Abstract
Gastrointestinal (GI) functional and motility disorders are highly prevalent and responsible for long-term morbidity and sometimes mortality in the affected patients. It is estimated that one in three persons has a GI functional or motility disorder. However, diagnosis and treatment of these widespread conditions remains challenging. This partly stems from the multisystem pathophysiology, including processing abnormalities in the central and peripheral (enteric) nervous systems and motor dysfunction in the GI wall. Interstitial cells of Cajal (ICCs) are central to the generation and propagation of the cyclical electrical activity and smooth muscle cells (SMCs) are responsible for electromechanical coupling. In these and other excitable cells voltage-sensitive ion channels (VSICs) are the main molecular units that generate and regulate electrical activity. Thus, VSICs are potential targets for intervention in GI motility disorders. Research in this area has flourished with advances in the experimental methods in molecular and structural biology and electrophysiology. However, our understanding of the molecular mechanisms responsible for the complex and variable electrical behavior of ICCs and SMCs remains incomplete. In this review, we focus on the slow waves and action potentials in ICCs and SMCs. We describe the constituent VSICs, which include voltage-gated sodium (NaV), calcium (CaV), potassium (KV, KCa), chloride (Cl–) and nonselective ion channels (transient receptor potentials [TRPs]). VSICs have significant structural homology and common functional mechanisms. We outline the approaches and limitations and provide examples of targeting VSICs at the pores, voltage sensors and alternatively spliced sites. Rational drug design can come from an integrated view of the structure and mechanisms of gating and activation by voltage or mechanical stress.
Keywords: functional GI, GI motility, ion channel, voltage gated ion channel, potassium voltage gated ion channel, sodium voltage gated ion channel, calcium voltage gated ion channel, chloride channel
Gastrointestinal functional and motility disorders
Gastrointestinal (GI) functional and motility disorders are common and commonly morbid. In the broadest sense, GI functional and motility disorders refer to a group of disorders whose symptomatology is related to motor, sensory or even secretory function of the GI tract. The term ‘functional’ refers to the subgroup of disorders that do not yet have a well-defined pathophysiology. This broad definition means that the symptoms are nonspecific and include nausea, vomiting, bloating, abdominal discomfort or pain, constipation or diarrhea. Some of the most common motility disorders encountered in practice are achalasia, gastroparesis, intestinal pseudo-obstruction and slow transit constipation. Common functional GI disorders such as functional dyspepsia and irritable bowel syndrome (IBS) are highly prevalent in the community. For example, IBS has an estimated prevalence of 15–22% in Western society and by itself accounts for 3% of all primary care visits and up to 60% of the referrals for secondary care [Ford et al. 2008]. GI motility disorders are also not standalone pathologies; they may be complications of other systemic illnesses, such as diabetes, which can result in diabetic gastroparesis in a subset of patients [Camilleri et al. 2011; Kashyap and Farrugia, 2010]. Finally, intestinal pseudo-obstruction and other less common GI motility disorders are associated with substantial mortality risk.
Pathophysiology
GI functional and motility disorders have a multifactorial pathophysiology. Pathologies responsible for these disorders span the central (CNS) and peripheral (enteric) (ENS) nervous systems, interstitial cells of Cajal (ICCs), smooth muscle cells (SMCs), and immune cells. For example, IBS involves a complex interplay between multiple potential pathologic factors, including abnormal pain signaling due to both central perception and peripheral sensitivity, infectious or postinfectious causes and disordered motility of the GI tract [Ford and Talley, 2011]. While the complex multivariate nature of these disorders is responsible for many diagnostic and therapeutic challenges [Ford and Talley, 2011], a particular advantage is that multisystem pathophysiology provides a rich source of potential targets. We defer the extensive discussion on targeting ion channels in the CNS and ENS to other excellent reviews [Gourine et al. 2009; Storr and Sharkey, 2007; Galligan, 2004, 2002; Cervero and Laird, 2003; Smith et al. 2003; Galligan and North, 1988]. Instead, we focus on the effectors of the GI tract, the motor cells: SMCs and ICCs. In the GI tract, the ICCs have several functions [Sarna, 2008] including generating and propagating electrical activity [Thomsen et al. 1998; Huizinga et al. 1995], setting SMC membrane potential [Farrugia et al. 2003], mediating neuronal input [Powley et al. 2008], and as mechanosensors [Won et al. 2005; Strege et al. 2003b]. The ICC and SMC system coordinates electromechanical coupling [Der-Silaphet et al. 1998] and mechanoelectrical feedback [Kraichely and Farrugia, 2007]. There are at least three distinct advantages of targeting the effector cells. First, SMCs and ICCs are essential for normal motility, so dysfunction in these cell types is most likely pathogenic [Farrugia, 2008]. Second, the final effector targets allow direct intervention, limiting side effects that hamper approaches involving upstream targets. Third, drug delivery to these cells may be facilitated by their location close to the gut lumen.
Electromechanical functions
GI tract wall organization underlies its electromechanical functions. Both cyclical and stimulated contractions of the GI tract require electrical excitation and excitation–contraction coupling. GI motility is the result of coordinated activity of extrinsic nerves, the ENS, immune cells, ICCs and SMCs. Yet, the GI tract is able to function independently of external neuronal input. We know that ICCs are fundamental for the generation and propagation of the electrical cyclical activity in the GI tract [Thomsen et al. 1998]. In the small intestine, ICCs around the myenteric (Auerbach’s) plexus (ICC-MYs) between the circular and longitudinal muscle layers are responsible for the generation the cyclical activity, known as slow waves [Kito et al. 2005]. In the colon submuscular ICCs (ICC-SMs) appear to be required for slow wave generation [Lee et al. 2009]. Slow waves are the cyclical electrical events that depolarize the ICCs for seconds from its resting membrane potential to a more positive voltage, but the resting membrane potential, amount of depolarization and frequency of the slow waves are variable through the GI tract [Hara et al. 1986]. Other ICC networks, such as intramuscular ICCs (ICC-IMs) propagate and amplify slow waves and thus regulate motility [Cho and Daniel, 2005] (Figure 1A). The slow waves are then transmitted to the SMCs, which also depolarize cyclically. The result of the SMC depolarization is activation of calcium entry and contraction, also known as excitation–contraction coupling. In the following sections we provide some details on the slow wave generation in the ICCs and propagation to the SMCs with particular focus on the involved voltage-sensitive ion channels (VSICs) and their potential as drug targets.
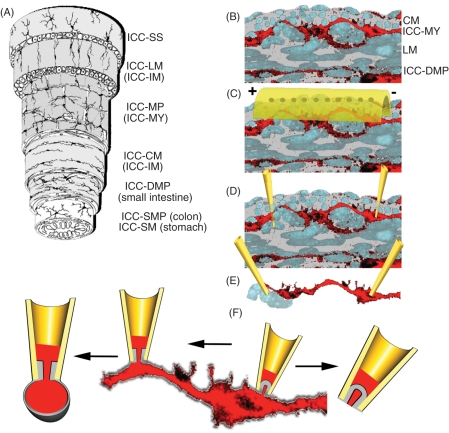
Figure 1.
Reductionist electrophysiology approaches for the study of ion channel activity in the gastrointestinal (GI) tract. (A) Diagram of the GI wall structure (reproduced with permission from [Hanani et al. 2005]) showing interstitial cells of Cajal (ICC) networks interspersed between the muscle layers and submucosa. (B) Cross section of a segment of murine small bowel with nuclei of the smooth muscle cells (SMCs) in gray and ICCs in red (circular muscle layer on top, mucosa on bottom). (C) Overlying the tissue is a set of extracellular electrodes. (D) Dissected segments may be impaled by intracellular electrodes so that single cell electrical activity is recorded, whether from ICCs (right electrode), SMCs (left electrode) or both. (E) Tissue may be further dissected into isolated small strands of muscle/ICCs. (F) Completely dissociated tissue or cells that heterologously express channels of interest may be voltage clamped in multiple configurations (from left to right: outside out, whole cell, cell attached and inside out).
Electrical activity can be studied at increasingly reductionist levels. A major objective in the study of ion channels of SMCs and ICCs is to identify the molecular identities and biophysical properties. A general multidisciplinary approach is a combination of electrophysiology, pharmacology and molecular biology. Ion channels can be classified by their ion selectivity (e.g. K+, Na+, Ca++, Cl–, nonselective cation), mode of activation (voltage, ligand, mechanical), and time-dependent properties of ion conduction (kinetics). Ion substitution and ion channel modulators can be used as early screening approaches for the presence of particular ion channel species [Barajas-Lopez et al. 1989]. However, the detailed study of the electrophysiological operation requires experimental approaches that span from the tissue level down to the molecular level. Extracellular recording electrodes can be used to record electrical activity at the tissue and organ levels [Egbuji et al. 2010] (Figure 1B) and tools are being developed for endoscopic use [Coleski and Hasler, 2004]. However, the study of the mechanisms that underlie electrical function at the organ level also requires examination on smaller scales. To this end, muscle strips may be obtained from animals and human surgical specimens. The strips may be dissected and can then be impaled using sharp microelectrodes (Figure 1C). The recorded electrical behavior is a composite of activities of ion channels of different families and from many surrounding cells [Hara et al. 1986]. These strips can be further dissociated into small bundles that allow closer inspection of the rhythmic behaviors, and often with an ability to control electrical parameters such as voltage (voltage clamp) [Beckett et al. 2004] (Figure 1D). Complete dissociation of the tissue allows detailed examination of single cells. The cells of interest can be identified visually or by specific molecular markers, such as Kit [Rich et al. 2002; Thomsen et al. 1998]. Individual cells can be sampled using electrophysiology in whole cell [Hamill et al. 1981] or single channel patch clamp modes [Neher and Sakmann, 1976] (Figure 1F). Finally, once the ion channels of interest are identified and cloned, these can be heterologously expressed in cell lines and fine details of channel operation can be studied [Beyder et al. 2010]. Single cell and patch approaches provide an experimental template for examination of potential pharmaceutical interventions, but these are notoriously low throughput. Recently, multipatch platforms have been developed to extend cellular electrophysiology studies into the high-throughput realm [Estacion et al. 2010].
Basic voltage-gated ion channel structure
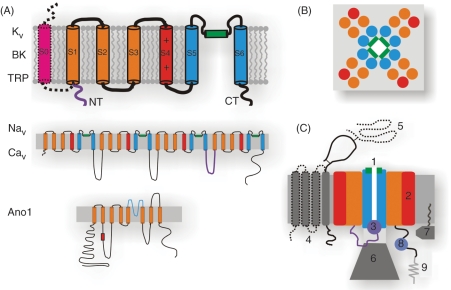
Voltage-gated ion channels gate predominantly in response to voltage. Other channels have some varying sensitivity for voltage, but their gating is dominated by other stimuli. We define both as VSICs and make them the focus of this review. The reasons for this broader channel selection are that VSICs have central roles in the physiology of GI motility and that they are structurally and functionally homologous. The basic building block of a VSIC is characterized by a single domain of a typical KV channel shown in Figure 2A (top). Functional potassium (KV), big K (BK) and transient receptor potentials (TRPs) are tetramers, while sodium (NaV) and calcium (CaV) channels are monomers with four homologous domains (Figure 2A, middle). The S1–S4 from each domain serve as the voltage sensors, and the charge on S4 determines the amount of voltage sensitivity (high for KV, NaV, CaV, low for TRP, for example). The S5 and S6 from the four domains fold to form the pore (blue), with the S5–S6 linker (green) serving as a selectivity filter. The molecular architecture of these channels is emerging as recent crystal structures are showing a cruciform layout with the voltage sensors making an extensive interaction with the surrounding lipids (Figure 2B). The newly discovered calcium-activated-chloride channel (Ano1) has a topology that is different from the traditional VSICs, specifically that the voltage sensor is on the intracellular loop, but little is otherwise known about this channel’s structure (Figure 2A, bottom). Multiple potential drug targets for VSICs are presented as a diagram in Figure 2C and are explained below.
Figure 2.
Fundamental aspects of the voltage-sensitive ion channel (VSIC) structures. (A) The building block of a VSIC is a six transmembrane (TM) subunit. The positively charged voltage sensor (S4, red) is supported in structure and function by S1-S3 (orange). The pore is lined by S5-S6 (blue) with P-loop being the selectivity filter (green). Big K (BK) channels have an additional S0 subunit (pink). The inactivation particle is formed by the N-terminus (purple). Potassium (KV), BK and transient receptor potentials (TRPs) are tetramers, while sodium (NaV) and calcium (CaV) channels are monomers with four linked homologous domains. Ano1 is structurally unique, having eight TMs with a pore region between TM 5 and 6, and a proposed voltage sensor on the intracellular loop between TM 2 and 3. (B) Top view of the VSIC of the K+, Ca+ and Na+ families folds into a cruciform shape with the four S5-6 forming the pore and four voltage sensor domains (S1-S4) in the periphery making substantial contact with the bilayer lipids. (C) Side view of the VSIC modulatory elements that may serve as targets: (1) selectivity filter and C-type inactivation, (2) voltage sensors, (3) N-type inactivation, (4) NaV β-subunit (solid gray), CaV γ-subunit (solid+dotted), (5) CaV α2-subunit, (6) KV β-subunit, (7) KChIP, (8) calmodulin (CAM)-binding and EF-hand for Ca++ binding, (9) cytoskeleton binding via PDZ (on C-terminus) and ankyrin (DII-DIII) linker domains for NaV1.5 for example.
Pacemaker ICCs generate and propagate slow waves to SMCs which produce contractions. The mechanisms of cyclical electrical activity in the GI tract have been studied extensively (Figure 3A). Nevertheless, the ion channel that gives rise to pacemaker current which initiates the slow wave is still not established. The main candidates are a calcium-inhibited nonselective cation channel [Koh et al. 2002], a calcium-activated chloride channel [Gomez-Pinilla et al. 2009; Zhu et al. 2009] or a combination of both [Namkung et al. 2011; Kito and Suzuki, 2003]. Pacemaker current depolarization turns on the local T-type Ca++ channels [Lee et al. 2007] (Figure 3E), and in humans and dogs also NaV1.5 [Strege et al. 2003a] (Figure 3D) producing further depolarization and the upstroke of the slow wave. The slow wave plateau and the repolarization phase are likely a combination of multiple channels. These include Ano1, which after its involvement in the upstroke should pass outward currents, repolarizing the membrane at potentials more positive than –50 mV [Zhu et al. 2009] (Figure 3C). K channels, including ERG [McKay et al. 2006], delayed rectifiers [Huizinga et al. 2004; Hatton et al. 2001], BK and Ca++ activated K channels also contribute to repolarization [Zhu and Huizinga, 2008] (Figure 3F). The resting membrane potential is maintained by a combination of the sodium, potassium and NS cation channels. ICCs also express L-type Ca channels whose function is still not established. Slow waves generated by the ICCs are passively conducted to the SMCs, SMC depolarization provides the stimulus for opening of the voltage-dependent L-type calcium channels, leads to a Ca++ mediated action potential that consists of a plasmalemmal Ca++ influx, and a further Ca++ release from the internal stores, so-called calcium-activated calcium release. Increase in the cytoplasmic calcium is the impetus for excitation–contraction coupling.
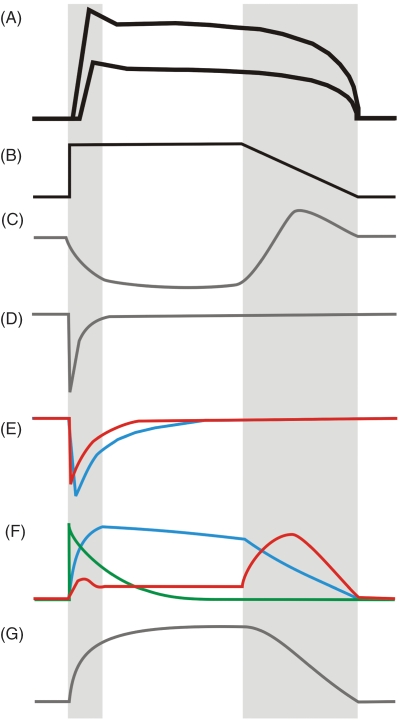
Figure 3.
Cartoon of voltage-sensitive ion channel conductances involved in formation of ICC and SMC slow waves. (A) Generic ICC and SMC slow waves with gray bars highlighting upstroke and repolarization. (B) A simplified ’slow wave’ voltage step protocol (from a holding potential of about -80 mV to 0 mV). Individual currents are shown in (C)-(G), with deflections downward and upward being inward (depolarizing), and outward (repolarizing) currents, respectively. (C) Ano1, (D) NaV currents, (E) CaV currents showing CaV1 or L-type (blue), CaV3 or T-type (red), (F) potassium currents showing KV3 and KV4 or A-type (green), KV1 or delayed rectifier and KCa1 or BK (blue), KV11.1 or ERG (red), (G) TRPM nonselective cation current.
Voltage-sensitive chloride selective channels
Voltage-sensitive Cl– channels have been described in the GI tract in the SMCs [Sun et al. 1992] and ICCs [Huizinga et al. 2002], with Ca++-activated Cl– current (CaCC) in various smooth muscle systems (as reviewed by Large and Wang [1996]). CaCC conductance was suggested as essential in slow wave generation, since a block of Cl– conductance resulted in the loss of slow wave, but the multiple molecular candidates did not pan out [Ferrera et al. 2010]. A recent determination that the CaCC anoctamin 1 (Ano1) encoded by TMEM16A [Caputo et al. 2008] in the muscularis propria is relatively specific to ICCs in mice and humans [Gomez-Pinilla et al. 2009]. ANO1 is vital for slow wave function in ICCs [Zhu et al. 2009], as mice lacking this gene fail to generate GI slow waves [Hwang et al. 2009]. Further, expression of this protein in heterologous systems results in voltage- and calcium-sensitive Cl– conductances [Schroeder et al. 2008] (Figure 3C). However, the situation is complex, since the topology map for these molecules lacks the traditional features of the voltage-sensitive channels [Sheridan et al. 2011], extensive alternative splicing alters sequence and function [Mazzone et al. 2011; Ferrera et al. 2009] and modulation by various stimuli makes these channels difficult to study [Tian et al. 2011].
Voltage-sensitive sodium selective channels
Various voltage-sensitive sodium selective channels. These channels are found in both the ICCs and SMCs of the circular smooth muscle layer of the human jejunum [Strege et al. 2003b; Holm et al. 2002; Ou et al. 2002], and dog [Strege et al. 2007], rat ileum [Smirnov et al. 1992] and gastric fundus [Muraki et al. 1991] as well as from the SMCs of rat and human colons [Xiong et al. 1993]. NaV channels are highly voltage sensitive inwardly rectifying with activation and inactivation kinetics on a millisecond scale (Figure 3D). Blocking of NaV channels by lidocaine and QX-314 reduced the rate of slow wave rise and increased slow wave duration, resulting in a decrease of slow wave frequency [Strege et al. 2003b]. Both TTX-sensitive [Smirnov et al. 1992] and TTX-resistant [Holm et al. 2002] NaV channels have been reported, suggesting that multiple NaV isoforms may be present in the GI SMCs and ICCs although it appears that the dominant channel is the TTX-resistant NaV1.5. The TTX-resistant current in human jejunum circular SMCs has been identified as NaV1.5, which is typically referred to as the ‘cardiac’ sodium channel [Ou et al. 2002]. It was found that patients with mutations in the SCN5A gene, which codes for NaV1.5, have more abdominal symptoms than controls. This finding was not accounted for by a prolonged QT interval [Locke et al. 2006]. SCN5A mutations are also prevalent in IBS [Saito et al. 2009b; Braak et al. 2008]. While the mechanism is not yet clear, a SCN5A mutation from an IBS patient resulted in smaller NaV1.5 currents and reduced mechanosensitivity [Saito et al. 2009a].
Nonselective cation channels
Gene chip studies show that TRPC1,2,4 and TRPM4,7 are expressed on ICC in mice [Chen et al. 2007]. Transient receptor potential (TRP) family channels are nonselective cation channels and are traditionally not considered to be in the VSIC family [Clapham, 2003]. However, we include them in this discussion because they have similar topology to the classical voltage-gated ion channels, TRPC (classic) and TRPM (melastatin) show substantial voltage sensitivity, and recent data suggest that TRPs may play a role in generation of slow waves although the exact molecular identity is still unclear (Figure 3G). TRPM7 in mouse-cultured ICC was reported as critical for slow waves [Kim et al. 2005] and in another study TRP4 were thought to be responsible for Ca++ influx, without which slow waves were abolished [Torihashi et al. 2002]. However, these data have yet to be widely reproduced.
Voltage-sensitive calcium selective channels
Voltage-sensitive calcium (CaV) channels L-type (CaV1) and T-type (CaV3) have been described in GI SMCs and ICCs. T-type Ca++ channels are also known as the low-voltage-gated, dihydropyridine (DHP)-resistant channels. CaV3.2 (α1h) voltage-gated calcium selective ion channels were identified as nifedipine resistant and mibefradil sensitive Ca++ currents that modulate the voltage-dependent Ca++ influx into the pacer units, activating them and entraining pacemaker response [Kim et al. 2002] (Figure 3E). The T-type channel blocker mibefradil decreases the slow wave amplitude [Hotta et al. 2007] and frequency [Kito et al. 2005]. These findings were also confirmed in the cacna1h knock-out mice [Gibbons et al. 2009]. The L-type Ca++ channels are also known as the high-voltage-gated, DHP-sensitive channels. The CaV1.2 (α1c) channels are densely expressed in the ICCs and SMCs [Chen et al. 2007]. These channels activate slower than the T-type channels on depolarization but have a higher total conductance in SMCs (Figure 3E). They provide the bulk of Ca++ required to initiate excitation–contraction [Corrias and Buist, 2007]. L-type currents are also known to be present in the ICCs [Kim et al. 2002]. Studies show that block of the L-type current has no significant effects on the slow waves [Dickens et al. 1999] but abolishes SMC mechanical activity [Farrugia, 1996]. Thus, constipation is a common side effect of the DHP antihypertensive medications, which block L-type currents [Farrugia, 1999]. On the other hand, the ubiquity of Ca++ channels in the GI tract is exploited by a novel antispasmodic otilonium bromide known to block both the L-type and multiple T-type subunits [Strege et al. 2010, 2004]. Since otilonium bromide is a quaternary ammonium that does not become systemically absorbed it may be used in the gut without systemic side effects [Evangelista, 2004].
Voltage-sensitive and calcium-activated potassium selective channels
Potassium channels make up half of the ion channel superfamily, and voltage-sensitive KV channels are the largest group [Wulff et al. 2009]. This rich diversity of K+ channels is reflected in the wide variety of potassium channels detected in ICCs and SMCs of the GI tract [Vogalis, 2000]. With the intracellular K+ concentration more than 10-fold higher than extracellular, K+ channel opening typically produces an outward K+ flux that drives the membrane potential in the negative direction toward K+ reversal potential. Consequently, as a broad oversimplification, K+ channels maintain resting potential, participate in the plateau current and repolarization of the slow wave [Farrugia, 1999]. GI SMCs and ICCs express a broad range of K+ channels with multiplicity of functional properties described by variable kinetics and some with sensitivities for secondary modulation by Ca++ and auxiliary proteins. This variability of K+ channels and currents in the GI tract is responsible for the flexibility of the plateau and repolarizing waveforms. In this review we focus on the six TM voltage sensitive K channels, and will refrain from discussion of the many two TM K channels, such as Kir, which also have significant roles (K channels in smooth muscle are reviewed by Vogalis [2000]).
Voltage-dependent K+ currents limit the upstroke, set the plateau and initiate repolarization (Figure 3F)
The upstroke of the slow wave is due to nonselective cation channels, Ca++, Cl– and Na+ channels as described above, and the upstroke of the AP is due to Ca++ influx through the L-type Ca++ channels and calcium-mediated calcium release. A-type K+ currents (IA) the earliest to activate after onset of depolarization and are responsible for limiting the slow wave upstroke. Inactivation of IA is of the order of a second [Hille, 2001], so these channels are also likely involved in part in the maintenance of the slow wave plateau phase [Barajas-Lopez et al. 1989]. In the GI tract, the locations and precise isoforms are variable, but there is good evidence that the KV4 [Amberg et al. 2003], KV3 [Ohya et al. 1997] families of channels are responsible for A-type current in the SMCs and perhaps ICCs [Parsons and Huizinga, 2010]. KV4 channels have especially diverse structures and make connections with an assortment of intracellular regulators, which significantly customize their behavior [Birnbaum et al. 2004]. Temporally following IA are slow delayed rectifier K+ (IK) currents and ether-à-go-go-related genes (eag). The channels responsible for IK current are TEA and 4-AP sensitive and commonly expressed in excitable cells. These are further divided into the rapid (IKr) and the slow (IKs) delayed rectifiers. Multiple KV1 family are expressed by GI SMCs, including Kv1.1 [Huizinga et al. 2004; Hatton et al. 2001], Kv1.2 [Hart et al. 1993], Kv1.5 [Overturf et al. 1994]. The delayed rectifier Kv1 subunits also have variable expression in the SMCs, and have an ability to form heterotetramers [Hatton et al. 2001]. This leads to a large variation of the on–off current kinetics observed experimentally and likely custom tailoring of the slow wave morphology. The eag code for voltage-sensitive K+ channels of three subfamilies (KV11 and KV12) that include eag, elk (eag-like), and erg (eag-related gene), with a common structural EAG domain in the N-terminus that regulates the rates of deactivation by binding to the S4–S5 linker [Schwarz and Bauer, 2004]. These channels have been found in SMCs of the esophagus [Akbarali et al. 1999], stomach [Ohya et al. 2002] and colon [Shoeb et al. 2003]. However, in the small intestine of a mouse ERG channels were detected of the ICC-MY but not on the SMC [Zhu et al. 2003]. ERG channels are inwardly rectifying ion channels with complex kinetics [Smith et al. 1996]. They inactivate quickly upon depolarization, then recover from inactivation upon repolarization, so they are likely involved in the repolarization phase of the slow waves [Schwarz and Bauer, 2004; Zhu et al. 2003]. ERG block by E-4031 and cisapride resulted in slowing of the slow wave activity [Akbarali et al. 1999]. hERG channels are notorious for complicating VSIC drug discovery efforts due to their promiscuous binding pocket that leads to inhibition of cardiac hERG, prolonging repolarization and predisposing to arrhythmias [Wulff et al. 2009]. GI prokinetic drug cisapride (5HT4 receptor agonist) was taken off the market due to its affinity for hERG [Rampe et al. 1997], but other more selective 5HT4 agonists are being designed specifically to minimize cross reactivity with hERG [Camilleri et al. 2009]. On the other hand, the difference between the cardiac and gut hERG is a truncation by a hundred residues in the gut [Shoeb et al. 2003], which may allow gut-specific targeting.
Calcium-activated K channels (KCa) decrease excitability
KCa channels are classified based on their conductance: small (SK), intermediate (IK) and big (BK). All of these channel types have been described in the GI smooth muscle [Farrugia, 1999]. BK channels are the only channels with intrinsic voltage-dependence, while IK and SK are not voltage sensitive outright, but appear so due to the voltage dependence of Ca++ influx. IK and SK channels are widely expressed in the SMCs and ICCs as well as in fibroblast-like cells (SK3) [Fujita et al. 2003], but due to the lack of voltage sensitivity we abstain from further discussion. The BK or maxi-KCa channels (KCa1.1) are homotetramers, but structurally unique in that they have an additional transmembrane segment S0 and a long C-terminus that forms a calcium binding bowl [Wu et al. 2010] (Figure 2A). While these channels are encoded by a single gene (slo1, KCNMA1), they undergo extensive processing leading to over 100 splice variants [Hille, 2001]. BK channels are ubiquitous in excitable cells, including the SMCs [Wang et al. 2010] and ICCs [Zhu and Huizinga, 2008]. Block of BK by ChTx has little impact on resting voltage but allows hyperexcitability [Hong et al. 1997]. Alternatively, BK activation reduced contractility of ileum smooth muscle [Dela Pena et al. 2009], so BK activators may be reasonable options for spasmodic motility disorders. It has also been shown that NO, which is produced in several places in the GI tissue activates the BK channels, increasing K+ influx and decreasing excitability [Zhu and Huizinga, 2008]. BK channels likely function as negative feedback regulators, assisting in repolarization and controlling excitability by primary modulation by Ca++ and secondary modulation by auxiliary subunits [Hagen et al. 2003], neural [Zhu and Huizinga, 2008] and mechanical [Wang et al. 2010] inputs.
Voltage-sensitive ion channels are viable targets for functional and motility disorders
VSICs are the essential players in the electrical and mechanical activity of the GI tract, and thus they are viable targets for functional and motility disorders. In the human genome VSICs constitute the largest group of signaling molecules after protein kinases and G-protein coupled receptors [Sharman et al. 2011]. Recent advances in electrophysiology and pharmacology are now being combined with the structural information from X-ray crystallography to provide specific molecular binding sites and conformational states that are worthy of pharmaceutical targeting [Cuello et al. 2010]. However, there are some difficulties in these approaches. Below, we begin with the limitations of targeting VSICs in GI functional and motility disorders and then provide potential solutions.
Complete understanding of the components of the slow wave and AP is lacking
At the most fundamental level, the development of rational drug targets is impeded by the lack of slow wave details and action potential mechanisms. A major difficulty is that dissociation of tissues required for identification and functional characterization of ion channels results in changes in the cells’ electrical activity. Techniques such as single-cell polymerase chain reaction (PCR) [Ou et al. 2002] and laser capture microdissection [Ou et al. 2003] are useful for isolation and study of ion channels from single cells. What is more, there are well-described differences in the ion channel types and isoforms across gut regions and species [Strege et al. 2007], which means that the database of the involved ion channels is extensive.
Multifactorial nature of the GI motility and functional disorders likely requires a combination of targets
Once we understand in detail the molecular components of the electrical activity and the functioning of the individual ion channels, we can begin to rationally address the prime targets for pharmaceutical intervention. At the present time, we only know that interventions to modulate channel function generally produce an altered cellular activity. However, most commonly those changes are difficult to predict and the outcomes at the tissue level are not clear. Thus, clear cut prime targets for particular motility disorders do not exist. We are beginning to understand which of the VSICs are indispensable for electrical activity. Even when prime VSIC targets are elucidated, their modulation would need to be state dependent and not an on–off type approach. In addition, given the multifactorial nature of motility disorders, successful therapeutic approaches are likely to be multifaceted, targeting the CNS, ENS and the effectors (SMCs and ICCs).
VSIC types are conserved across electrically active organs
The GI tract is electrically active and functionally similar to the other excitable tissues, such as the cardiac, neuronal, skeletal and other smooth muscles (e.g. bladder and blood vessels). There are obvious major differences in the roles for these organs, but from the standpoint of electrical function the distinction is only with respect to the resting voltage, amplitude and kinetics of the cyclical electrical activity. Owing to the similarities in functional requirements and the fact that the evolutionary tolerance of dysfunctional electrical systems is low, there is significant redundancy in the molecular components of the electrical systems. As the ion gradients have little variation, the upstroke of electrical activity (depolarization) involves NaV and CaV channels, while the KV channels are typically responsible for repolarization. The families of NaV and KV channels have several members, and there are some that are found exclusively in certain tissues, but all of the channels expressed in the GI tract tissue have been described elsewhere. This overlapping channel distribution means that drugs with systemic circulation targeted to GI tissues will likely also affect the same VSICs in other tissues.
VSICs have significant structural homology
A variety of voltage-sensitive ion channels exists. Drugs that have targeted VSICs have suffered from cross reactivity with other VSICs [Wulff et al. 2009]. As noted in Figure 2, there is significant structural conservation, which is one of the reasons for some cross reactivity for the drugs developed to target-specific channels. On the other hand, recent studies have shown that both the pores and voltage-sensor functional domains can confer their specific properties when spliced onto other channels [Alabi et al. 2007]. Thus, as for the other molecular targets even small perturbations in sequence [DeSimone et al. 2009], the surrounding lipid composition [Lundbaek et al. 2004] and mechanical state of the bilayer [Schmidt and Mackinnon, 2008] have been shown to significantly affect drug function.
While the limitations of voltage-gated ion channels as molecular targets in GI functional and motility disorders are real, there are potential solutions for drug developers to consider. We provide details of the approaches for targeting specific VSIC structural and functional motifs below.
Ion channel pores as drug targets
The pores of VSICs have specific ion selectivity (K+, Na+, Ca+, Cl–) and may therefore serve as specific drug targets. The best known is the selectivity filter and pore (green and blue, respectively in Figure 2) of a K+ channel (KcsA) which balance the needs for high selectivity and rapid conduction [Doyle et al. 1998]. In this example, the selectivity filter selects K+ ions, strips them of surrounding water molecules and ushers them through the selectivity filter (green) and then into a water-filled pore cavity for rehydration (blue) [Berneche and Roux, 2001]. The selectivity and pores of the other ion channels share some homology [Lipkind and Fozzard, 2000] and mechanism, but mutations of only single residues in the selectivity filter sometimes allow significant shifts in selectivity [Heinemann et al. 1992]. Permeation through the channels can be affected by either blocking the inner water-filled pore or by binding near the external face of the pore near the selectivity filter. Drugs such as TEA for K+ channels wedge into the water-filled pore to block conduction. This process is similar to the natural fast-inactivation (N-type) of these channels by the ball-and-chain intracellular block (purple in Figure 2) [del Camino et al. 2000]. While the block by these molecules is a physical obstruction of the water-filled inner vestibule, its structure in the NaV, CaV and KV is different enough that blockers generally do not cross react across families [Hille, 2001]. On the other side of the filter, the best known drugs that target the extracellular face of the pore are toxins such as TTX for NaV [Hille, 2001] and α-KTx for KV [Avdonin et al. 2000]. An alternative idea to affect permeation and the selectivity filter is through C-type (slow) inactivation, which requires restructuring of the selectivity filter such that ion flow is interrupted (green in Figure 2) [Cuello et al. 2010]. It is likely that a simple pore block is not a selective enough approach, since entire families of currents are affected and the effects are potentially devastating. In contrast, targeting the kinetics of both N- and C-type inactivation is an attractive approach to modulate permeation through the pore.
Voltage sensors as drug targets
The hallmark of VSICs is that opening and closing transitions are closely linked to changes in transmembrane voltage, often responding to changes of a few millivolts. The voltage-sensing mechanism deserves intense scientific attention, since modulation of the voltage sensor function allows elaborate regulation of ion channel function. In most simple terms, each subunit posses a voltage sensor which is a transmembrane helix (S4) with a few (2–5) positively charged arginine or lysine residues (red in Figure 2) and the supporting transmembrane segments (S1–S3; orange in Figure 2) [Long et al. 2005b]. The transmembrane voltage does work on the charged S4 helix displacing it across the lipid bilayer, and this movement is coupled to the swinging of the intracellular activation gate opening the pore and allowing ion conduction [Long et al. 2005a]. Venomous organisms have utilized the power of voltage-sensor modification [Escoubas and Rash, 2004]. Researchers have in turn used small peptide toxins from the venoms to study and specifically target the voltage sensors [Bosmans and Swartz, 2010]. Voltage sensor toxins are gating modifiers, essentially modulating the function of the voltage sensors, making voltage sensor operation either more or less stable. This is an overly simplified view. Detailed studies of the voltage sensor operation support a voltage-sensing mechanism that involves multiple conformational states [Bezanilla, 2008]. Each of these states can serve as a target for therapeutic intervention.
Ancillary subunits as drug targets
The VSIC α-subunits form fully functional ion channels. However, ancillary subunits and other modifiers (e.g. kinases and calmodulin) play a large role in determining ion channel function in vivo (Figure 2C) [Abriel, 2007]. These proteins constitute possible drug targets, as there is a variety of isoforms whose expression is frequently tissue specific. There is also flexibility in the approach for modulation by regulating either the levels of these proteins or their binding and activity at the α-subunits.
Biophysical considerations for pharmacologic targeting of VSICs
An alternative to the approach of targeting structural motifs is one of targeting specific functional aspects. Ion channels exist in many states in addition to the closed, open, and inactivated. For example, the voltage-sensing mechanism described above actually involves multiple conformational states [Bezanilla, 2008] and each of these states can serve as a target for therapeutic intervention. Moreover, conformational shifts during gating alter the affinity of some drugs for their binding sites, known as the ‘modulated receptor’ hypothesis [Hille, 1977]. An example of modulated receptor action is mibefradil binding to T-type Ca++ channels [Bezprozvanny and Tsien, 1995], which allows differential targeting specific to the voltage state of the system. In a guarded receptor model, the drug binding site cycles between completely accessible and inaccessible as the channels open and close, respectively. Then drug effect is use dependent and this effect may be utilized in the treatment of arrhythmias. An example is lidocaine binding by NaV1.5 during cardiac tachyarrhythmias [Nau and Wang, 2004], or perhaps a different hyperactive channel in gastric tachyarrhythmia [Lammers et al. 2008].
Mechano-electrical considerations
We now know that VSICs have an impact on the mechanical state of the surrounding membrane [Beyder and Sachs, 2009] and in return many of the VSICs are mechanically sensitive [Schmidt and Mackinnon, 2008]. Mechanically sensitive VSICs include those in the SMCs and ICCs [Kraichely and Farrugia, 2007], such as NaV1.5 [Beyder et al. 2010; Strege et al. 2003b], L-type Ca++ [Kraichely et al. 2009; Lyford et al. 2002], BK [Wang et al. 2010; Qi et al. 2005] and KV1 and KV4 [Laitko et al. 2006]. The molecular mechanisms for mechanical sensitivity by VSICs are being elucidated and likely involve voltage sensors [Beyder et al. 2010] and channel gates [Beyder and Sachs, 2009; Laitko et al. 2006]. Alteration in mechanical sensitivity may be responsible for pathology in a subset of IBS patients [Saito et al. 2009a] and thus may serve as another viable target for VSICs.
The detailed structural information revealed from crystallography or electron microscopy [Wang and Sigworth, 2009] continues to emerge and is now being combined into the body of biophysical knowledge to understand the mechanisms of VSIC function [Nishida et al. 2007]. Other experimental approaches aim to fill the gaps between the static structural information and electrophysiology. Examples are nuclear magnetic resonance (NMR) [Shah et al. 2006], electron para magnetic resonance (EPR) [Cordero-Morales et al. 2006], fluorescence resonance energy transfer (FRET) and other fluorescent approaches [Posson et al. 2005]. When integrated together those data may provide detailed targets for VSICs.
Defining other molecular domains in VSICs
There is Mounting evidence that ion channels are regulated at the genetic and epigenetic level [Shao et al. 2009] and by posttranslational modification [Akbarali et al. 2010]. Ion channels, including VSICs, are specifically spliced and levels are tightly managed in different tissues and pathologic states [Mazzone et al. 2011; Shi et al. 2011; Wang et al. 2010; Walker et al. 2001; Bielefeldt, 1999; Ohya et al. 1997]. Ion channel splicing and posttranslational modification often produces channels with altered function, whether due to alteration of the voltage-dependence properties, current selectivity and flux, or binding to the regulatory domains. The implications are twofold. First, custom processing of VSICs that alters functional performance on the molecular level also alters function at the cellular and tissue level. For example, hERG channels may be spliced differently in the gut and the heart, with the functional changes demonstrated in the gut hERG channels [Shoeb et al. 2003]. There is new evidence that alternative splicing of Ano1 in diabetic gastroparesis leads to functional consequences [Mazzone et al. 2011]. Second, such variations provide site- and disease-specific targets. An example of a successful splice targeting is the tissue-specific DHP block of the vascular L-type Ca++ channel due to alternative splicing of the DIS6 segments [Welling et al. 1997]. As our understanding of the alternative splicing mechanisms continues to advance other potential targets will emerge. These will allow control at the genetic and epigenetic levels as well as splice variant specific modulation by phosphorylation [Ahern et al. 2005] and interaction with G-proteins [Yarbrough et al. 2002].
Future directions
The continuous development of experimental tools for the study of electrophysiology in GI tissues has produced a deep understanding of electrical activity down to the molecular level. However, this progress has yet to bring the clinical tools that can diagnose and monitor GI motility disorders. In order to continue toward that goal we need to further our efforts to understand the mechanisms from the single molecule to the tissue levels, and then to build rational models and noninvasive ways to test those models. VSICs should figure prominently in this plan, since their function is vital to GI motility. In fact, their properties are a focus of intense study. Computational models are beginning to emerge that once populated with some experimental data on VSICs will be able to predict complex activity at the cellular and tissue levels [Faville et al. 2008; Corrias and Buist, 2007] (for a recent review see Lees-Green et al. [2011]). Furthermore, the development is continuing toward minimally invasive measurements of electrical activity in vivo [Egbuji et al. 2010]. These efforts may lead a state where for GI motility disorders we will be able to measure electrical activity in vivo and compare with the predicted normal, pointing out specific molecular pathologies and thus therapeutic targets.
Summary
Pathologies involving ICCs and SMCs lead to GI functional and motility disorders. Slow waves and action potentials are the signaling events that underpin normally functioning ICCs and SMCs. Voltage-sensitive ion channels are indispensable for slow waves and action potentials. Many of the voltage-sensitive ion channel species involved in the electrical activity of these two cell types have been discovered and their functions elucidated in remarkable but not yet sufficient detail. These molecules serve as potential exciting targets for pharmaceutical interventions.
Acknowledgments
The authors would like to thank Seth Eisenman for technical help and Dr Simon Gibbons for thoughtful discussions and comments on the manuscript.
Footnotes
This work was supported by the NIH (grant numbers DK52766 and DK84567).
The authors declare no conflicts of interest in preparing this article.
References
- Abriel H. (2007) Cardiac sodium channel Nav1.5 and its associated proteins. Arch Mal Coeur Vaiss 100: 787–793 [PubMed] [Google Scholar]
- Ahern C.A., Zhang J.F., Wookalis M.J., Horn R. (2005) Modulation of the cardiac sodium channel NaV1.5 by Fyn, a Src family tyrosine kinase. Circ Res 96: 991–998 [DOI] [PubMed] [Google Scholar]
- Akbarali H.I.,E,G.H., Ross G.R., Kang M. (2010) Ion channel remodeling in gastrointestinal inflammation. Neurogastroenterol Motil 22: 1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarali H.I., Thatte H., He X.D., Giles W.R., Goyal R.K. (1999) Role of HERG-like K(+) currents in opossum esophageal circular smooth muscle. Am J Physiol 277: C1284–C1290 [DOI] [PubMed] [Google Scholar]
- Alabi A.A., Bahamonde M.I., Jung H.J., Kim J.I., Swartz K.J. (2007) Portability of paddle motif function and pharmacology in voltage sensors. Nature 450: 370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg G.C., Koh S.D., Imaizumi Y., Ohya S., Sanders K.M. (2003) A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol 284: C583–C595 [DOI] [PubMed] [Google Scholar]
- Avdonin V., Nolan B., Sabatier J.M., De Waard M., Hoshi T. (2000) Mechanisms of Maurotoxin Action on Shaker Potassium Channels. Biophys J 79: 776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Lopez C., Den Hertog A., Huizinga J.D. (1989) Ionic basis of pacemaker generation in dog colonic smooth muscle. J Physiol 416: 385–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett E.A., Bayguinov Y.R., Sanders K.M., Ward S.M., Hirst G.D. (2004) Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J Physiol 559: 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneche S., Roux B. (2001) Energetics of ion conduction through the K+ channel. Nature 414: 73–77 [DOI] [PubMed] [Google Scholar]
- Beyder A., Rae J.L., Bernard C., Strege P.R., Sachs F., Farrugia G. (2010) Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J Physiol 588: 4969–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyder A., Sachs F. (2009) Electromechanical coupling in the membranes of Shaker-transfected HEK cells. Proc Natl Acad Sci U S A 106: 6626–6631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. (2008) How membrane proteins sense voltage. Nat Rev Mol Cell Biol 9: 323–332 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Tsien R.W. (1995) Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967). Mol Pharmacol 48: 540–549 [PubMed] [Google Scholar]
- Bielefeldt K. (1999) Molecular diversity of voltage-sensitive calcium channels in smooth muscle cells. J Lab Clin Med 133: 469–477 [DOI] [PubMed] [Google Scholar]
- Birnbaum S.G., Varga A.W., Yuan L.L., Anderson A.E., Sweatt J.D., Schrader L.A. (2004) Structure and function of Kv4-family transient potassium channels. Physiol Rev 84: 803–833 [DOI] [PubMed] [Google Scholar]
- Bosmans F., Swartz K.J. (2010) Targeting voltage sensors in sodium channels with spider toxins. Trends Pharmacol Sci 31: 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak B., Klooker T.K., Scholvinck D., Hofman N., Wilde A., Boeckxstaens G.E. (2008) Abdominal Symptoms in Patients with Long Qt Syndrome and a “Gain of Function” Mutation in the Nav1.5 Sodium Channel. Gastroenterology 134(4, Suppl. 1): A–A683 [Google Scholar]
- Camilleri M., Beyens G., Kerstens R., Robinson P., Vandeplassche L. (2009) Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterol Motil 21: e1256–e1117 [DOI] [PubMed] [Google Scholar]
- Camilleri M., Bharucha A.E., Farrugia G. (2011) Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol 9: 5–12; quiz e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., et al. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594 [DOI] [PubMed] [Google Scholar]
- Cervero F., Laird J.M. (2003) Role of ion channels in mechanisms controlling gastrointestinal pain pathways. Curr Opin Pharmacol 3: 608–612 [DOI] [PubMed] [Google Scholar]
- Chen H., Ordog T., Chen J., Young D.L., Bardsley M.R., Redelman D., et al. (2007) Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics 31: 492–509 [DOI] [PubMed] [Google Scholar]
- Cho W.J., Daniel E.E. (2005) Proteins of interstitial cells of Cajal and intestinal smooth muscle, colocalized with caveolin-1. Am J Physiol Gastrointest Liver Physiol 288: G571–G585 [DOI] [PubMed] [Google Scholar]
- Clapham D.E. (2003) TRP channels as cellular sensors. Nature 426: 517–524 [DOI] [PubMed] [Google Scholar]
- Coleski R., Hasler W.L. (2004) Directed endoscopic mucosal mapping of normal and dysrhythmic gastric slow waves in healthy humans. Neurogastroenterol Motil 16: 557–565 [DOI] [PubMed] [Google Scholar]
- Cordero-Morales J.F., Cuello L.G., Zhao Y., Jogini V., Cortes D.M., Roux B., et al. (2006) Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol 13: 311–318 [DOI] [PubMed] [Google Scholar]
- Corrias A., Buist M.L. (2007) A quantitative model of gastric smooth muscle cellular activation. Ann Biomed Engng 35: 1595–1607 [DOI] [PubMed] [Google Scholar]
- Cuello L.G., Jogini V., Cortes D.M., Perozo E. (2010) Structural mechanism of C-type inactivation in K(+) channels. Nature 466: 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino D., Holmgren M., Liu Y., Yellen G. (2000) Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature 403: 321–325, PM:10659852 [DOI] [PubMed] [Google Scholar]
- Dela Pena I.C., Yoon S.Y., Kim S.M., Lee G.S., Park C.S., Kim Y.C., et al. (2009) Inhibition of intestinal motility by the putative BK(Ca) channel opener LDD175. Arch Pharm Res 32: 413–420 [DOI] [PubMed] [Google Scholar]
- Der-Silaphet T., Malysz J., Hagel S., Larry Arsenault A., Huizinga J.D. (1998) Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology 114: 724–736 [DOI] [PubMed] [Google Scholar]
- DeSimone C.V., Lu Y., Bondarenko V.E., Morales M.J. (2009) S3b amino acid substitutions and ancillary subunits alter the affinity of Heteropoda venatoria toxin 2 for Kv4.3. Mol Pharmacol 76: 125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens E.J., Hirst G.D., Tomita T. (1999) Identification of rhythmically active cells in guinea-pig stomach. J Physiol 514: 515–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D.A., Cabral J.M., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., et al. (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69–77 [DOI] [PubMed] [Google Scholar]
- Egbuji J.U., O’Grady G., du P., Cheng L.K., Lammers W.J., Windsor J.A., et al. (2010) Origin, propagation and regional characteristics of porcine gastric slow wave activity determined by high-resolution mapping. Neurogastroenterol Motil 22: e292–e300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas P., Rash L. (2004) Tarantulas: eight-legged pharmacists and combinatorial chemists. Toxicon 43: 555–556 [DOI] [PubMed] [Google Scholar]
- Estacion M., Choi J.S., Eastman E.M., Lin Z., Li Y., Tyrrell L., et al. (2010) Can robots patch-clamp as well as humans? Characterization of a novel sodium channel mutation. J Physiol 588: 1915–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista S. (2004) Quaternary ammonium derivatives as spasmolytics for irritable bowel syndrome. Curr Pharm Des 10: 3561–3568 [DOI] [PubMed] [Google Scholar]
- Farrugia G. (1996) Modulation of ionic currents in isolated canine and human jejunal circular smooth muscle cells by fluoxetine. Gastroenterology 110: 1438–1445 [DOI] [PubMed] [Google Scholar]
- Farrugia G. (1999) Ionic conductances in gastrointestinal smooth muscles and interstitial cells of Cajal. Ann Rev Physiol 61: 45–84 [DOI] [PubMed] [Google Scholar]
- Farrugia G. (2008) Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil 20(Suppl. 1): 54–63 [DOI] [PubMed] [Google Scholar]
- Farrugia G., Lei S., Lin X., Miller S.M., Nath K.A., Ferris C.D., et al. (2003) A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci U S A 100: 8567–8570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faville R.A., Pullan A.J., Sanders K.M., Smith N.P. (2008) A biophysically based mathematical model of unitary potential activity in interstitial cells of Cajal. Biophys J 95: 88–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera L., Caputo A., Galietta L.J. (2010) TMEM16A protein: a new identity for Ca(2+)-dependent Cl channels. Physiology (Bethesda) 25: 357–363 [DOI] [PubMed] [Google Scholar]
- Ferrera L., Caputo A., Ubby I., Bussani E., Zegarra-Moran O., Ravazzolo R., et al. (2009) Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem 284: 33360–33368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A.C., Talley N.J. (2011) IBS in 2010: Advances in pathophysiology, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 8: 76–78 [DOI] [PubMed] [Google Scholar]
- Ford A.C., Talley N.J., Veldhuyzen van Zanten S.J., Vakil N.B., Simel D.L., Moayyedi P. (2008) Will the history and physical examination help establish that irritable bowel syndrome is causing this patient’s lower gastrointestinal tract symptoms?. JAMA 300: 1793–1805 [DOI] [PubMed] [Google Scholar]
- Fujita A., Takeuchi T., Jun H., Hata F. (2003) Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci 92: 35–42 [DOI] [PubMed] [Google Scholar]
- Galligan J.J. (2002) Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil 14: 611–623 [DOI] [PubMed] [Google Scholar]
- Galligan J.J. (2004) Enteric P2X receptors as potential targets for drug treatment of the irritable bowel syndrome. Br J Pharmacol 141: 1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan J.J., North R.A. (1988) Drug receptors on single enteric neurons. Life Sci 43: 2183–2192 [DOI] [PubMed] [Google Scholar]
- Gibbons S.J., Strege P.R., Lei S., Roeder J.L., Mazzone A., Ou Y., et al. (2009) The alpha1H Ca2+ channel subunit is expressed in mouse jejunal interstitial cells of Cajal and myocytes. J Cell Mol Med 13: 4422–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla P.J., Gibbons S.J., Bardsley M.R., Lorincz A., Pozo M.J., Pasricha P.J., et al. (2009) Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296: G1370–G1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine A.V., Wood J.D., Burnstock G. (2009) Purinergic signalling in autonomic control. Trends Neurosci 32: 241–248 [DOI] [PubMed] [Google Scholar]
- Hagen B.M., Bayguinov O., Sanders K.M. (2003) Beta 1-subunits are required for regulation of coupling between Ca2+ transients and Ca2+-activated K+ (BK) channels by protein kinase C. Am J Physiol Cell Physiol 285: C1270–C1280 [DOI] [PubMed] [Google Scholar]
- Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv Eur J Physiol 391: 85–100 [DOI] [PubMed] [Google Scholar]
- Hanani M., Farrugia G., Komuro T. (2005) Intercellular coupling of interstitial cells of Cajal in the digestive tract. Int Rev Cytol 242: 249–282 [DOI] [PubMed] [Google Scholar]
- Hara Y., Kubota M., Szurszewski J.H. (1986) Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol 372: 501–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P.J., Overturf K.E., Russell S.N., Carl A., Hume J.R., Sanders K.M., et al. (1993) Cloning and expression of a Kv1.2 class delayed rectifier K+ channel from canine colonic smooth muscle. Proc Natl Acad Sci U S A 90: 9659–9663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton W.J., Mason H.S., Carl A., Doherty P., Latten M.J., Kenyon J.L., et al. (2001) Functional and molecular expression of a voltage-dependent K(+) channel (Kv1.1) in interstitial cells of Cajal. J Physiol 533: 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S.H., Terlau H., Stuhmer W., Imoto K., Numa S. (1992) Calcium channel characteristics conferred on the sodium channel by single mutations. Nature 356: 441–443 [DOI] [PubMed] [Google Scholar]
- Hille B. (1977) Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69: 497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. (2001) Ion Channels of Excitable Membranes, 3rd edn, Sinauer Associates, Inc.: Sunderland, MA [Google Scholar]
- Holm A.N., Rich A., Miller S.M., Strege P., Ou Y., Gibbons S., et al. (2002) Sodium current in human jejunal circular smooth muscle cells. Gastroenterology 122: 178–187 [DOI] [PubMed] [Google Scholar]
- Hong S.J., Roan Y.F., Chang C.C. (1997) Spontaneous activity of guinea pig ileum longitudinal muscle regulated by Ca(2+)-activated K+ channel. Am J Physiol 272: G962–G971 [DOI] [PubMed] [Google Scholar]
- Hotta A., Okada N., Suzuki H. (2007) Mibefradil-sensitive component involved in the plateau potential in submucosal interstitial cells of the murine proximal colon. Biochem Biophys Res Commun 353: 170–176 [DOI] [PubMed] [Google Scholar]
- Huizinga J.D., Golden C.M., Zhu Y., White E.J. (2004) Ion channels in interstitial cells of Cajal as targets for neurotransmitter action. Neurogastroenterol Motil 16(Suppl. 1): 106–111 [DOI] [PubMed] [Google Scholar]
- Huizinga J.D., Thuneberg L., Kluppel M., Malysz J., Mikkelsen H.B., Bernstein A. (1995) W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349 [DOI] [PubMed] [Google Scholar]
- Huizinga J.D., Zhu Y., Ye J., Molleman A. (2002) High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology 123: 1627–1636 [DOI] [PubMed] [Google Scholar]
- Hwang S.J., Blair P.J., Britton F.C., Odriscoll K.E., Hennig G., Bayguinov J.R., et al. (2009) Expression of anoctamin 1Tmem16a by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap P., Farrugia G. (2010) Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut 59: 1716–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Lim H.H., Yang D.K., Jun J.Y., Chang I.Y., Park C.S., et al. (2005) Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology 129: 1504–1517 [DOI] [PubMed] [Google Scholar]
- Kim Y.C., Koh S.D., Sanders K.M. (2002) Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol 541: 797–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y., Suzuki H. (2003) Electrophysiological properties of gastric pacemaker potentials. J Smooth Muscle Res 39: 163–173 [DOI] [PubMed] [Google Scholar]
- Kito Y., Ward S.M., Sanders K.M. (2005) Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol 288: C710–C720 [DOI] [PubMed] [Google Scholar]
- Koh S.D., Jun J.Y., Kim T.W., Sanders K.M. (2002) A Ca(2+)-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol 540: 803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraichely R.E., Farrugia G. (2007) Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol Motil 19: 245–252 [DOI] [PubMed] [Google Scholar]
- Kraichely R.E., Strege P.R., Sarr M.G., Kendrick M.L., Farrugia G. (2009) Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum. Am J Physiol Gastrointest Liver Physiol 296: G833–G839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitko U., Juranka P.F., Morris C.E. (2006) Membrane stretch slows the concerted step prior to opening in a Kv channel. J Gen Physiol 127: 687–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers W.J., Ver Donck L., Stephen B., Smets D., Schuurkes J.A. (2008) Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology 135: 1601–1611 [DOI] [PubMed] [Google Scholar]
- Large W.A., Wang Q. (1996) Characteristics and physiological role of the Ca(2+)-activated Cl- conductance in smooth muscle. Am J Physiol 271: C435–C454 [DOI] [PubMed] [Google Scholar]
- Lee H.T., Hennig G.W., Fleming N.W., Keef K.D., Spencer N.J., Ward S.M., et al. (2007) The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology 132: 1852–1865 [DOI] [PubMed] [Google Scholar]
- Lee H.T., Hennig G.W., Park K.J., Bayguinov P.O., Ward S.M., Sanders K.M., et al. (2009) Heterogeneities in ICC Ca2+ activity within canine large intestine. Gastroenterology 136: 2226–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Green R., Du P., O’Grady G., Beyder A., Farrugia G., Pullan A.J. (2011) Biophysically-based modelling of the interstitial cells of Cajal: Current status and future perspectives. Front Comput Physiol, Med, submitted [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind G.M., Fozzard H.A. (2000) KcsA crystal structure as framework for a molecular model of the Na(+) channel pore. Biochemistry 39: 8161–8170 [DOI] [PubMed] [Google Scholar]
- Locke G.R., 3rd, Ackerman M.J., Zinsmeister A.R., Thapa P., Farrugia G. (2006) Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am J Gastroenterol 101: 1299–1304 [DOI] [PubMed] [Google Scholar]
- Long S.B., Cambell E.B., MacKinnon R. (2005a) Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309: 897–903 [DOI] [PubMed] [Google Scholar]
- Long S.B., Cambell E.B., MacKinnon R. (2005b) Voltage sensor of kv1.2: Structural basis of electromechanical coupling. Science 309: 903–908 [DOI] [PubMed] [Google Scholar]
- Lundbaek J.A., Birn P., Hansen A.J., Sogaard R., Nielsen C., Girshman J., et al. (2004) Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J Gen Physiol 123: 599–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford G.L., Strege P.R., Shepard A., Ou Y., Ermilov L., Miller S.M., et al. (2002) alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol 283: C1001–C1008 [DOI] [PubMed] [Google Scholar]
- Mazzone A., Bernard C.E., Strege P.R., Beyder A., Galietta L.J., Pasricha P.J., et al. (2011) Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem 286(15): 13393–13403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay C.M., Ye J., Huizinga J.D. (2006) Characterization of depolarization-evoked ERG K currents in interstitial cells of Cajal. Neurogastroenterol Motil 18: 324–333 [DOI] [PubMed] [Google Scholar]
- Muraki K., Imaizumi Y., Watanabe M. (1991) Sodium currents in smooth muscle cells freshly isolated from stomach fundus of the rat and ureter of the guinea-pig. J Physiol 442: 351–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung W., Phuan P.W., Verkman A.S. (2011) TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem 286: 2365–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau C., Wang G.K. (2004) Interactions of local anesthetics with voltage-gated Na+ channels. J Membrane Biol 201: 1–8 [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. (1976) Single-channel currents recorded from membrane of denervated frog muscle-fibers. Nature 260: 799–802 [DOI] [PubMed] [Google Scholar]
- Nishida M., Cadene M., Chait B.T., MacKinnon R. (2007) Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J 26: 4005–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S., Asakura K., Muraki K., Watanabe M., Imaizumi Y. (2002) Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol 282: G277–G287 [DOI] [PubMed] [Google Scholar]
- Ohya S., Tanaka M., Oku T., Asai Y., Watanabe M., Giles W.R., et al. (1997) Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel alpha-subunit, Kv4.3 in the rat. FEBS Lett 420: 47–53 [DOI] [PubMed] [Google Scholar]
- Ou Y., Gibbons S.J., Miller S.M., Strege P.R., Rich A., Distad M.A., et al. (2002) SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil 14: 477–486 [DOI] [PubMed] [Google Scholar]
- Ou Y., Strege P., Miller S.M., Makielski J., Ackerman M., Gibbons S.J., et al. (2003) Syntrophin gamma 2 regulates SCN5A gating by a PDZ domain-mediated interaction. J Biol Chem 278: 1915–1923 [DOI] [PubMed] [Google Scholar]
- Overturf K.E., Russell S.N., Carl A., Vogalis F., Hart P.J., Hume J.R., et al. (1994) Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am J Physiol 267: C1231–C1238 [DOI] [PubMed] [Google Scholar]
- Parsons S.P., Huizinga J.D. (2010) Transient outward potassium current in ICC. Am J Physiol Gastrointest Liver Physiol 298: G456–G466 [DOI] [PubMed] [Google Scholar]
- Posson D.J., Ge P., Miller C., Bezanilla F., Selvin P.R. (2005) Small vertical movement of a K+ channel voltage sensor measured with luminescence energy transfer. Nature 436: 848–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley T.L., Wang X.Y., Fox E.A., Phillips R.J., Liu L.W., Huizinga J.D. (2008) Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil 20: 69–79 [DOI] [PubMed] [Google Scholar]
- Qi Z., Chi S., Su X., Naruse K., Sokabe M. (2005) Activation of a mechanosensitive BK channel by membrane stress created with amphipaths. Mol Membrane Biol 22: 519–527 [DOI] [PubMed] [Google Scholar]
- Rampe D., Roy M.L., Dennis A., Brown A.M. (1997) A mechanism for the proarrhythmic effects of cisapride (Propulsid): high affinity blockade of the human cardiac potassium channel HERG. FEBS Lett 417: 28–32 [DOI] [PubMed] [Google Scholar]
- Rich A., Hanani M., Ermilov L.G., Malysz J., Belzer V., Szurszewski J.H., et al. (2002) Physiological study of interstitial cells of Cajal identified by vital staining. Neurogastroenterol Motil 14: 189–196 [DOI] [PubMed] [Google Scholar]
- Saito Y.A., Strege P.R., Tester D.J., Locke G.R.,III, Talley N.J., Bernard C.E., et al. (2009a) Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol - Gastrointest Liver Physiol 296: G211–G218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y.A., Tester D.J., Mazzone A., Beyder A., Locke G.R.,III, Talley N.J., et al. (2009b) Sodium channel mutations in irritable bowel syndrome. In: Proceedings of the Neurogastroenterology and Motility Conference, Chicago, IL [Google Scholar]
- Sarna S.K. (2008) Are interstitial cells of Cajal plurifunction cells in the gut?. Am J Physiol Gastrointest Liver Physiol 294: G372–G390 [DOI] [PubMed] [Google Scholar]
- Schmidt D., Mackinnon R. (2008) Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci U S A 105: 19276–19281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B.C., Cheng T., Jan Y.N., Jan L.Y. (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.R., Bauer C.K. (2004) Functions of erg K+ channels in excitable cells. J Cell Mol Med 8: 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V.N., Wingo T.L., Weiss K.L., Williams C.K., Balser J.R., Chazin W.J. (2006) Calcium-dependent regulation of the voltage-gated sodium channel hH1: intrinsic and extrinsic sensors use a common molecular switch. Proc Natl Acad Sci U S A 103: 3592–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D., Okuse K., Djamgoz M.B. (2009) Protein-protein interactions involving voltage-gated sodium channels: Post-translational regulation, intracellular trafficking and functional expression. Int J Biochem Cell Biol 41: 1471–1481 [DOI] [PubMed] [Google Scholar]
- Sharman J.L., Mpamhanga C.P., Spedding M., Germain P., Staels B., Dacquet C., et al. (2011) IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucleic Acids Res 39(Database issue): D534–D538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J.T., Worthington E.N., Yu K., Gabriel S.E., Hartzell H.C., Tarran R. (2011) Characterization of the oligomeric structure of the Ca2+-activated Cl- channel Ano1/TMEM16A. J Biol Chem 286: 1381–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X.Z., Lin Y.M., Powell D.W., Sarna S.K. (2011) Pathophysiology of motility dysfunction in bowel obstruction: role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol 300: G99–G108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb F., Malykhina A.P., Akbarali H.I. (2003) Cloning and functional characterization of the smooth muscle ether-a-go-go-related gene K+ channel. Potential role of a conserved amino acid substitution in the S4 region. J Biol Chem 278: 2503–2514 [DOI] [PubMed] [Google Scholar]
- Smirnov S.V., Zholos A.V., Shuba M.F. (1992) Potential-dependent inward currents in single isolated smooth muscle cells of the rat ileum. J Physiol 454: 549–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.L., Baukrowitz T., Yellen G. (1996) The inward rectification mechanism of the HERG cardiac potassium channel. Nature 379: 833–836 [DOI] [PubMed] [Google Scholar]
- Smith T.K., Kang S.H., Vanden Berghe P. (2003) Calcium channels in enteric neurons. Curr Opin Pharmacol 3: 588–593 [DOI] [PubMed] [Google Scholar]
- Storr M.A., Sharkey K.A. (2007) The endocannabinoid system and gut-brain signalling. Curr Opin Pharmacol 7: 575–582 [DOI] [PubMed] [Google Scholar]
- Strege P.R., Evangelista S., Lyford G.L., Sarr M.G., Farrugia G. (2004) Otilonium bromide inhibits calcium entry through L-type calcium channels in human intestinal smooth muscle. Neurogastroenterol Motil 16: 167–173 [DOI] [PubMed] [Google Scholar]
- Strege P.R., Holm A.N., Rich A., Miller S.M., Ou Y., Sarr M.G., et al. (2003a) Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am J Physiol Cell Physiol 284: C60–C66 [DOI] [PubMed] [Google Scholar]
- Strege P.R., Mazzone A., Kraichely R.E., Sha L., Holm A.N., Ou Y., et al. (2007) Species dependent expression of intestinal smooth muscle mechanosensitive sodium channels. Neurogastroenterol Motil 19: 135–143 [DOI] [PubMed] [Google Scholar]
- Strege P.R., Ou Y., Sha L., Rich A., Gibbons S.J., Szurszewski J.H., et al. (2003b) Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 285: G1111–G1121 [DOI] [PubMed] [Google Scholar]
- Strege P.R., Sha L., Beyder A., Bernard C.E., Perez-Reyes E., Evangelista S., et al. (2010) T-type Ca(2+) channel modulation by otilonium bromide. Am J Physiol Gastrointest Liver Physiol 298: G706–G713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.P., Supplisson S., Torres R., Sachs G., Mayer E. (1992) Characterization of large-conductance chloride channels in rabbit colonic smooth muscle. J Physiol 448: 355–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen L., Robinson T.L., Lee J.C., Farraway L.A., Hughes M.J., Andrews D.W., et al. (1998) Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med 4: 848–851 [DOI] [PubMed] [Google Scholar]
- Tian Y., Kongsuphol P., Hug M., Ousingsawat J., Witzgall R., Schreiber R., et al. (2011) Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J 25: 1058–1068 [DOI] [PubMed] [Google Scholar]
- Torihashi S., Fujimoto T., Trost C., Nakayama S. (2002) Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J Biol Chem 277: 19191–19197 [DOI] [PubMed] [Google Scholar]
- Vogalis F. (2000) Potassium channels in gastrointestinal smooth muscle. J Auton Pharmacol 20: 207–219 [DOI] [PubMed] [Google Scholar]
- Walker R.L., Hume J.R., Horowitz B. (2001) Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol Cell Physiol 280: C1184–C1192 [DOI] [PubMed] [Google Scholar]
- Wang L., Sigworth F.J. (2009) Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature 461: 292–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Huang H., Hou D., Liu P., Wei H., Fu X., et al. (2010) Mechanosensitivity of STREX-lacking BKCa channels in the colonic smooth muscle of the mouse. Am J Physiol Gastrointest Liver Physiol 299: G1231–G1240 [DOI] [PubMed] [Google Scholar]
- Welling A., Ludwig A., Zimmer S., Klugbauer N., Flockerzi V., Hofmann F. (1997) Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res 81: 526–532 [DOI] [PubMed] [Google Scholar]
- Won K.J., Sanders K.M., Ward S.M. (2005) Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A 102: 14913–14918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Yang Y., Ye S., Jiang Y. (2010) Structure of the gating ring from the human large-conductance Ca(2+)-gated K(+) channel. Nature 466: 393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H., Castle N.A., Pardo L.A. (2009) Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discovery 8: 982–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Sperelakis N., Noffsinger A., Fenoglio-Preiser C. (1993) Fast Na+ current in circular smooth muscle cells of the large intestine. Pflugers Arch 423: 485–491 [DOI] [PubMed] [Google Scholar]
- Yarbrough T.L., Lu T., Lee H.C., Shibata E.F. (2002) Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res 90: 443–449 [DOI] [PubMed] [Google Scholar]
- Zhu M.H., Kim T.W., Ro S., Yan W., Ward S.M., Koh S.D., et al. (2009) A Ca2+-activated Cl- conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Golden C.M., Ye J., Wang X.Y., Akbarali H.I., Huizinga J.D. (2003) ERG K+ currents regulate pacemaker activity in ICC. Am J Physiol Gastrointest Liver Physiol 285: G1249–G1258 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Huizinga J.D. (2008) Nitric oxide decreases the excitability of interstitial cells of Cajal through activation of the BK channel. J Cell Mol Med 12(5A): 1718–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]