Abstract
Stomach cancer is one of the most common cancers worldwide, despite its declining overall incidence. Although there are differences in incidence, etiology and pathological factors, most studies do not separately analyze cardia and noncardia gastric cancer. Surgery is the only potentially curative treatment for advanced, resectable gastric cancer, but locoregional relapse rate is high with a consequently poor prognosis. To improve survival, several preoperative and postoperative treatment strategies have been investigated. Whereas perioperative chemotherapy and postoperative chemoradiation (CRT) are considered standard therapy in the Western world, in Asia postoperative monochemotherapy with S-1 is often used. Several other therapeutic options, although generally not accepted as standard treatment, are postoperative combination chemotherapy, hyperthermic intraperitoneal chemotherapy and preoperative radiotherapy and CRT. Postoperative combination chemotherapy does show a statistically significant but clinically equivocal survival advantage in several meta-analyses. Hyperthermic intraperitoneal chemotherapy is mainly performed in Asia and is associated with a higher postoperative complication rate. Based on the currently available data, the use of postoperative radiotherapy alone and the use of intraoperative radiotherapy should not be advised in the treatment of resectable gastric cancer. Western randomized trials on gastric cancer are often hampered by slow or incomplete accrual. Reduction of toxicity for preoperative and especially postoperative treatment is essential for the ongoing improvement of gastric cancer care.
Keywords: gastric cancer, surgery, chemotherapy, radiotherapy, standard of care
Introduction
Epidemiology
Gastric cancer is a major problem worldwide: it is the second leading cause of cancer death, affecting approximately 1 million new individuals per year [Kamangar et al. 2006]. Whereas the incidence in males is twice as high as in females, there is also a marked geographic variation. Highest incidence rates occur in North-East Asia (up to 70 per 100,000), Eastern Europe and much of the eastern part of South America, while lowest incidence rates are seen in North America (8 per 100,000), Africa and South and West Asia [Yamaoka et al. 2008]. Stomach cancers can anatomically be classified as noncardia (fundus, corpus and antrum) and cardia cancers, with noncardia cancers constituting the majority of all gastric cancers worldwide. Whereas the incidence of noncardia gastric cancer has declined over the past decades [Kelley and Duggan, 2003; Howson et al. 1986], there has been a rapid increase in the incidence of cardia gastric cancer until the early 1990s, which has not persisted in the current century [Steevens et al. 2010a; Wu et al. 2009; Pohl and Welch, 2005].
Carcinogenesis
Two distinct histologic types of gastric cancer have been defined by Lauren: an intestinal type, which is characterized by irregular tubular structures in areas of mucosal inflammation; and a diffuse type, which can be characterized by discohesive cells and pools of mucus [Lauren, 1965]. Gastric carcinogenesis of the intestinal type is thought to be a multifactorial process involving irritation of the mucosa by environmental factors, acid secretion and bacterial nitrite and N-nitroso compounds production from dietary nitrates. The intestinal type gastric cancer is mostly found in the distal stomach and typically arises through the Correa’s cascade, progressing from the successive steps of normal gastric epithelium infected by Helicobacter pylori, leading to acute and chronic gastritis, atrophic gastritis, intestinal metaplasia, dysplasia and finally gastric carcinoma [Correa, 1992; Correa et al. 1975]. Very little is known about the development of diffuse gastric cancer, although in the autosomal dominantly inherited syndrome of hereditary diffuse gastric cancer (HDGC), loss of polarity of gastric stem or progenitor cells has been suggested to lead to the formation of foci of signet ring cells that invade the lamina propria [Humar and Guilford, 2009; Carneiro et al. 2004].
Etiology
The childhood environment is an important factor in the risk of developing gastric cancer [Coggon et al. 1990; Kolonel et al. 1981].
Environmental risk factors for noncardia gastric cancer include H. pylori infection [Hansen et al. 2007; Eslick, 2006; Helicobacter and Cancer Collaborative Group, 2001], high intake of salt and salt-preserved foods [Tsugane and Sasazuki, 2007; Machida-Montani et al. 2004], low intake of vegetables and fruits [Nouraie et al. 2005], tobacco smoking [Steevens et al. 2010b; Ladeiras-Lopes et al. 2008], and achlorhydria [Svendsen et al. 1986]. Gastric atrophy has been positively associated with noncardia gastric cancer [Derakhshan et al. 2008; Hansen et al. 2007]. For cardia cancer, described risk factors are male sex, white race [Devesa et al. 1998], smoking and obesity [Abnet et al. 2008; Hjartaker et al. 2008], and gastroesophageal reflux disease [Derakhshan et al. 2008].
Of all cancers of the stomach, about 10% arise in individuals with a family history of gastric cancer [La Vecchia et al. 1992]. HDGC develops in subjects with a germline mutation in one allele of the E-cadherin gene (CDH1) [Guilford et al. 1999]. During a recent consensus meeting of the International Gastric Cancer Linkage Consortium, updated results on carriers of 58 families with a CDH1 mutation showed a more than 80% lifetime risk of developing diffuse gastric cancer [Fitzgerald et al. 2010]. Familial preponderance has been described in other familial cancer syndromes, such as Lynch syndrome [Gylling et al. 2007], Li–Fraumeni syndrome [Varley et al. 1995], and Peutz–Jeghers syndrome [Giardiello et al. 2000; Boardman et al. 1998] as well. In these families the intestinal type of gastric cancer prevails.
Staging
In the Western world, staging is performed according to the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) [UICC, 2009]. The Japanese Gastric Cancer Association has its own staging system of gastric carcinoma [Japanese Gastric Cancer Association, 1998]. Until recently, the Japanese staging of nodal status (N) was based on location of the positive nodes. Nowadays both Japanese and Western systems are based on the number of positive lymph nodes, which seems to be more reproducible, provided that a minimum number of 15 lymph nodes are removed and analyzed [Karpeh et al. 2000].
Tumors of the gastroesophageal junction (GEJ) are often misclassified as either gastric when they should be esophageal, or vice versa. In 2000, Siewert and colleagues proposed a classification based on anatomic location: type I (adenocarcinoma of the distal esophagus), type II (cardia carcinoma, arising from the GEJ), and type III (subcardial gastric carcinoma infiltrating the GEJ and esophagus from below) [Siewert et al. 2000]. In the latest, 7th edition of the TNM classification, tumors of the GEJ are all classified as esophageal cancer based on the worse prognosis of cardia and GEJ tumors as compared to mid and distal gastric tumors[Rusch et al. 2010]. Differences in stage grouping between the 6th and 7th edition of the AJCC staging system for gastric cancer are shown in Table 1 [Edge et al. 2010; Greene et al. 2002].
Table 1.
Stage grouping for gastric cancer according to the 6th (2002) and 7th (2010) edition of the AJCC staging system [Edge et al. 2010; Greene et al. 2002].
| 6th Edition AJCC staging system |
7th Edition AJCC staging system |
||||||
|---|---|---|---|---|---|---|---|
| Stage | T | N | M | Stage | T | N | M |
| 0 | Tis | N0 | M0 | 0 | Tis | N0 | M0 |
| IA | T1 | N0 | M0 | IA | T1 | N0 | M0 |
| IB | T1 | N1 | M0 | IB | T1 | N1 | M0 |
| T2 | N0 | M0 | T2 | N0 | M0 | ||
| II | T1 | N2 | M0 | IIA | T1 | N2 | M0 |
| T2 | N1 | M0 | T2 | N1 | M0 | ||
| T3 | N0 | M0 | T3 | N0 | M0 | ||
| IIB | T1 | N3 | M0 | ||||
| T2 | N2 | M0 | |||||
| T3 | N1 | M0 | |||||
| T4a | N0 | M0 | |||||
| IIIA | T2 | N2 | M0 | IIIA | T2 | N3 | M0 |
| T3 | N1 | M0 | T3 | N2 | M0 | ||
| T4 | N0 | M0 | T4a | N1 | M0 | ||
| IIIB | T3 | N2 | M0 | IIIB | T3 | N3 | M0 |
| T4a | N2 | M0 | |||||
| T4b | N0/1 | M0 | |||||
| IIIC | T4a | N3 | M0 | ||||
| T4b | N2/3 | M0 | |||||
| IV | T4 | N1-3 | M0 | IV | Any T | Any N | M1 |
| T1-3 | N3 | M0 | |||||
| Any T | Any N | M1 | |||||
T, tumor classification; N, nodal status; M, metastases status.
Survival
As more than half of the patients in the Western world present with stage III or IV gastric cancer, overall prognosis is poor [Hundahl et al. 2000]. A recent survey shows that 5-year survival in all gastric cancer patients in Europe is only 24.1% [Sant et al. 2009]. Survival for all patients in the US is comparable: in the period 1999–2005, survival was 26.5%. For patients with metastatic disease at initial presentation, 5-year survival is <5% [Horner et al. 2009]. In patients treated with surgery in the US in the period 1985–1996, stage-specific (AJCC 6th edition) 5-year survival was 58% for stage IB, 34% for stage II, 20% for stage IIIA and 8% for stage IIIB [Hundahl et al. 2000]. In contrast, Japan has 5-year survival rates of approximately 60% [Kamangar et al. 2006]. This difference has been addressed to mass screening programs using photofluorography [Hamashima et al. 2008], differences in tumor biology and location with more intestinal subtypes and distal locations, and stage migration due to higher lymph node yield in Japanese series [Bunt et al. 1995]. In a comparative analysis between a US and a Korean center, multivariate analysis applying different patient and tumor characteristics and the number of resected lymph nodes, shows a higher disease-specific survival for Korean patients as compared with US patients (hazard ratio [HR] 1.3), suggesting the possibility of an intrinsic biologic difference between gastric cancer in the US and Korea [Strong et al. 2010].
Recurrence patterns
With increasing cancer stage, the risk of locoregional relapse increases, thus diminishing survival. In a combined analysis of several autopsies series, eventually 80–93% of all patients developed locoregional relapse [Gunderson, 2002]. A retrospective study on 367 patients with clinically complete recurrence data in a single center revealed that 54% of recurrences were locoregional, whereas distant sites were involved in 51%. Of all recurrences, 79% developed within the first 2 years [D’Angelica et al. 2004]. In a single-center study performed during 1949–1971, reoperations as second-look procedures in 107 previously resected gastric cancer patients, both symptomatic and asymptomatic, revealed locoregional failure in 23% as the only site of relapse [Gunderson and Sosin, 1982]. Data from a US randomized trial showed the highest relapse in locoregional sites, even after postoperative chemoradiation had been administered [Macdonald et al. 2001].
Surgical treatment
Resection is a prerequisite for the curative treatment of localized gastric cancer. It can be divided into three major approaches: endoscopic (sub) mucosal resection (EMR) or dissection (ESD), minimally invasive surgery, and open gastrectomy. Endoscopic mucosal resection is only used for the treatment of early gastric cancer (EGC), which is defined as a tumor of the stomach limited to the mucosa or submucosa regardless of lymph node metastases [Kitaoka et al. 1984]. This topic is not further covered in this review.
Laparoscopic surgery
Minimal invasive surgery for the treatment of gastric cancer is mainly performed in Korea and Japan, with the majority of patients treated for early and distal gastric cancer. However, with increasing laparoscopic experience and improvement in instrumentation, more extensive procedures and treatment of more advanced gastric cancers is becoming more common. Although laparoscopic gastrectomy has been performed since 1991, only four, mostly single-center, randomized controlled trials comparing the technique with open gastrectomy have been reported [Kim et al. 2010; Hayashi et al. 2005; Huscher et al. 2005; Lee and Han, 2005; Kitano et al. 2002]. Laparoscopic gastrectomy has been discussed in two reviews which indicate oncologic equivalency and safety based on the current small patient numbers [Shehzad et al. 2007; Shiraishi et al. 2006]. Large multicenter randomized controlled trials are necessary to establish the role of laparoscopy in the treatment of gastric cancer.
Extent of gastric resection and margins
Total gastrectomy is the indicated treatment for tumors located in the proximal or middle third of the stomach [Maruyama et al. 1996]. As compared with a total gastrectomy, a proximal gastrectomy for proximal gastric cancer is associated with a markedly higher rate of complications such as anastomotic stenosis and weight loss [An et al. 2008]. For distal gastric cancer, a distal gastrectomy is the recommended therapy provided that an adequate margin can be obtained. Two randomized trials investigated the impact of total versus distal gastrectomy for distal gastric cancer, and showed no difference in postoperative morbidity, mortality, or overall survival with more extensive resection [Bozzetti et al. 1999; Gouzi et al. 1989].
Microscopically positive resection margins (R1) are associated with a significantly worse prognosis as compared with a microscopically radical (R0) resection, especially in patients with early stage disease [Cho et al. 2007; Kim et al. 1999]. An Italian study investigated the minimal margin that should be obtained to ensure radical surgery in T3–4 tumors, and suggested a minimum margin of 6 cm [Bozzetti, 2001]. Data from the Netherlands show that survival in patients with an R1 resection is comparable with patients with positive cytology after abdominal washing [Songun et al. 1996], indicating that intra-operative frozen-section examination is mandatory for potentially curative resections of gastric cancer.
Lymph node dissection
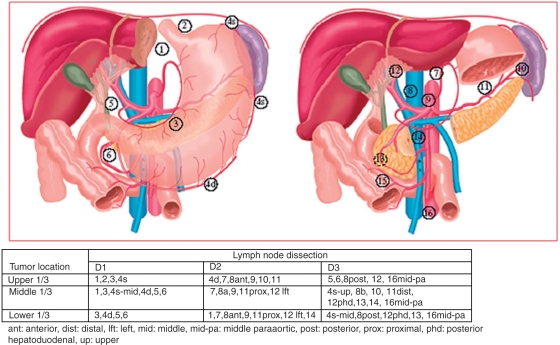
As the primary tumor penetrates more deeply through the wall of the stomach, the risk of lymph node metastases increases. The Japanese Classification of Gastric Carcinoma [Japanese Gastric Cancer Association, 1998] defined 16 different lymph node stations surrounding the stomach (Figure 1), which are divided into three groups, each group further away from the primary tumor site. In a D1 dissection, the stomach (total or distal) plus the perigastric lymph nodes are removed. For a D2 dissection, additional removal of the nodes along the left gastric, the common hepatic, the splenic and the left hepatoduodenal artery is performed as well as some stations that are different for proximal, middle and distal tumors. With a D3 dissection, an even more extended lymphadenectomy is performed, including paraaortic and posterior hepatoduodenal nodes. For adequate staging a minimum of 15 lymph nodes should be evaluated [Karpeh et al. 2000].
Figure 1.
Lymph node stations as defined by the Japanese Research Society for Gastric Cancer [Japanese Gastric Cancer Association, 1998], with nodal stations defined for each type of lymph node dissection. Originally published by the American Society of Clinical Oncology [Dikken et al. 2010].
Three prospective randomized trials have been performed that compared D1 with D2 lymph node dissection [Hartgrink et al. 2004a; Cuschieri et al. 1999; Dent et al. 1988]. In an early trial, 43 patients were randomized between a D1 or D2 dissection, and with a median follow up of 3.1 years no differences in survival were detected [Dent et al. 1988]. A British trial that randomized 400 patients for D1 or D2 dissection, showed equal 5-year survival rates (35% versus 33%), but increased postoperative mortality and morbidity in the D2 group (13% versus 7% and 46% versus 28%) [Cuschieri et al. 1999, 1996]. In the Dutch Gastric Cancer Group Trial (DGCT), 711 patients underwent a D1 or D2 gastrectomy. Initial results showed an increased morbidity (25% versus 43%) and mortality (4% versus 10%) in the D2 group, which could be partially attributed to the higher number of splenectomies and pancreatectomies in this group [Bonenkamp et al. 1999], while there was no significant difference in 11-year survival rates (30% versus 35%) [Hartgrink et al. 2004a]. However, a recent update revealed that gastric cancer-related death rate after a median follow up of 15.2 years was significantly higher in the D1 group (48%) compared with the D2 group (37%) [Songun et al. 2010], indicating that a D2 dissection is the recommended type of surgery in Western countries, especially when postoperative mortality can be avoided.
In Japan, a D2 lymph node dissection is seen as standard treatment for curative resections [Nakajima, 2002]. Convinced of the benefits of extended lymph node dissection, Japanese surgeons consider it generally unethical towards patients to run a randomized trial including an arm with a D1 lymph node dissection. A Japanese trial randomizing 523 patients for D2 alone or D2 combined with paraaortic node dissection showed no significant difference in 5-year survival while there was a trend towards more surgery-related complications in the paraaortic group (28% versus 21%) [Sasako et al. 2008; Sano et al. 2004]. In a Taiwanese study with 221 patients, for the first time the benefit of a D3 over a D1 lymph node dissection was detected: 5-year overall survival was significantly higher in the D3 group (60% versus 54%) [Wu et al. 2006].
In conclusion, in Western countries there has been an extensive debate on the role of a D2 lymph node dissection, which can now be considered a recommended type of surgery for advanced gastric cancer, with removal of at least 15 lymph nodes for adequate staging. In Asian countries at least a D2 dissection is performed.
Accepted adjuvant and neoadjuvant therapies
Because adequate locoregional or systemic control is difficult to obtain with resection alone, surgery can be combined with adjuvant or neoadjuvant treatment. A distinction between accepted and nonstandard adjuvant and neoadjuvant therapies is provided in Table 2. Randomized studies on adjuvant and neoadjuvant treatment of gastric cancer are summarized in Table 3.
Table 2.
Currently available treatment strategies for advanced, resectable gastric cancer.
| Therapy | Supporting data | Comments | |
|---|---|---|---|
| Accepted standard therapy | Postoperative chemotherapy | Sakuramoto et al. [2007] | In Asia S-1 monotherapy is considered standard therapy in resectable gastric cancer |
| Postoperative chemoradiotherapy | Macdonald et al. [2001], Kim et al. [2005] | ||
| Perioperative chemotherapy | Cunningham et al. [2006], Boige and Pignon [2007] | Low compliance for postoperative chemotherapy | |
| Nonstandard or encouraging therapy | Preoperative chemotherapy | Hartgrink et al. [2004b], Schuhmacher et al. [2009] | Underpowered studies |
| Postoperative combination chemotherapy | Sun et al. [2009] | Only positive in meta-analyses, absolute survival benefit ≤ 5% | |
| Hyperthermic Intraperitoneal Chemotherapy |
Yan et al. [2007] | Small studies, high postoperative complication rate, mainly performed in Asia | |
| Preoperative radiotherapy | Fiorica et al. [2007], Valentini et al. [2009] | ||
| Preoperative chemoradiotherapy | Ajani et al. [2006a, 2005, 2004] | Only phase II studies | |
| No role or inadequate data | Postoperative radiotherapy | Valentini et al. [2009] | Meta-analysis with a limited number of studies and heterogeneous design |
| Intraoperative radiotherapy | Sindelar et al. [1993], Kramling et al. [1996], Skoropad et al. [2000] | Underpowered studies | |
Table 3.
Randomized studies on preoperative and postoperative therapy in gastric cancer.
| Trial | N | Stage | Loc | Cardia | Intervention arm | Control arm | Treatment compliance (months of treatment) | Median follow up (years) | Survival (intervention versus control) | Relapse-free survival (intervention versus control) | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Postoperative chemotherapy | |||||||||||
| Sakuramoto et al. [2007] | 1059 | II-IIIB | GC | 1%2 | SURG: R0, D2-D3 | SURG: R0, D2-D3 | 66% (12) | 2.9 | 3y80% versus 70% (P=0.003) | 3y72% versus 60% (P<0.001) | |
| - 1 y cycles of 6 wks: | |||||||||||
| - 4 wk 40 mg/m2 S-1 bid, 2 wk off | |||||||||||
| Postoperative chemoradiotherapy | |||||||||||
| Macdonald et al. [2001] | 556 | Ib-IV | GC | 7%3 | SURG: R0, D0-2 | SURG: D0-2 | 64% | 5 | 5y40% versus 28% (P=0.005) | 5y31% versus 25% (P<0.001) | 54% D0 36% D1 10% D2 |
| - 5-FU 425 mg/m2/day plus L 20 mg/m2/day for 5 days | |||||||||||
| - 45Gy EBRT, 5 wks, 1,8Gy/day | |||||||||||
| - 5-FU 400 mg/m2/day + L 20 mg/m2/day on first 4 and last 3 days of EBRT | |||||||||||
| - after 1 month: 2 5-day cycles of 5-FU and L | |||||||||||
| Kim et al. [2005] | 1000 | Ib-IV | GC | 10%3 | SURG: R0, D2 CT/EBRT: same as Macdonald et al. [2001] |
SURG: R0, D2 | 75% | 5.5 | 5y57% versus 51% (P=0.02) | 5yHR 0.8 (P=0.02) | Observational study |
| Combined preoperative and postoperative treatment | |||||||||||
| Cunningham et al. [2006] | 503 | II-IV | ALE, GEJ, GC | 11.5%3 | 3 preoperative and 3 postoperative cycles of 3 weeks | SURG: 69.3% R0, D1-2 | 42% (6) | 2 | 5y36% versus 23% P=0.009 | HR 0.66 | |
| - E 50 mg/m2 day 1 | |||||||||||
| - C 60 mg/m2 day 1 | |||||||||||
| - 5-FU 21 days gastrectomy (R0 66.4%, D1-D2) | |||||||||||
| Boige and Pignon [2007] | 224 | - | ALE, GEJ, GC | 64% GEJ | 2-3 cycles of 28 days- C 100 mg/m2- 5-FU 800 mg/m2, day 1-5 gastrectomy (84% R0) | SURG: 73% R0 | 48% (3) | 5.7 | 5y38% versus 24% | 5y34% versus 21% | Preliminary data |
| Postoperative/intraoperative radiotherapy | |||||||||||
| Hallissey et al. [1994] | 436 | II-III | GC | - | SURGgroup 1: MMC 4 mg/m2, F 30 mg/m2, 5-FU 600 mg/m2 iv, 8 cycles every 3 wks group 2: EBRT: 25x1,8Gy in 35 days + 1x 5 Gy boost | SURG | 1) 42%2) 66% | 7 | 5y19% versus 12% versus 20% (NS) | - | |
| Sindelar et al. [1993] | 41 | I-IV | GC | - | SURG IORT 20 Gy | st. I-II: SURG st. III-IV: SURG + EBRT 50 Gy in 5–6 wk | - | 7 | 25 m versus 21 m (NS) | 12 m versus 16 m (NS) | |
| Kramling et al. [1996] | 115 | - | - | - | SURG + IORT 28 Gy | SURG | - | 2.5 | 27m versus 31 m(NS) | - | |
| Skoropad et al. [2000] | 78 | - | GC, GEJ | 23%3 | SURG: R0, D1-5x4 Gy pre-op EBRT- 20 Gy IORT | SURG: R0, D1 | 100% | - | HR 1.03 (NS) | - | |
| Preoperative chemotherapy | |||||||||||
| Hartgrink et al. [2004b] | 59 | I-IV | GC | - | 4 monthly courses of- MTX 1500 mg/m2, 5-FU 1500mg/m2- L 30 mg every 6h, 8x- Dox 30 mg/m2 gastrectomy (46% R0) | gastrectomy (59% R0) | 56% (4) | 6.9 | 5y21% versus 34% | - | trial stopped preliminary after interim analysis |
| Schuhmacher et al. [2009] | 144 | T3-4 | GC, GEJ | 53% | 2 48-day cycles of- L 500 mg/m2 weekly- 5-FU 2000 mg/m2/24h weekly- C 50 mg/m2/1h biweeklySURG | Gastrectomy (66.7% r0) | 63% (2) | 4.4 | HR 0.84 (p = 0.065) | - | Closed early due to poor accrual. Preliminary data |
| Biffi et al. [2010] | 69 | Ib-IV | GC | - | 4 cycles of- Doc 75 mg/m2 1x- C 75 mg/m2 1x- 5-FU 300 mg/m2/day for 2 weeksSURG | SURG4 cycles of- Doc 75 mg/m2 1x- C 75 mg/m2 1x- 5-FU 300 mg/m2/day for 2 wk | 75% (2) versus 34% (2) | - | - | - | |
| Preoperative radiotherapy | |||||||||||
| Zhang et al. [1998] | 370 | I-IV | cardia | 100%2 | EBRT 20x2 Gy in 4 weeksSURG: 80% R0 | SURG: 61.8% R0 | - | 10 | 5y30% versus 20% | - | |
| Skoropad et al. [2002] | 102 | I-IV | GC | 29%2 | EBRT 5x4 Gy in 5d SURG: 89% R0 |
SURG: 80% R0 | 100% (5d) | - | 5y39% versus 30% (NS) | - | |
| Shchepotin et al. [1994] | 293 | T2-4N any | GC | - | (1) EBRT 4x5 Gy in 4d (2) EBRT 4x5 Gy + hyperthermia in 4 days |
(3) SURG | - | - | 5y45% (1) versus 30% (3)51% (2) versus 30% (3) | - | |
| SURG | |||||||||||
| Preoperative chemoradiotherapy | |||||||||||
| No phase III trials | |||||||||||
Specification of cardia cancer: 1true cardia, 2stomach divided into two parts (upper/lower), 3stomach divided into three parts (upper/middle/lower).
3y: 3-year survival, 5y: 5-year survival.
GC: gastric cancer, GEJ: cancer of the gastroesophageal junction, ALE: adenocarcinoma of the lower esophagus, EBRT: external beam radiotherapy, SURG: surgery, IORT: intraoperative radiotherapy.
Dox: doxorubicin, F: doxyfluridine, L: leucovorin, MMC: mitomycin C, MTX: methotrexate, C: cisplatin, Doc: docetaxel, 5FU: 5-fluorouracil; E: epirubicin wk: weeks, y: years, m: months, NS: nonsignificant.
Postoperative chemotherapy
Adjuvant chemotherapy may eliminate occult residual locoregional or metastatic disease after surgery. More than 30 randomized trials have been performed evaluating adjuvant chemotherapy in gastric cancer over the past two decades. Although the earlier trials were small, during the last decade trials with up to 400 patients have been performed in Southern Europe. Most find a small survival benefit, which is mostly nonsignificant [Cascinu et al. 2007; De Vita et al. 2007; Nitti et al. 2006; Bouche et al. 2005; Bajetta et al. 2002]. Different treatment regimens were tested, including 5-fluorouracil-based chemotherapy with or without anthracyclines, with or without mitomycin C, and platinum with etoposide. Most of these studies are included in several meta-analyses [Sun et al. 2009; Hu et al. 2002; Janunger et al. 2002; Panzini et al. 2002; Mari et al. 2000; Hermans et al. 1993; Earle and Maroun, 1999], which all except for one [Hermans et al. 1993] show a small, significant increase in survival for adjuvant chemotherapy of 3–5% (Table 4). However, the benefit of this increase in daily clinical practice is modest.
Table 4.
Meta-analyses on adjuvant chemotherapy.
| Number of trials | Number of patients | Mortality risk | 95% Confidence interval | West/East | |
|---|---|---|---|---|---|
| Hermans et al. [1993] | 11 | 2096 | 0.88 (OR) | 0.72–1.08 | Both |
| Earle and Maroun [1999] | 13 | 1990 | 0.80 (OR) | 0.66–0.97 | West |
| Mari et al. [2000] | 20 | 3658 | 0.82 (RR) | 0.75–0.89 | Both |
| Hu et al. [2002] | 14 | 4543 | 0.56 (OR) | 0.40–0.79 | Both |
| Panzini et al. [2002] | 18 | 3118 | 0.72 (OR) | 0.62–0.84 | Both |
| Janunger et al. [2002] | 21 | 3962 | 0.84 (OR) | 0.74–0.96 | Both |
| Sun et al. [2009] | 12 | 3809 | 0.78 (OR) | 0.71–0.85 | Both |
OR: odds ratio, RR: relative risk.
Sakuramoto and colleagues were the first to show a significant benefit in overall survival for postoperative chemotherapy in a large, adequately powered trial performed in an Asian patient population. In this study 1059 patients with stage II/III gastric cancer were randomized following at least D2 and R0 resection between surgery alone or surgery plus S-1 (oral fluoropyrimidine) for 12 months. Compliance after 12 months of chemotherapy was 66%. After 3 years, overall survival (80% versus 70%) and relapse-free survival (72% versus 60%) were significantly higher in the chemotherapy group [Sakuramoto et al. 2007]. Experience with S-1 in Western populations is limited to a combination chemotherapy study in patients with advanced, untreated gastroesophageal cancer [Ajani et al. 2010].
Overall, many early trials showed no or little advantage of postoperative chemotherapy. However, meta-analyses indicate a statistically significant but clinically equivocal survival benefit for adjuvant chemotherapy. Whereas Western trials focus on multidrug regimens, in Japan S-1 is considered to be of superior value. Compliance for postoperative chemotherapy remains a problem: in most Western studies 4–6 months of combination chemotherapy gives compliance rates from 87% to 43%, with hematological and gastrointestinal toxicities as the main reasons for not completing the treatment schedule. None of the randomized trials distinguished between cardia or noncardia cancer.
Postoperative chemoradiotherapy
Radiosensitizing drugs, such as 5-fluorouracil, have been added to radiotherapy with the intent to enhance the cytotoxic effect of radiotherapy on locoregional occult residual disease and to reduce locoregional relapse. Four early randomized trials showed the benefit of 5-fluorouracil-based chemoradiotherapy (CRT) over surgery alone [GITSG, 1990, 1982; Klaassen et al. 1985; Moertel et al. 1984], while another early study was negative[Dent et al. 1979]. However, patient numbers in these studies were small (N = 62–191), limiting the value of this observation.
The key trial supporting the role of adjuvant CRT was the US Intergroup 0116 trial [Macdonald et al. 2001], in which 556 patients with stage Ib to IV gastric cancer who had received an R0 resection were randomized to no further treatment or postoperative CRT. Adjuvant treatment consisted of one cycle of 5-fluorouracil, leucovorin and 45 Gy of radiation with 7 days of 5-fluorouracil administered in 5 weeks, followed by two more cycles of 5-fluorouracil plus leucovorin. Treatment compliance in the CRT group was 64%; 17% stopped treatment because of mostly hematologic and gastrointestinal side effects. Major reasons for premature discontinuation in the other patients were early disease progression or patient’s request. Overall survival at 5 years was significantly higher in the CRT group (40% versus 28%), which was confirmed in a recent update with follow up of over 10 years [Macdonald et al. 2009]. Owing to the results of this trial, postoperative CRT is currently a standard option in the United States for patients undergoing curative resection of stage Ib–IV gastric cancer who did not receive neoadjuvant therapy [Ajani et al. 2006a]. However, the study has been criticized for the complexity of the CRT protocol, the limited interaction between chemotherapy and radiotherapy, the lack of surgical quality control, and because patients were highly selected (only R0 resections with adequate postoperative recovery). Furthermore, CRT might have compensated for the low number of extended lymph node dissections, with only 10% of the patients undergoing a D2 dissection and 54% receiving a D0 dissection.
At the same time, an observational study from South Korea compared 446 patients who underwent D2 gastrectomy with 544 patients that underwent D2 gastrectomy followed by CRT per the Intergroup 0116 protocol [Kim et al. 2005]. After a median follow-up of 66 months, there was a significant benefit in survival in the CRT group (57% versus 51%), indicating the potentially beneficial role of postoperative CRT also after extended lymphadenectomy. A Dutch observational study comparing 694 patients who underwent D1 or D2 surgery with 91 patients who underwent postoperative fluoropyrimidine-based CRT showed improved local control in the CRT group after a D1 dissection, but not following a D2 dissection [Dikken et al. 2010]. After an R1 resection, postoperative chemoradiation was significantly associated with better survival.
In a meta-analysis of postoperative CRT, 5-year overall survival is significantly higher with CRT as compared to surgery alone (odds ratio [OR] 0.45, 95% confidence interval [CI] 0.32–0.64). Despite a higher frequency of severe and life-threatening toxicities in the CRT group, overall compliance for the CRT was 73%. The majority of patients in this analysis are nonetheless derived from the Intergroup trial [Fiorica et al. 2007].
Several phase I/II studies on CRT with new types of chemotherapy have been performed to improve the interaction between chemotherapy and radiotherapy. A study from Germany in which patients were treated with 45 Gy of radiotherapy plus folinic acid, 5-fluorouracil, paclitaxel and cisplatin, showed that this four-drug regimen had an acceptable toxicity profile [Kollmannsberger et al. 2005]. Three studies from the Netherlands demonstrated the feasibility of radiotherapy combined with daily capecitabine and cisplatin [Jansen et al. 2009, 2007a, 2007b]. Radiotherapy fields contained the gastric bed and the anastomosis, with lymph node regions depending on the location of the primary tumor A side study on renal toxicity in 44 patients from these studies showed that there is a progressive relative functional impairment of the left kidney after postoperative CRT for gastric cancer, emphasizing that radiotherapy doses to the kidney should be minimized by using newer techniques such as intensity modulated radiotherapy (IMRT) in order to reduce toxicity while gaining the full benefit of survival of postoperative CRT [Jansen et al. 2007c].
In conclusion, postoperative CRT shows an advantage in survival over surgery alone, but the question remains as to whether this effect persists after an extended lymphadenectomy and radical resection. New treatment regimens on CRT opting for equal or better efficacy and reduced toxicity are currently under investigation.
Perioperative chemotherapy
The most important limitation of postoperative therapy is the impaired patient performance status after a gastrectomy that can hamper or even prevent delivery of the planned adjuvant treatment [Bozzetti et al. 2007]. Part of this is caused by the nutritional status and insufficient nutritional support that is given in this patient group prone to major weight loss [Bozzetti et al. 2007, 2001]. For this reason, the concept of neoadjuvant treatment might be a valuable alternative, while the postoperative therapy still can be administered when tolerated. The main goal of giving neoadjuvant chemotherapy is to treat micrometastatic disease at an early stage and to improve resectability by tumor downsizing and downstaging [Cunningham et al. 2006].
In the beginning of the 1990s the concept of perioperative chemotherapy was tested for its feasibility in a small study, showing a compliance rate of 72% and an acceptable toxicity profile [Ajani et al. 1991]. The MRC Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial, randomized 503 patients with advanced (more than submucosal), resectable adenocarcinoma of the stomach, esophagogastric junction, or lower esophagus for surgery and perioperative chemotherapy versus surgery alone. Chemotherapy consisted of three preoperative and three postoperative cycles of epirubicin, cisplatin and 5-fluorouracil. R0 resection rates were 66% and 69% for the two groups in favor of the chemotherapy group, and 40% of all resections were D2 lymph node dissections. Whereas 86% of the patients completed the preoperative chemotherapy schedule, only 55% started postoperative chemotherapy and subsequently 42% completed all six courses. The most important reasons for not starting or finishing postoperative chemotherapy were early progressive disease or death, patient’s request and postoperative complications. With a median follow up of 48 months, 5-year overall survival was significantly higher in the chemotherapy group (36% versus 23%) with no differences according to tumor site. No differences in postoperative morbidity and mortality were observed between the two treatment groups [Cunningham et al. 2006].
A French prospective trial randomized 224 patients with adenocarcinoma of the stomach (25%), the GEJ (64%) or lower esophagus (11%) between chemotherapy plus surgery (N = 113) or surgery alone (N = 111). Chemotherapy consisted of two or three cycles of preoperative 5-fluorouracil and cisplatin and was continued after surgery in case of response to preoperative chemotherapy or stable disease with pN+. Compliance for the preoperative therapy was 87%, whereas 48% of the patients completed the total regimen. With a median follow up of 5.7 years, 5-year overall and disease-free survival were significantly higher in the chemotherapy group (38% versus 24% and 34% versus 21%) [Boige and Pignon, 2007]. Although the final report of this initially in 2007 presented study has still to be awaited, the results are quite similar to the MAGIC study with better outcomes with perioperative chemotherapy when compared with surgery alone.
Only a few studies have been performed on preoperative chemotherapy without postoperative treatment. In a randomized trial from the Netherlands, 59 patients were treated with surgery alone (N = 30) or chemotherapy with 5-fluorouracil, doxorubicin and methotrexate (FAMTX) followed by surgery (N = 29). This trial was discontinued before total accrual was achieved because of poor accrual and a low R0 resection rate in the neoadjuvant group. With a median follow up of 83 months, this study did not show a difference in overall survival [Hartgrink et al. 2004b]. An EORTC study randomized 144 patients between surgery versus surgery preceded by folinic acid, 5-fluorouracil and cisplatin. Again, due to poor accrual, the trial was closed early. Although the R0 resection rate was actually lower in the neoadjuvant chemotherapy group (82% versus 67%), there was no difference in overall survival [Schuhmacher et al. 2009].
Based on these underpowered studies, it is difficult to draw conclusions about the role of preoperative chemotherapy without postoperative therapy.
The choice between established treatment paradigms
Whereas adjuvant chemotherapy with S-1 is an established regimen in Japan, the Western debate currently focuses on the use of postoperative chemoradiation versus perioperative chemotherapy. While the Intergroup 0116 study only included patients with an R0 resection and adequate postoperative recovery, the MAGIC study included all patients that were eligible for curative surgery. Therefore, the results of the Intergroup 0116 [Macdonald et al. 2001] and MAGIC [Cunningham et al. 2006] studies are incomparable with regards to treatment adherence and survival. In both studies, most toxicities were hematological or gastrointestinal, but due to a different way of reporting on the number of adverse effects, toxicity profiles cannot be compared either. However, what these studies do indicate is that the toxicity profile of the chemotherapy and radiation regimen is critical for the individual patient to complete therapy, and consequently for trials to complete accrual.
To compare preoperative with postoperative chemotherapy, a Swiss/Italian study randomized 70 patients for docetaxel, cisplatin and 5-fluorouracil either before or after surgery. This trial closed early because of poor accrual. In the neoadjuvant group, 75% completed the whole treatment schedule, as compared with 34% in the postoperative group (66% started with postoperative chemotherapy). Neoadjuvant chemotherapy could be delivered with a higher dose intensity without decreasing the chances for radical surgery or an increase in perioperative mortality [Biffi et al. 2010].
Based on these results, preoperative chemotherapy should be considered standard treatment in patients with advanced (more than submucosal), resectable gastric cancer. With a significantly higher compliance rate as compared with postoperative therapy, it not only reduces tumor burden, but also increases the chance for an R0 resection. When tolerated, adjuvant therapy should also be administered, but no standard regimen for this has been established. Patients with (distant) micrometastases will benefit more from systemic chemotherapy, but so far there is no adequate diagnostic modality or molecular marker to identify distant micrometastases. A different approach on predicting the efficacy of postoperative chemotherapy is grading histological response in the resection specimen after preoperative chemotherapy. Such a response, however, has not proven to be associated with survival in a US study [Mansour et al. 2007]. Patients at high risk for a local recurrence, for example patients who undergo an R1 resection, may benefit most from postoperative chemoradiation [Dikken et al. 2010], although this has not been addressed in a prospective study yet.
Questions on the use of postoperative chemotherapy or chemoradiation, after preoperative chemotherapy and surgery, are prospectively addressed in the Dutch CRITICS trial (see http://www.critics.nl), in which patients receive three cycles of preoperative ECC (epirubicin, cisplatin, and capecitabine), followed by D1+ surgery (D2 dissection without a splenectomy or pancreatectomy). Postoperative therapy consists of another three cycles of ECC, or CRT with capecitabine and cisplatin without epirubicin.
Nonstandard adjuvant and neoadjuvant therapies
Intraperitoneal chemotherapy
With a curative resection for gastric cancer, positive peritoneal washings occur in 7% of the patients [Ribeiro et al. 2006], whereas more than 50% will develop a peritoneal carcinomatosis at some point during follow up. Risk factors for positive cytology include serosal invasion and lymph node metastases [Bonenkamp et al. 1996]. The concept of intraoperative intraperitoneal chemotherapy (IPC) has been tested in several trials on gastric cancer. IPC can be combined with hyperthermia (HIPC) and can also be administered directly after surgery (early postoperative intraperitoneal chemotherapy [EPIC]).
Most trials on IPC are included in a meta-analysis, which reports on studies where patients received normothermic IPC, HIPC, or EPIC with or without postoperative systemic chemotherapy. Patient numbers of the 10 included, and mostly Asian, studies varied from 67 to 268. This meta-analysis showed a significant improvement in survival with HIPC alone (HR = 0.60, 95% CI 0.43–0.83) and HIPC combined with EPIC (HR = 0.45, 95% CI 0.29–0.68). There was also a trend towards improved survival with IPC, but this was not significant in combination with either EPIC alone or delayed (after recovery from surgery) postoperative intraperitoneal chemotherapy. Intraperitoneal chemotherapy was associated with higher risks of neutropenia and intra-abdominal abscess [Yan et al. 2007].
A more recent large Korean study, that was reported in abstract form only and was not included in the meta-analysis, randomized 640 patients with serosa-positive, but M0 resectable gastric cancer to adjuvant systemic mitomycin C and doxifluridine with or without IPC with cisplatin. With a median follow up of 3.5 years, overall survival was significantly higher in the IPC group (71% versus 60%) [Kang and Chang, 2008]. This study can be criticized because of differences in the adjuvant chemotherapy schedule [Kang et al. 2008].
Summarizing, HIPC in Asian trials is associated with a significant benefit in survival, at the cost of an increased postoperative complication rate. Therefore, this treatment modality is used with restraint in Western countries, and is considered an investigational strategy, not intended for standard daily practice.
Postoperative/intraoperative radiotherapy
Several studies investigated the effect of postoperative and intraoperative radiotherapy. A British randomized study with 436 patients found no difference in 5-year survival between surgery alone, surgery plus radiotherapy (45–50 Gy) or surgery plus chemotherapy (mitomycin C, doxorubicin, and 5-fluorouracil) postoperatively. Compliance for the protocol-defined dose in the radiotherapy group was 66%, with poor patient condition and withdrawal of consent as the most important reasons for failure [Hallissey et al. 1994]. A meta-analysis reporting on preoperative and postoperative radiotherapy also revealed no significant difference for postoperative radiation [Valentini et al. 2009].
Intraoperative radiotherapy (IORT) has been tested in several relatively small trials. In an American randomized trial, 41 patients were treated with surgery (control arm: early stages) and postoperative radiotherapy (control arm: advanced stages), or with surgery and IORT (experimental arm: all stages). Locoregional recurrence rates were lower for the IORT group (44% versus 92%, p < 0.001), but this did not translate into a difference in survival. There were no differences in complication rates [Sindelar et al. 1993]. A German study that randomized 115 patients for surgery or surgery plus IORT (1 × 28 Gy) also did not show a significant difference in overall survival [Kramling et al. 1996]. A Russian study, however, did show longer survival after IORT in a post hoc subgroup analysis: 78 patients received either preoperative radiotherapy (5 × 4 Gy) followed by surgery with 20 Gy IORT, or surgery alone. Although there was no survival difference between the two groups, for patients with T3–4 disease or lymph node involvement a significant benefit in survival for the radiotherapy group was reported [Skoropad et al. 2000].
Based on these underpowered studies, adjuvant radiotherapy as single modality following surgery has no role in routine daily clinical practice. IORT might be further investigated in patients with unfavorable tumor characteristics.
Preoperative radiotherapy
In a Chinese prospective randomized trial, 370 patients with cardia gastric cancer were randomized for surgery alone or preoperative radiotherapy (20 × 2 Gy in 4 weeks) followed by surgery after 2–4 weeks. The 5-year survival rates were 30% for the RT group as compared with 20% for the surgery alone group with a higher R0 resection rate in the RT group and no statistical difference in postoperative mortality and morbidity. Increased pathologic response rate to radiotherapy correlated with increased survival [Zhang et al. 1998].
A Russian study randomized 102 patients with resectable gastric cancer to radiotherapy (5 × 4 Gy in 1 week) plus surgery within 5 days or surgery only. Tolerance of the radiotherapy scheme was acceptable. The difference in 5-year overall survival between the two groups (39% versus 30%) did not reach statistical significance. Subgroup analysis showed a tendency towards better survival in the radiotherapy group in locally advanced gastric cancer (T4 and tumor positive lymph nodes) [Skoropad et al. 2002]. To investigate the effect of hyperthermia added to preoperative radiotherapy, a Ukrainian–American study randomized 293 patients between surgery, surgery preceded by radiotherapy (4 × 5 Gy), and surgery with a similar short course of preoperative radiotherapy and hyperthermia. Radiotherapy showed no significant benefit over surgery alone, but hyperthermia in combination with the radiotherapy significantly improved 5-year survival compared with surgery alone (51% versus 30%) [Shchepotin et al. 1994].
A meta-analysis based on the abovementioned three trials showed an advantage of neoadjuvant radiotherapy over surgery alone in 3- and 5-year survival (OR 0.57 and 0.62) [Fiorica et al. 2007]. Another meta-analysis on preoperative, intraoperative, and postoperative radiotherapy showed a significant increase in 3- and 5-year survival as well (RR 1.26) with most survival benefit using the preoperative approach [Valentini et al. 2009].
In summary, data on neoadjuvant radiotherapy are still limited, but suggest an advantage in survival over surgery alone. The largest trial has been performed in patients from a high incidence area with exclusively cardia cancer.
Preoperative chemoradiotherapy
Currently, most accruing randomized trials focus on perioperative chemotherapy and postoperative chemo(radio)therapy. However, several phase I/II studies have combined the administration of neoadjuvant chemotherapy with neoadjuvant radiotherapy [Ajani et al, 2006b, 2005, 2004; Allal et al. 2005]. Although results are promising with different chemotherapy schedules all containing 5-fluorouracil and cisplatin, multicenter phase III trials are necessary in order to evaluate whether this treatment strategy can improve survival.
Conclusion and future perspectives
Surgery remains the primary curative treatment for locally advanced gastric cancer. A D2 dissection is the recommended type of surgery in Western countries, while in the East at least a D2 lymph node dissection is performed. Despite the effort to improve surgical quality, locoregional relapse rate remains high with a consequent poor prognosis.
Currently accepted adjuvant and neoadjuvant therapies include adjuvant chemotherapy, postoperative chemoradiation, and perioperative chemotherapy. Adjuvant chemotherapy is mainly given in Japan with S-1, but has not been evaluated in the West because of limited experience with S-1 in Western patients. The Western debate focuses on the use of postoperative chemoradiation versus perioperative chemotherapy, but due to different inclusion criteria, the results of the Intergroup 0116 and MAGIC trials are incomparable with regards to treatment adherence and survival. These studies do indicate, however, that the toxicity profile of the chemotherapy and radiation regimen is critical for patient compliance, and study accrual. Based on the superior compliance of preoperative chemotherapy as compared with postoperative chemotherapy or radiation, preoperative chemotherapy should be considered standard treatment in patients with advanced, resectable gastric cancer. When tolerated, postoperative treatment should also be administered, but no standard regimen for this has been established. After an R1 resection postoperative chemoradiation might improve survival, but it has not been compared in a prospective randomized manner with postoperative chemotherapy.
Several currently accruing or yet unpublished trials (Table 5) focus on the choice of the optimal postoperative treatment. In the Dutch CRITICS trial, patients receive three cycles of preoperative chemotherapy (ECC) followed by surgery, after which they receive another three cycles of ECC, or postoperative chemoradiation. The Korean ARTIST trial, which finished accrual, randomized patients who received a D2 dissection between postoperative chemotherapy (cisplatin and capecitabine) and postoperative chemoradiation. No preoperative therapy was administered. Feasibility data of this study were reported at ASCO-GI 2009 showing good toxicity profiles with compliance rates of 75% versus 82%, respectively. Survival data of this trial have to be awaited [Lee et al. 2009]. With the low cure rates of the currently accepted therapies, several of the currently accruing Western trials focus on improved chemotherapy schedules: in the British MAGIC-B trial, bevacizumab is added to perioperative epirubicin, cisplatin, and capecitabine. A very recent protocol change has included another arm with panitumumab instead of bevacizumab. The US CALGB 80101 compares the Intergroup regimen (radiation, 5-FU, leucovorin) with radiation, epirubicin, cisplatin and 5-FU and has finished accrual, but final outcomes of this study have to be awaited.
Table 5.
Current phase III trials for treatment of gastric cancer.
| Trial | Treatment setting | Treatment arms | Number of patients required |
|---|---|---|---|
| CRITICS Dutch Colorectal Cancer Group | Stage Ib-IV gastric cancer | Perioperative ECC (threecycles pre and post) | 788 |
| Neo-adjuvant ECC (threecycles), surgery, then adjuvant CC CRT | |||
| Magic-B British MRC | Stage Ib-IV resectable adenocarcinoma of the stomach or Type III GEJ | Perioperative ECC (three cyclespre- and post-) | 1100 |
| Perioperative ECC plus bevacizumab (three cycles pre- and post-) Maintenance bevacizumab 6x | |||
| ARTIST | ≥D2 resected stage | Adjuvant CC | 490 |
| Samsung Medical Centre NCT00323830 | Ib-IV gastric cancer | Adjuvant CC CRT | |
| Intergroup CALGB 80101 (completed) | Stage Ib-IV resected adenocarcinoma of the stomach or GEJ | Adjuvant 5-FU CRT Adjuvant ECF CRT |
824 |
| Tokyo Metropolitan Oncology Group (NCT00687843) |
Stage II-IIIB gastric cancer | Adjuvant Tegafur-gimeracil-oteracil (TS-1) | 480 |
| Adjuvant TS-1 + PSK (Krestin) | |||
| Hokuriku-Kinki Immunochemotherapy Study Group (NCT00216034) | Stage II-IIIA gastric cancer | Adjuvant Tegafur-gimeracil-oteracil (TS-1) Adjuvant TS-1 + PSK (Krestin) |
280 |
| Japan Clinical Oncology Group JCOG 0501 (NCT00252161) |
Borrmann Type 4 and Large Type 3 Gastric Cancer |
≥D2 resection alone Neoadjuvant S-1 plus cisplatinthen ≥D2 resection |
300 |
| CLASSIC | Stage II-IIIb gastric cancer | D2 resection | 1024 |
| Sanofi-Aventis, South Korea | D2 resection, adjuvant capecitabine, oxiplatin | ||
Explanations of acronyms: ECC: epirubicin, cisplatin, capecitabine; CC: cisplatin, capecitabine; CRT: chemoradiotherapy; GEJ: gastroesophageal junction; 5-FU: 5-fluorouracil; ECF: epirubicin, cisplatin, 5-FU
Western randomized controlled trials on gastric cancer are often hampered by slow or incomplete accrual. Reduction of toxicity for preoperative and especially postoperative treatment and adequate nutritional support is essential for the ongoing improvement of gastric cancer care. Currently accruing Asian trials mainly focus on improved adjuvant chemotherapy with or without immunotherapy.
Most of the studies covered in the current review mention the rate of cardia cancer in the trial population. However, subgroup analyses for cardia versus noncardia cancer are rarely performed. Owing to the differences in epidemiological, etiological and histological factors, this topic warrants further attention.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts of interest in preparing this article.
References
- Abnet C.C., Freedman N.D., Hollenbeck A.R., Fraumeni J.F., Jr, Leitzmann M., Schatzkin A. (2008) A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 44: 465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajani J., Bekaii-Saab T., D’Amico T.A., Fuchs C., Gibson M.K., Goldberg M., et al. (2006a) Gastric Cancer Clinical Practice Guidelines. J Natl Compr Canc Netw 4: 350–366 [DOI] [PubMed] [Google Scholar]
- Ajani J.A., Mansfield P.F., Crane C.H., Wu T.T., Lunagomez S., Lynch P.M., et al. (2005) Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol 23: 1237–1244 [DOI] [PubMed] [Google Scholar]
- Ajani J.A., Mansfield P.F., Janjan N., Morris J., Pisters P.W., Lynch P.M., et al. (2004) Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol 22: 2774–2780 [DOI] [PubMed] [Google Scholar]
- Ajani J.A., Ota D.M., Jessup J.M., Ames F.C., McBride C., Boddie A., et al. (1991) Resectable gastric carcinoma. An evaluation of preoperative and postoperative chemotherapy. Cancer 68: 1501–1506 [DOI] [PubMed] [Google Scholar]
- Ajani J.A., Rodriguez W., Bodoky G., Moiseyenko V., Lichinitser M., Gorbunova V., et al. (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28: 1547–1553 [DOI] [PubMed] [Google Scholar]
- Ajani J.A., Winter K., Okawara G.S., Donohue J.H., Pisters P.W., Crane C.H., et al. (2006b) Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol 24: 3953–3958 [DOI] [PubMed] [Google Scholar]
- Allal A.S., Zwahlen D., Brundler M.A., de Peyer R., Morel P., Huber O., et al. (2005) Neoadjuvant radiochemotherapy for locally advanced gastric cancer: long-term results of a phase I trial. Int J Radiat Oncol Biol Phys 63: 1286–1289 [DOI] [PubMed] [Google Scholar]
- An J.Y., Youn H.G., Choi M.G., Noh J.H., Sohn T.S., Kim S. (2008) The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg 196: 587–591 [DOI] [PubMed] [Google Scholar]
- Bajetta E., Buzzoni R., Mariani L., Beretta E., Bozzetti F., Bordogna G., et al. (2002) Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann Oncol 13: 299–307 [DOI] [PubMed] [Google Scholar]
- Biffi R., Fazio N., Luca F., Chiappa A., Andreoni B., Zampino M.G., et al. (2010) Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol 16: 868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman L.A., Thibodeau S.N., Schaid D.J., Lindor N.M., McDonnell S.K., Burgart L.J., et al. (1998) Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med 128: 896–899 [DOI] [PubMed] [Google Scholar]
- Boige V., Pignon J.P. (2007) Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. In: Proceedings of the ASCO Annual Meeting [Google Scholar]
- Bonenkamp J.J., Hermans J., Sasako M., van de Velde C.J., Welvaart K., Songun I., et al. (1999) Extended lymph-node dissection for gastric cancer. N Engl J Med 340: 908–914 [DOI] [PubMed] [Google Scholar]
- Bonenkamp J.J., Songun I., Hermans J., van de Velde C.J. (1996) Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg 83: 672–674 [DOI] [PubMed] [Google Scholar]
- Bouche O., Ychou M., Burtin P., Bedenne L., Ducreux M., Lebreton G., et al. (2005) Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol 16: 1488–1497 [DOI] [PubMed] [Google Scholar]
- Bozzetti F. (2001) Principles of surgical radicality in the treatment of gastric cancer. Surg Oncol Clin N Am 10: 833–854, ix [PubMed] [Google Scholar]
- Bozzetti F., Braga M., Gianotti L., Gavazzi C., Mariani L. (2001) Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet 358: 1487–1492 [DOI] [PubMed] [Google Scholar]
- Bozzetti F., Gianotti L., Braga M., Di Carlo V., Mariani L. (2007) Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr 26: 698–709 [DOI] [PubMed] [Google Scholar]
- Bozzetti F., Marubini E., Bonfanti G., Miceli R., Piano C., Gennari L. (1999) Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 230: 170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt A.M., Hermans J., Smit V.T., van de Velde C.J., Fleuren G.J., Bruijn J.A. (1995) Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol 13: 19–25 [DOI] [PubMed] [Google Scholar]
- Carneiro F., Huntsman D.G., Smyrk T.C., Owen D.A., Seruca R., Pharoah P., et al. (2004) Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol 203: 681–687 [DOI] [PubMed] [Google Scholar]
- Cascinu S., Labianca R., Barone C., Santoro A., Carnaghi C., Cassano A., et al. (2007) Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst 99: 601–607 [DOI] [PubMed] [Google Scholar]
- Cho B.C., Jeung H.C., Choi H.J., Rha S.Y., Hyung W.J., Cheong J.H., et al. (2007) Prognostic impact of resection margin involvement after extended (D2/D3) gastrectomy for advanced gastric cancer: a 15-year experience at a single institute. J Surg Oncol 95: 461–468 [DOI] [PubMed] [Google Scholar]
- Coggon D., Osmond C., Barker D.J. (1990) Stomach cancer and migration within England and Wales. Br J Cancer 61: 573–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P. (1992) Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52: 6735–6740 [PubMed] [Google Scholar]
- Correa P., Haenszel W., Cuello C., Tannenbaum S., Archer M. (1975) A model for gastric cancer epidemiology. Lancet 2: 58–60 [DOI] [PubMed] [Google Scholar]
- Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J., Nicolson M., et al. (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355: 11–20 [DOI] [PubMed] [Google Scholar]
- Cuschieri A., Fayers P., Fielding J., Craven J., Bancewicz J., Joypaul V., et al. (1996) Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 347: 995–999 [DOI] [PubMed] [Google Scholar]
- Cuschieri A., Weeden S., Fielding J., Bancewicz J., Craven J., Joypaul V., et al. (1999) Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 79: 1522–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelica M., Gonen M., Brennan M.F., Turnbull A.D., Bains M., Karpeh M.S. (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 240: 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita F., Giuliani F., Orditura M., Maiello E., Galizia G., Di Martino N., et al. (2007) Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann Oncol 18: 1354–1358 [DOI] [PubMed] [Google Scholar]
- Dent D.M., Madden M.V., Price S.K. (1988) Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 75: 110–112 [DOI] [PubMed] [Google Scholar]
- Dent D.M., Werner I.D., Novis B., Cheverton P., Brice P. (1979) Prospective randomized trial of combined oncological therapy for gastric carcinoma. Cancer 44: 385–391 [DOI] [PubMed] [Google Scholar]
- Derakhshan M.H., Malekzadeh R., Watabe H., Yazdanbod A., Fyfe V., Kazemi A., et al. (2008) Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut 57: 298–305 [DOI] [PubMed] [Google Scholar]
- Devesa S.S., Blot W.J., Fraumeni J.F., Jr (1998) Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 83: 2049–2053 [PubMed] [Google Scholar]
- Dikken J.L., Jansen E.P., Cats A., Bakker B., Hartgrink H.H., Kranenbarg E.M., et al. (2010) Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 28: 2430–2436 [DOI] [PubMed] [Google Scholar]
- Earle C.C., Maroun J.A. (1999) Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer 35: 1059–1064 [DOI] [PubMed] [Google Scholar]
- Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Trotti A. (2010) AJCC Cancer Staging Manual, 7th edn, Springer: New York [Google Scholar]
- Eslick G.D. (2006) Helicobacter pylori infection causes gastric cancer? A review of the epidemiological, meta-analytic, and experimental evidence. World J Gastroenterol 12: 2991–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorica F., Cartei F., Enea M., Licata A., Cabibbo G., Carau B., et al. (2007) The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev 33: 729–740 [DOI] [PubMed] [Google Scholar]
- Fitzgerald R.C., Hardwick R., Huntsman D., Carneiro F., Guilford P., Blair V., et al. (2010) Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 47: 436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello F.M., Brensinger J.D., Tersmette A.C., Goodman S.N., Petersen G.M., Booker S.V., et al. (2000) Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology 119: 1447–1453 [DOI] [PubMed] [Google Scholar]
- GITSG (1982) A comparison of combination chemotherapy and combined modality therapy for locally advanced gastric carcinoma. Gastrointestinal Tumor Study Group. Cancer 49: 1771–1777 [DOI] [PubMed] [Google Scholar]
- GITSG (1990) The concept of locally advanced gastric cancer. Effect of treatment on outcome. The Gastrointestinal Tumor Study Group. Cancer 66: 2324–2330 [DOI] [PubMed] [Google Scholar]
- Gouzi J.L., Huguier M., Fagniez P.L., Launois B., Flamant Y., Lacaine F., et al. (1989) Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg 209: 162–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene F.L., Page D.L., Fleming I.D., Fritz A.G., Balch C.M., Haller D.G., et al. (2002) AJCC Cancer Staging Manual, 6th edn, Springer: New York [Google Scholar]
- Guilford P.J., Hopkins J.B., Grady W.M., Markowitz S.D., Willis J., Lynch H., et al. (1999) E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat 14: 249–255 [DOI] [PubMed] [Google Scholar]
- Gunderson L.L. (2002) Gastric cancer–patterns of relapse after surgical resection. Semin Radiat Oncol 12: 150–161 [DOI] [PubMed] [Google Scholar]
- Gunderson L.L., Sosin H. (1982) Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 8: 1–11 [DOI] [PubMed] [Google Scholar]
- Gylling A., Abdel-Rahman W.M., Juhola M., Nuorva K., Hautala E., Jarvinen H.J., et al. (2007) Is gastric cancer part of the tumour spectrum of hereditary non-polyposis colorectal cancer? A molecular genetic study. Gut 56: 926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallissey M.T., Dunn J.A., Ward L.C., Allum W.H. (1994) The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet 343: 1309–1312 [DOI] [PubMed] [Google Scholar]
- Hamashima C., Shibuya D., Yamazaki H., Inoue K., Fukao A., Saito H., et al. (2008) The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol 38: 259–267 [DOI] [PubMed] [Google Scholar]
- Hansen S., Vollset S.E., Derakhshan M.H., Fyfe V., Melby K.K., Aase S., et al. (2007) Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut 56: 918–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgrink H.H., van de Velde C.J., Putter H., Bonenkamp J.J., Klein Kranenbarg E., Songun I., et al. (2004a) Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22: 2069–2077 [DOI] [PubMed] [Google Scholar]
- Hartgrink H.H., van de Velde C.J., Putter H., Songun I., Tesselaar M.E., Kranenbarg E.K., et al. (2004b) Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol 30: 643–649 [DOI] [PubMed] [Google Scholar]
- Hayashi H., Ochiai T., Shimada H., Gunji Y. (2005) Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surgical Endos Other Intervent Techniques 19: 1172–1176 [DOI] [PubMed] [Google Scholar]
- Helicobacter and Cancer Collaborative Group (2001) Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 49: 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans J., Bonenkamp J.J., Boon M.C., Bunt A.M., Ohyama S., Sasako M., et al. (1993) Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol 11: 1441–1447 [DOI] [PubMed] [Google Scholar]
- Hjartaker A., Langseth H., Weiderpass E. (2008) Obesity and diabetes epidemics: cancer repercussions. Adv Exp Med Biol 630: 72–93 [DOI] [PubMed] [Google Scholar]
- Horner M.J., Ries L.A.G., Krapcho M., Neyman N., Aminou R., Howlader N., et al. (2009) SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; Available at: http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- Howson C.P., Hiyama T., Wynder E.L. (1986) The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev 8: 1–27 [DOI] [PubMed] [Google Scholar]
- Hu J.K., Chen Z.X., Zhou Z.G., Zhang B., Tian J., Chen J.P., et al. (2002) Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol 8: 1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humar B., Guilford P. (2009) Hereditary diffuse gastric cancer: a manifestation of lost cell polarity. Cancer Sci 100: 1151–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundahl S.A., Phillips J.L., Menck H.R. (2000) The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 88: 921–932 [PubMed] [Google Scholar]
- Huscher C.G.S., Mingoli A., Sgarzini G., Sansonetti A., Di Paola M., Recher A., et al. (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer - Five-year results of a randomized prospective trial. Ann Surg 241: 232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E.P., Boot H., Dubbelman R., Bartelink H., Cats A., Verheij M. (2007a) Postoperative chemoradiotherapy in gastric cancer—a Phase I/II dose-finding study of radiotherapy with dose escalation of cisplatin and capecitabine chemotherapy. Br J Cancer 97: 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E.P., Boot H., Dubbelman R., Verheij M., Cats A. (2009) Postoperative chemoradiotherapy in gastric cancer–a phase I-II study of radiotherapy with dose escalation of weekly cisplatin and daily capecitabine chemotherapy. Ann Oncol, in press [DOI] [PubMed] [Google Scholar]
- Jansen E.P., Boot H., Saunders M.P., Crosby T.D., Dubbelman R., Bartelink H., et al. (2007b) A phase I-II study of postoperative capecitabine-based chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 69: 1424–1428 [DOI] [PubMed] [Google Scholar]
- Jansen E.P., Saunders M.P., Boot H., Oppedijk V., Dubbelman R., Porritt B., et al. (2007c) Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 67: 781–785 [DOI] [PubMed] [Google Scholar]
- Janunger K.G., Hafstrom L., Glimelius B. (2002) Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg 168: 597–608 [DOI] [PubMed] [Google Scholar]
- Japanese Gastric Cancer Association (1998) Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer 1: 10–24 [DOI] [PubMed] [Google Scholar]
- Kamangar F., Dores G.M., Anderson W.F. (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24: 2137–2150 [DOI] [PubMed] [Google Scholar]
- Kang Y., Chang H. (2008) Postoperative adjuvant chemotherapy for grossly serosa-positive advanced gastric cancer: A randomized phase III trial of intraperitoneal cisplatin and early mitomycin-C plus long-term doxifluridine plus cisplatin (iceMFP) versus mitomycin-C plus short-term doxifluridine (Mf) (AMC 0101) (NCT00296322). In: ASCO Annual Meeting 2008 [Google Scholar]
- Kang Y., Chang H., Min Y., Zang D., Kim G., Yang D., et al. (2008) A randomized phase III trial comparing mitomycin-C plus short-term doxifluridine (Mf) versus mitomycin-C plus long-term doxifluridine plus cisplatin (MFP) after curative resection of advanced gastric cancer (AMC 0201). In: ASCO Annual Meeting [Google Scholar]
- Karpeh M.S., Leon L., Klimstra D., Brennan M.F. (2000) Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg 232: 362–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J.R., Duggan J.M. (2003) Gastric cancer epidemiology and risk factors. J Clin Epidemiol 56: 1–9 [DOI] [PubMed] [Google Scholar]
- Kim H.H., Hyung W.J., Cho G.S., Kim M.C., Han S.U., Kim W., et al. (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251: 417–420 [DOI] [PubMed] [Google Scholar]
- Kim S., Lim D.H., Lee J., Kang W.K., MacDonald J.S., Park C.H., et al. (2005) An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys 63: 1279–1285 [DOI] [PubMed] [Google Scholar]
- Kim S.H., Karpeh M.S., Klimstra D.S., Leung D., Brennan M.F. (1999) Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg 3: 24–33 [DOI] [PubMed] [Google Scholar]
- Kitano S., Shiraishi N., Fujii K., Yasuda K., Inomata M., Adachi Y. (2002) A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 131(1 Suppl): S306–S311 [DOI] [PubMed] [Google Scholar]
- Kitaoka H., Yoshikawa K., Hirota T., Itabashi M. (1984) Surgical treatment of early gastric cancer. Jpn J Clin Oncol 14: 283–293 [PubMed] [Google Scholar]
- Klaassen D.J., MacIntyre J.M., Catton G.E., Engstrom P.F., Moertel C.G. (1985) Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol 3: 373–378 [DOI] [PubMed] [Google Scholar]
- Kollmannsberger C., Budach W., Stahl M., Schleucher N., Hehr T., Wilke H., et al. (2005) Adjuvant chemoradiation using 5-fluorouracil/folinic acid/cisplatin with or without paclitaxel and radiation in patients with completely resected high-risk gastric cancer: two cooperative phase II studies of the AIO/ARO/ACO. Ann Oncol 16: 1326–1333 [DOI] [PubMed] [Google Scholar]
- Kolonel L.N., Nomura A.M., Hirohata T., Hankin J.H., Hinds M.W. (1981) Association of diet and place of birth with stomach cancer incidence in Hawaii Japanese and Caucasians. Am J Clin Nutr 34: 2478–2485 [DOI] [PubMed] [Google Scholar]
- Kramling H.J., Wilkowski R., Duhmke E., Cramer C., Willich N., Schildberg F.W. (1996) [Adjuvant intraoperative radiotherapy of stomach carcinoma]. Langenbecks Arch Chir Suppl Kongressbd 113: 211–213 [PubMed] [Google Scholar]
- La Vecchia C., Negri E., Franceschi S., Gentile A. (1992) Family history and the risk of stomach and colorectal cancer. Cancer 70: 50–55 [DOI] [PubMed] [Google Scholar]
- Ladeiras-Lopes R., Pereira A.K., Nogueira A., Pinheiro-Torres T., Pinto I., Santos-Pereira R., et al. (2008) Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control 19: 689–701 [DOI] [PubMed] [Google Scholar]
- Lauren P. (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64: 31–49 [DOI] [PubMed] [Google Scholar]
- Lee J., Kang W., Lim D. (2009) Phase III trial of adjuvant capecitabine/cisplatin (XP) compared with capecitabine/cisplatin/RT (XPRT) in resected gastric cancer with D2 nodal dissection (ARTIST trial): Safety analysis. In: Proceedings of the ASCO Gastrointestinal Cancers Symposium [Google Scholar]
- Lee J.H., Han H.S. (2005) A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surgical Endosc Other Intervent Techniques 19: 168–173 [DOI] [PubMed] [Google Scholar]
- Macdonald J.S., Benedetti J., Smalley S., Haller D., Hundahl S., Jessup J., et al. (2009) Chemoradiation of resected gastric cancer: A 10-year follow-up of the phase III trial INT0116 (SWOG 9008). Proceedings of the 2009 ASCO Annual Meeting J Clin Oncol 27(15 Suppl): abstract 4515 [Google Scholar]
- Macdonald J.S., Smalley S.R., Benedetti J., Hundahl S.A., Estes N.C., Stemmermann G.N., et al. (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345: 725–730 [DOI] [PubMed] [Google Scholar]
- Machida-Montani A., Sasazuki S., Inoue M., Natsukawa S., Shaura K., Koizumi Y., et al. (2004) Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer 7: 46–53 [DOI] [PubMed] [Google Scholar]
- Mansour J.C., Tang L., Shah M., Bentrem D., Klimstra D.S., Gonen M., et al. (2007) Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer?. Ann Surg Oncol 14: 3412–3418 [DOI] [PubMed] [Google Scholar]
- Mari E., Floriani I., Tinazzi A., Buda A., Belfiglio M., Valentini M., et al. (2000) Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente). Ann Oncol 11: 837–843 [DOI] [PubMed] [Google Scholar]
- Maruyama K., Sasako M., Kinoshita T., Sano T., Katai H. (1996) Surgical treatment for gastric cancer: the Japanese approach. Semin Oncol 23: 360–368 [PubMed] [Google Scholar]
- Moertel C.G., Childs D.S., O’Fallon J.R., Holbrook M.A., Schutt A.J., Reitemeier R.J. (1984) Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol 2: 1249–1254 [DOI] [PubMed] [Google Scholar]
- Nakajima T. (2002) Gastric cancer treatment guidelines in Japan. Gastric Cancer 5: 1–5 [DOI] [PubMed] [Google Scholar]
- Nitti D., Wils J., Dos Santos J.G., Fountzilas G., Conte P.F., Sava C., et al. (2006) Randomized phase III trials of adjuvant FAMTX or FEMTX compared with surgery alone in resected gastric cancer. A combined analysis of the EORTC GI Group and the ICCG. Ann Oncol 17: 262–269 [DOI] [PubMed] [Google Scholar]
- Nouraie M., Pietinen P., Kamangar F., Dawsey S.M., Abnet C.C., Albanes D., et al. (2005) Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiol Biomarkers Prev 14: 2087–2092 [DOI] [PubMed] [Google Scholar]
- Panzini I., Gianni L., Fattori P.P., Tassinari D., Imola M., Fabbri P., et al. (2002) Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori 88: 21–27 [PubMed] [Google Scholar]
- Pohl H., Welch H.G. (2005) The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97: 142–146 [DOI] [PubMed] [Google Scholar]
- Ribeiro U., Jr, Safatle-Ribeiro A.V., Zilberstein B., Mucerino D., Yagi O.K., Bresciani C.C., et al. (2006) Does the intraoperative peritoneal lavage cytology add prognostic information in patients with potentially curative gastric resection?. J Gastrointest Surg 10: 170–176, discussion 176-177 [DOI] [PubMed] [Google Scholar]
- Rusch V.W., Rice T.W., Crowley J., Blackstone E.H., Rami-Porta R., Goldstraw P. (2010) The seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals: the new era of data-driven revisions. J Thorac Cardiovasc Surg 139: 819–821 [DOI] [PubMed] [Google Scholar]
- Sakuramoto S., Sasako M., Yamaguchi T., Kinoshita T., Fujii M., Nashimoto A., et al. (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357: 1810–1820 [DOI] [PubMed] [Google Scholar]
- Sano T., Sasako M., Yamamoto S., Nashimoto A., Kurita A., Hiratsuka M., et al. (2004) Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy—Japan Clinical Oncology Group study 9501. J Clin Oncol 22: 2767–2773 [DOI] [PubMed] [Google Scholar]
- Sant M., Allemani C., Santaquilani M., Knijn A., Marchesi F., Capocaccia R. (2009) EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer 45: 931–991 [DOI] [PubMed] [Google Scholar]
- Sasako M., Sano T., Yamamoto S., Kurokawa Y., Nashimoto A., Kurita A., et al. (2008) D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359: 453–462 [DOI] [PubMed] [Google Scholar]
- Schuhmacher C., Schlag P., Lordick F., Hohenberger W., Heise J., Haag C. (2009) Neoadjuvant chemotherapy versus surgery alone for locally advanced adenocarcinoma of the stomach and cardia: Randomized EORTC phase III trial #40954. In: Proceedings of the ASCO Annual Meeting [Google Scholar]
- Shchepotin I.B., Evans S.R., Chorny V., Osinsky S., Buras R.R., Maligonov P., et al. (1994) Intensive preoperative radiotherapy with local hyperthermia for the treatment of gastric carcinoma. Surg Oncol 3: 37–44 [DOI] [PubMed] [Google Scholar]
- Shehzad K., Mohiuddin K., Nizami S., Sharma H., Khan I.M., Memon B., et al. (2007) Current status of minimal access surgery for gastric cancer. Surg Oncol 16: 85–98 [DOI] [PubMed] [Google Scholar]
- Shiraishi N., Yasuda K., Kitano S. (2006) Laparoscopic gastrectomy with lymph node dissection for gastric cancer. Gastric Cancer 9: 167–176 [DOI] [PubMed] [Google Scholar]
- Siewert R., Feith M., Werner M., Stein H. (2000) Adenocarcinoma of the esophagogastric junction results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 232: 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindelar W.F., Kinsella T.J., Tepper J.E., DeLaney T.F., Maher M.M., Smith R., et al. (1993) Randomized trial of intraoperative radiotherapy in carcinoma of the stomach. Am J Surg 165: 178–186, discussion 186-177 [DOI] [PubMed] [Google Scholar]
- Skoropad V., Berdov B., Zagrebin V. (2002) Concentrated preoperative radiotherapy for resectable gastric cancer: 20-years follow-up of a randomized trial. J Surg Oncol 80: 72–78 [DOI] [PubMed] [Google Scholar]
- Skoropad V.Y., Berdov B.A., Mardynski Y.S., Titova L.N. (2000) A prospective, randomized trial of pre-operative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer. Eur J Surg Oncol 26: 773–779 [DOI] [PubMed] [Google Scholar]
- Songun I., Bonenkamp J.J., Hermans J., van Krieken J.H., van de Velde C.J. (1996) Prognostic value of resection-line involvement in patients undergoing curative resections for gastric cancer. Eur J Cancer 32A: 433–437 [DOI] [PubMed] [Google Scholar]
- Songun I., Putter H., Kranenbarg E.M., Sasako M., van de Velde C.J. (2010) Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol, in press [DOI] [PubMed] [Google Scholar]
- Steevens J., Botterweck A.A., Dirx M.J., van den Brandt P.A., Schouten L.J. (2010a) Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol 22: 669–678 [DOI] [PubMed] [Google Scholar]
- Steevens J., Schouten L.J., Goldbohm R.A., van den Brandt P.A. (2010b) Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut 59: 39–48 [DOI] [PubMed] [Google Scholar]
- Strong V.E., Song K.Y., Park C.H., Jacks L.M., Gonen M., Shah M., et al. (2010) Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 251: 640–646 [DOI] [PubMed] [Google Scholar]
- Sun P., Xiang J.B., Chen Z.Y. (2009) Meta-analysis of adjuvant chemotherapy after radical surgery for advanced gastric cancer. Br J Surg 96: 26–33 [DOI] [PubMed] [Google Scholar]
- Svendsen J.H., Dahl C., Svendsen L.B., Christiansen P.M. (1986) Gastric cancer risk in achlorhydric patients. A long-term follow-up study. Scand J Gastroenterol 21: 16–20 [DOI] [PubMed] [Google Scholar]
- Tsugane S., Sasazuki S. (2007) Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer 10: 75–83 [DOI] [PubMed] [Google Scholar]
- UICC (2009) TNM Classification of Malignant Tumors, 7th edn, Wiley-Blackwell: New York [Google Scholar]
- Valentini V., Cellini F., Minsky B.D., Mattiucci G.C., Balducci M., D’Agostino G., et al. (2009) Survival after radiotherapy in gastric cancer: systematic review and meta-analysis. Radiother Oncol 92: 176–183 [DOI] [PubMed] [Google Scholar]
- Varley J.M., McGown G., Thorncroft M., Tricker K.J., Teare M.D., Santibanez-Koref M.F., et al. (1995) An extended Li–Fraumeni kindred with gastric carcinoma and a codon 175 mutation in TP53. J Med Genet 32: 942–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.W., Hsiung C.A., Lo S.S., Hsieh M.C., Chen J.H., Li A.F., et al. (2006) Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 7: 309–315 [DOI] [PubMed] [Google Scholar]
- Wu H., Rusiecki J.A., Zhu K., Potter J., Devesa S.S. (2009) Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev 18: 1945–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kato M., Asaka M. (2008) Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med 47: 1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T.D., Black D., Sugarbaker P.H., Zhu J., Yonemura Y., Petrou G., et al. (2007) A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 14: 2702–2713 [DOI] [PubMed] [Google Scholar]
- Zhang Z.X., Gu X.Z., Yin W.B., Huang G.J., Zhang D.W., Zhang R.G. (1998) Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)—report on 370 patients. Int J Radiat Oncol Biol Phys 42: 929–934 [DOI] [PubMed] [Google Scholar]