of several mAbs directed against the NCRD of SP-D. These have proved useful in determining functionally important regions of the protein and demonstrating the role of crosslinking of NCRD trimers in antiviral activity [32, 33]. We have previously reported that the mAbs can be grouped into those that inhibit antiviral activity of SP-D against IAV (246-02, -03, -05, and -07) and those that do not [32]. Two of the non-blocking mAbs (246-04 and -08) strongly increase the antiviral activity of NCRD trimer preparations of SP-D, by cross-linking and enhancing binding of the NCRD to the virus [32]. We now show that the 246-08 binds to conglutinin strongly and CL-46 to a limited extent (Table 3). The rest of the mAbs in this group did not bind to any of the serum collectins above 5% of control (data not shown). Dr. Kuroki has developed other antibodies that recognize the NCRD of human SP-D [34-36]. We also show that the 6B2 produced by Dr. Kuroki cross-reacts with serum collectins (especially CL-46).
The RAK+R343I, RAK+R343V, R343I, R343V and RAK mutants all retained full binding to mAbs 246-08, 246-04 and 6B2 (Table 2), indicating that these mAbs probably bind to areas of the CRD distant from the lectin site. These findings are consistent with the fact that these mAbs do not block the binding activity of SP-D to IAV (see [32] and Table 3). We compared these results to those obtained with the blocking mAb, 246-02. The RAK insertion strongly diminished binding of this mAb, whereas binding was not affected by the R343V substitution. Of interest, the combined mutant RAK+R343V had restored binding to 246-02. We also tested the 3C3-C-20 mAb graciously provided by Ronald Scheule of Genzyme Inc. (Boston, MA). 3C3-C-20 blocked the antiviral activity of full length SP-D (i.e., HA inhibiting concentration of SP-D dodecamers was 37±4 ng/ml, whereas no inhibition was seen up to 1μg/ml after pre-incubation of SP-D with 3C3-C-20; n=4; p<0.001). As in the case of mAb 246-02, the binding of 3C3-C-20 was greatly diminished by the RAK insertion and restored to baseline by the combined RAK+R343V mutations (Table 2). These findings suggest that the combined mutant restores structural features recognized by these mAbs that are lost in the RAK insertion mutant.
Table 2. Binding of anti-SP-D mAbs to bovine serum collectin NCRDs and to mutant version of hSP-D-NCRD.
| NCRD | 6B2 | 246-04 | 246-08 | 246-02 | 3C3-C-20 |
|---|---|---|---|---|---|
| hSP-D-NCRD | 100 a | 100 | 100 | 100 | 100 |
| CL-46 | 30±3 | 2±1 | 12±2 | ||
| CL-43 | 10±6 | 2±1 | 3±0.6 | ||
| Conglutinin | 9±1.2 | 1.5±1.2 | 95±17 | ||
| RAK | 91±4 a | 91±9 | 90±13 | 40±2* | 13±6* |
| R343V | 102±5 | 105±3 | 105±1 | 105±1 | |
| RAK+R343V | 104±1 | 104±3 | 99±2 | 103±3 | 90±4 |
Results shown are percent of control binding (control being binding of mAbs to wild type hSP-D-NCRD) and are mean±SEM for 3 or more experiments using ELISA. Binding to hSP-D-NCRD is given as 100%. The dilution of mAbs was 1:1000.
Indicates where results for mutant NCRDs were significantly lower than for wild type hSP-D-NCRD control
Values were not determined for spaces that were left blank.
Table 3 shows the HA inhibitory activity of the bovine serum collectins in comparison to that of the wild type human SP-D NCRD. CL-43, CL-46 and conglutinin NCRDs all had measurable HA inhibitory activity, while hSP-D-NCRD did not at least up to a concentration of 50μg/ml. We also tested HA inhibitory activity by the NCRDs after cross-linking of the various collectins with mAbs. We have previously reported that the 246-04 and 246-08 mAbs increase HA activity of hSP-D-NCRD [32]; however, in the current study no enhancement of activity of conglutinin was found (Table 3). This was particularly surprising because it expressed the 246-08 epitope very strongly in the solid-phase binding assay. In contrast, mAb 246-08 increased the activity of the CL-46 NCRD. We now show that the 6B2 mAb also increases HA inhibitory activity of hSP-D-NCRD (Table 3). The 6B2 mAb also strongly increased HA inhibitory activity of CL-46 and CL-43 NCRDs (consistent the data in Table 2 showing binding of this mAb to these proteins). As shown in Table 3, cross-linking of the mutant NCRDs derived from SP-D with the enhancing mAbs 246-04, 246-08 or 6B2 increased their HA inhibitory activity as well. The 246-08 and 6B2 mAbs had stronger enhancing activity than 246-04 in these assays.
Discussion
Bovine serum collectin NCRDs have increased antiviral activity compared with the SP-D NCRD: role of residues flanking the lectin site
Despite the genetic and structural relationships between SP-D and bovine serum collectins, there are significant differences in ligand recognition and in key residues surrounding the primary carbohydrate binding site. As compared to trimeric subunits of SP-D, CL-43 trimers show greater interactions with mannan and IAV [16]. In addition, conglutinin dodecamers have distinct monosaccharide recognition properties and greater antiviral activity than SP-D dodecamers [15] and the NCRD of conglutinin has been reported to have antiviral activity while that of SP-D does not [37, 38].
We now directly compare NCRDs preparations of CL-43, conglutinin and CL-46, and find that all of them have intrinsically greater antiviral activity than the human SP-D. The viral neutralizing and HA inhibiting activities of the CL-46 NCRD have not been previously reported on and appear as strong, or stronger than, the other bovine serum collectins. One caveat is that the CL-46 protein was prepared in a different manner from the other NCRDs; however, we have previously tested a variety of human SP-D NCRD preparations and they have all lacked (or had minimal) HA inhibiting or neutralizing activity [20, 21, 39]. Thus, it is likely that the antiviral activity of the CL-46 NCRD significantly exceeds that of SP-D. We also confirm the substantially greater mannan binding activity of CL-43.
We attempted to determine the structural differences that could account for increased antiviral activity of these proteins. The ridges around the primary carbohydrate binding site show considerable divergence among collectins, perhaps in response to a need to recognize different pathogens. One obvious difference between all serum collectins and SP-A or SP-D is the presence of a hydrophobic residue at position 343. We have shown that the R343V or R343I mutants of hSP-D-NCRD have greatly increased antiviral activity compared to the wild type hSP-D-NCRD [29]; hence, this is one important difference accounting for the increased antiviral activity of bovine serum collectin NCRDs. Another difference relates to the presence of small amino acid insertions immediately N-terminal to residue 325. Since CL-43 had particularly strong mannan binding and antiviral activity, for this paper we produced and tested addition of the RAK sequence to the R343V (or R343I) mutant of hSP-D-NCRD. Although the combined mutations greatly increased mannan binding activity, antiviral activity was decreased as compared to R343V (or R343I). This finding indicates that the mechanisms of binding to mannan and to IAV, while similar, are not identical and involve a complex interplay between residues on the two ridges that flank the primary carbohydrate binding site. High mannose oligosaccharides on the IAV hemagglutinin are important for recognition and neutralization by SP-D [6]. Important differences in the detailed structure of oligomannose sugar chains on IAV and mannan, or in the macromolecular patterns of sugars of mannose rich sugars on IAV and mannan, may account for the differences in recognition of these ligands by specific NCRDS.
It is of interest that binding of mAbs 246-02 and 3C3-C-20, which is reduced to RAK, is partially or fully restored for RAK+R343V, implying that the combination of the insertion and substitution restore a structural feature in hSP-D-NCRD that is recognized by these mAbs. We plan, in future studies, to determine the crystal structures of these and other mutant versions of the SP-D NCRD. Although the RAK+R343V (or I) double mutants did not result in increased antiviral activity compared to single mutants, we are pursuing other strategies including substitutions for D325 in combination with the R343V substitution and have found increased activity (unpublished data). Hence, we still feel the approach of altering residues on the ridges flanking both sides of the lectin site is a productive approach to developing NCRDs that could be of therapeutic use in IAV.
Conserved epitopes on bovine serum collectins and SP-D and potentiation of antiviral activity of NCRDs with monoclonals recognizing these epitopes
Studies of the bovine serum collectin genes strongly suggest that they evolved through duplication of the SP-D gene in ruminants [40]. Although there are areas of significant sequence divergence, particularly in the N-terminal domains, the C-terminal lectin domains show generally high homology with SP-D. Of interest, we now show that two mAbs, 6B2 and 246-08, significantly cross-react with bovine serum collectins. We cannot yet identify the specific sequences recognized by 6B2, 246-04 or -08; however, they appear to be distinct from each other based on varied binding to serum collectin NCRDs. Binding to 246-04, 246-08 and 6B2 is not affected by changes in key residues around the lectin site (see Table 2) or by deletion of the neck [32, 41]. It is possible, therefore, that there are conserved structural motifs on the back or side surfaces of NCRDs of SP-D and bovine collectin CRDs. This hypothesis will need to be tested by comparative crystallographic analysis. The conservation of the 246-08 and 6B2 epitopes in serum collectins indicates that they are structurally and/or functionally important sites.
We have previously shown that mAbs 246-04 and 246-08 enhance activity of hSP-D-NCRD through cross-linking [32]. We now demonstrate that 6B2 can also enhance the antiviral activity of NCRDs, probably through a similar cross-linking mechanism. SP-D appears to be particularly dependent on cooperative interactions among NCRD heads for antiviral activity, whereas some other activities are retained to a greater degree in wild type hSP-D-NCRD trimers [42-44]. Activating antibodies could be used in combination with treatment with NCRDs to increase their host defense activity. Note that cross-linking of the R343V variant of hSP-D-NCRD with either mAb 246-08 or 6B2 results in very potent antiviral activity, which approaches the activity of native dodecamers (see Table 3).
Summary
Despite genetic and structural relationships between the NCRDs of bovine serum collectins and human SP-D, the bovine serum collectin NCRDs all have significantly increased ability to inhibit IAV. We demonstrate that the CL-43 NCRD and a mutant version of the human SP-D NCRD incorporating key distinctive features surrounding the lectin site of CL-43 (RAK+R343I) have greatly increased binding to mannan. The combined effect of the hydrophobic substitution at R343 and the RAK insertion adjacent to D325 alters both ridges surrounding the primary carbohydrate binding site leading to substantially greater mannan binding than occurs with either R343I or RAK alone. This indicates that the extended binding surface flanking the primary binding site can strongly modulate binding to important polysaccharide ligands. Unexpectedly, the RAK+R343I (or V) combined mutants had reduced viral binding and inhibiting activity compared to R343I (or V) single mutants. This also suggests that oligosaccharide structures on mannan and IAV differ enough to result in differing recognition by some NCRDs. MAbs recognizing conserved epitopes on the lateral or back surface of the CRD of SP-D and bovine serum collectins are able to cross-link these NCRDs and further increase their antiviral activity. The finding that there are cross-reactive epitopes in the NCRDs of SP-D and bovine collectins will be useful in efforts to identify binding sites of these functionally enhancing mAbs. Future studies will involve development of other combined mutants (e.g., with substitutions of D325 and R343) in efforts to specifically increase antiviral activity further.
Acknowledgments
This work was supported by NIH Grant AI-83222 (KLH, ECC, and JH) and Grant HL069031 (KLH).
References
- 1.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 2.Kingma PS, Zhang L, Ikegami M, Hartshorn K, McCormack FX, Whitsett JA. Correction of pulmonary abnormalities in Sftpd-/- mice requires the collagenous domain of surfactant protein D. J Biol Chem. 2006;281:24496–24505. doi: 10.1074/jbc.M600651200. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Hartshorn K, Crouch E, Ikegami M, Whitsett J. Complementation of pulmonary abnormalities in SP-D (-/-) mice with an SP-D/Conglutinin fusion protein. J Biol Chem. 2002;277:22453–22459. doi: 10.1074/jbc.M201632200. [DOI] [PubMed] [Google Scholar]
- 4.LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol. 2001;167:5868–5873. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- 5.Hawgood S, Brown C, Edmondson J, et al. Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J Virol. 2004;78:8565–8572. doi: 10.1128/JVI.78.16.8565-8572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartshorn KL, White MR, Voelker DR, Coburn J, Zaner K, Crouch EC. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem J. 2000;351(Pt 2):449–458. [PMC free article] [PubMed] [Google Scholar]
- 7.Reading PC, Pickett DL, Tate MD, Whitney PG, Job ER, Brooks AG. Loss of a single N-linked glycan from the hemagglutinin of influenza virus is associated with resistance to collectins and increased virulence in mice. Respir Res. 2009;10:117. doi: 10.1186/1465-9921-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigerust DJ, Ulett KB, Boyd KL, Madsen J, Hawgood S, McCullers JA. N-linked glycosylation attenuates H3N2 influenza viruses. J Virol. 2007;81:8593–8600. doi: 10.1128/JVI.00769-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Head J, Kosma P, et al. Recognition of heptoses and the inner core of bacterial lipopolysaccharides by surfactant protein d. Biochemistry. 2008;47:710–720. doi: 10.1021/bi7020553. [DOI] [PubMed] [Google Scholar]
- 11.Crouch E, McDonald B, Smith K, et al. Critical role of Arg/Lys343 in the species-dependent recognition of phosphatidylinositol by pulmonary surfactant protein D. Biochemistry. 2007;46:5160–5169. doi: 10.1021/bi700037x. [DOI] [PubMed] [Google Scholar]
- 12.Ikegami M, Grant S, Korfhagen T, Scheule RK, Whitsett JA. Surfactant protein-D regulates the postnatal maturation of pulmonary surfactant lipid pool sizes. J Appl Physiol. 2009;106:1545–1552. doi: 10.1152/japplphysiol.91567.2008. [DOI] [PubMed] [Google Scholar]
- 13.Botas C, Poulain F, Akiyama J, et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci. 1998;95:11869–11874. doi: 10.1073/pnas.95.20.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White MR, Crouch E, Chang D, et al. Enhanced antiviral and opsonic activity of a human mannose-binding lectin and surfactant protein D chimera. J Immunol. 2000;165:2108–2115. doi: 10.4049/jimmunol.165.4.2108. [DOI] [PubMed] [Google Scholar]
- 15.Hartshorn KL, Sastry KN, Chang D, White MR, Crouch EC. Enhanced anti-influenza activity of a surfactant protein D and serum conglutinin fusion protein. Am J Physiol Lung Cell Mol Physiol. 2000;278:L90–98. doi: 10.1152/ajplung.2000.278.1.L90. [DOI] [PubMed] [Google Scholar]
- 16.Hartshorn KL, Holmskov U, Hansen S, et al. Distinctive anti-influenza properties of recombinant collectin 43. Biochem J. 2002;366:87–96. doi: 10.1042/BJ20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartshorn KL, Collamer M, Auerbach M, Myers JB, Pavlotsky N, Tauber AI. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988;141:1295–1301. [PubMed] [Google Scholar]
- 18.Hartley CA, Jackson DC, Anders EM. Two distinct serum mannose-binding lectins function as beta inhibitors of influenza virus: identification of bovine serum beta inhibitor as conglutinin. J Virol. 1992;66:4358–4363. doi: 10.1128/jvi.66.7.4358-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartshorn K, Chang D, Rust K, White M, Heuser J, Crouch E. Interactions of recombinant human pulmonary surfactant protein D and SPD multimers with influenza A. Amer J Physiol. 1996;271:L753–762. doi: 10.1152/ajplung.1996.271.5.L753. [DOI] [PubMed] [Google Scholar]
- 20.Crouch EC, Smith K, McDonald B, et al. Species differences in the carbohydrate binding preferences of surfactant protein D. Am J Respir Cell Mol Biol. 2006;35:84–94. doi: 10.1165/rcmb.2005-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crouch E, Tu Y, Briner D, et al. Ligand specificity of human surfactant protein D: expression of a mutant trimeric collectin that shows enhanced interactions with influenza A virus. J Biol Chem. 2005;280:17046–17056. doi: 10.1074/jbc.M413932200. [DOI] [PubMed] [Google Scholar]
- 22.Hartshorn KL, White MR, Tecle T, Sorensen GL, Holmskov U, Crouch EC. Viral Aggregating and Opsonizing Activity in Collectin Trimers. Am J Physiol Lung Cell Mol Physiol. 2010;298:L79–88. doi: 10.1152/ajplung.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen S, Holm D, Moeller V, et al. CL-46, a Novel Collectin Highly Expressed in Bovine Thymus and Liver. J Immunol. 2002;169:5726–5734. doi: 10.4049/jimmunol.169.10.5726. [DOI] [PubMed] [Google Scholar]
- 24.Madsen J, Kliem A, Tornoe I, Skjolt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164:5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 25.Inoue T, Matsuura E, Nagata A, et al. Enzyme-linked immunosorbent assay for human pulmonary surfactant protein D. J Immunol Methods. 1994;173:157–164. doi: 10.1016/0022-1759(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 26.Hartshorn KL, Crouch EC, White MR, et al. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartshorn K, Sastry K, Chang D, White M, Crouch E. Enhanced antinfluenza activity of a recombinant pulmonary surfactant protein D and serum conglutinin fusion protein. Amer J Physiol. 2000;278:L90–98. doi: 10.1152/ajplung.2000.278.1.L90. [DOI] [PubMed] [Google Scholar]
- 28.Anders EM, Hartley CA, Jackson DC. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci U S A. 1990;87:4485–4489. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crouch E, Hartshorn K, Horlacher T, et al. Recognition of Mannosylated Ligands and Influenza A Virus by Human SP-D: Contributions of an Extended Site and Residue 343. Biochemistry. 2009;48:3335–3345. doi: 10.1021/bi8022703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen MJ, Laederach A, Reilly PJ, Mason RJ, Voelker DR. Arg343 in human surfactant protein D governs discrimination between glucose and N-acetylglucosamine ligands. Glycobiology. 2004 doi: 10.1093/glycob/cwh088. cwh088. [DOI] [PubMed] [Google Scholar]
- 31.Hakansson K, Lim N, Hoppe H, Reid K. Crystal structure of the trimeric alpha helical coiled-coil and the three lectin domains of human lung surfactant protein D. Structure. 1999;24:255–264. doi: 10.1016/s0969-2126(99)80036-7. [DOI] [PubMed] [Google Scholar]
- 32.Tecle T, White MR, Sorensen G, et al. Critical role for cross-linking of trimeric lectin domains of surfactant protein D in antiviral activity against influenza A virus. Biochem J. 2008;412:323–329. doi: 10.1042/BJ20071663. [DOI] [PubMed] [Google Scholar]
- 33.Hartshorn KL, White MR, Tecle T, et al. Reduced influenza viral neutralizing activity of natural human trimers of surfactant protein D. Respir Res. 2007;8:9. doi: 10.1186/1465-9921-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie X, Nishitani C, Yamazoe M, et al. Pulmonary Surfactant Protein D Binds MD-2 through the Carbohydrate Recognition Domain>. Biochemistry. 2008 doi: 10.1021/bi8010175. [DOI] [PubMed] [Google Scholar]
- 35.Yamazoe M, Nishitani C, Takahashi M, et al. Pulmonary surfactant protein D inhibits lipopolysaccharide (LPS)-induced inflammatory cell responses by altering LPS binding to its receptors. J Biol Chem. 2008;283:35878–35888. doi: 10.1074/jbc.M807268200. [DOI] [PubMed] [Google Scholar]
- 36.Konishi M, Nishitani C, Mitsuzawa H, et al. Alloiococcus otitidis is a ligand for collectins and Toll-like receptor 2, and its phagocytosis is enhanced by collectins. Eur J Immunol. 2006;36:1527–1536. doi: 10.1002/eji.200535542. [DOI] [PubMed] [Google Scholar]
- 37.Eda S, Suzuki Y, Kase T, et al. Recombinant bovine conglutinin, lacking N-terminal and collagenous domains, has less conglutination activity but is able to inhibit haemagglutination activity of influenza A virus. Biochem J. 1996;1996:43–48. doi: 10.1042/bj3160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eda S, Suzuki Y, Kawai T, et al. Structure of a truncated human surfactant protein D is less effective in agglutinating bacteria than the native structure and fails to inhibit haemagglutination by influenza A virus. Biochem J. 1997;323:393–399. doi: 10.1042/bj3230393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tacken PJ, Hartshorn KL, White MR, et al. Effective targeting of pathogens to neutrophils via chimeric surfactant protein D/anti-CD89 protein. J Immunol. 2004;172:4934–4940. doi: 10.4049/jimmunol.172.8.4934. [DOI] [PubMed] [Google Scholar]
- 40.Liou LS, Sastry R, Hartshorn KL, et al. Bovine conglutinin gene exon structure reveals its evolutionary relationship to surfactant protein-D. J Immunol. 1994;153:173–180. [PubMed] [Google Scholar]
- 41.Ohya M, Nishitani C, Sano H, et al. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry. 2006;45:8657–8664. doi: 10.1021/bi060176z. [DOI] [PubMed] [Google Scholar]
- 42.Palaniyar N, Clark H, Nadesalingam J, Shih MJ, Hawgood S, Reid KB. Innate immune collectin surfactant protein D enhances the clearance of DNA by macrophages and minimizes anti-DNA antibody generation. J Immunol. 2005;174:7352–7358. doi: 10.4049/jimmunol.174.11.7352. [DOI] [PubMed] [Google Scholar]
- 43.Clark H, Palaniyar N, Hawgood S, Reid KBM. A recombinant fragment of human surfactant protein D reduces alveolar macrophage apoptosis and pro-inflammatory cytokines in mice developing pulmonary emphysema. Ann N Y Acad Sci. 2003;1010:113–116. doi: 10.1196/annals.1299.019. [DOI] [PubMed] [Google Scholar]
- 44.Strong P, Reid K, Clark H. Intranasal delivery of a truncated recombinant human SP-D is effective at down-regulating allergic hypersensitivity in mice sensitized to allergens of aspergillus fumigatus. Clin Exp Immunol. 2002;130:19–24. doi: 10.1046/j.1365-2249.2002.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



