Abstract
X-linked dyskeratosis congenita (X-DC) is caused by mutations in the housekeeping nucleolar protein dyskerin. Amino acid changes associated with X-DC are remarkably heterogeneous. Peripheral mononuclear blood cells and fibroblasts isolated from X-DC patients harbor lower steady-state telomerase RNA (TER) levels and shorter telomeres than healthy age-matched controls. Previously, we showed that retroviral expression of recombinant TER, together with expression of recombinant telomerase reverse transcriptase, restored telomere maintenance and proliferative capacity in X-DC patient cells. Using rare X-DC isoforms (▵L37 and A386T dyskerin), we showed that telomere maintenance defects observed in X-DC are solely due to decreased steady-state levels of TER. Disease-associated reductions in steady-state TER levels cause deficiencies in telomere maintenance. Here, we confirm these findings in other primary X-DC patient cell lines coding for the most common (A353V dyskerin) and more clinically severe (K314R and A353V dyskerin) X-DC isoforms. Using cell lines derived from these patients, we also examined the steady-state levels of other hinge-ACA motif RNAs and did not find differences in their in vivo accumulations. We show, for the first time, that purified telomerase holoenzyme complexes from different X-DC cells have normal catalytic activity. Our data confirm that dyskerin promotes TER stability in vivo, endorsing the development of TER supplementation strategies for the treatment of X-DC.
INTRODUCTION
Dyskeratosis congenita (DC) is a rare, heritable telomere biology disorder characterized by the diagnostic triad of nail dystrophy, reticular skin hyperpigmentation and oral leukoplakia (1–3). Bone marrow failure is the leading cause of mortality of DC. Patients with DC are also at high risk of cancer and other complications related to poor tissue renewal capacity (1,2,4,5). Autosomal dominant, autosomal recessive and X-linked recessive forms of the disease have been described and linked to genes that encode components of telomerase and telomere-associating proteins (6–11). Hoyeraal–Hreidarsson syndrome (HHS) is a severe form of DC (12,13). In addition to the diagnostic triad and bone marrow failure, patients with HHS exhibit slow prenatal and postnatal growth, cerebellar hypoplasia, microcephaly, developmental delay and immunologic abnormalities. HHS is a multisystem disorder with significantly earlier morbidity and disease onset than DC. Death often occurs within the first decade of life (12,13).
X-DC arises mostly from non-synonymous (missense) amino acid substitutions within the housekeeping protein dyskerin (DKC1) (14) (Supplementary Material, Fig. S1). Many HHS cases are also associated with specific missense mutations in dyskerin (12,15–17). Dyskerin is a pseudouridine synthase that catalyzes the isomerization of uridine to pseudouridine in non-coding RNAs (18,19). Being an essential gene, targeted deletion of dyskerin is embryonically lethal in mice (20). As a pseudouridine synthase, dyskerin binds to small nucleolar RNA (snoRNA) and small Cajal body RNA (scaRNA) bearing the signature hinge-ACA motif (H/ACA) (18,21,22). These snoRNA and scaRNA then act as sequence-specific guides for dyskerin to bind, and then modify targeted RNAs, including ribosomal and spliceosomal RNAs (23,24). Dyskerin is also an integral component of the telomerase holoenzyme. Dyskerin binds to the telomerase RNA (TER) H/ACA domain and protects the in vivo stability of TER (6,22). Evidence also indicates that TER harbors pseudouridine modification sites, and in vitro modification of these sites provides structural stability to TER (25).
Clinical presentations of X-DC are variable but correlate with very short telomeres in peripheral blood leukocytes (26,27). Surveys of X-DC patient samples revealed a universal reduction in steady-state TER levels (6,28,29). There are more than 30 known non-synonymous mutations of dyskerin associated with X-DC, and a subset of these are associated with HHS (30) (Supplementary Material, Fig. S1). These missense mutations cluster in two distinct regions: the first cluster (amino acids 30–70) is found near the amino-terminal of the protein, adjacent to the catalytic core and the TruB domains, and the second cluster (amino acids 310–420) overlaps with the RNA-binding PUA domain (31). A recent report posits that residues along these two mutation clusters form the binding surfaces for the H/ACA pre-ribonucleoprotein complex assembly factor Shq1 (32). Shq1 is a biogenesis factor, acting as a chaperone for dyskerin, preceding the formation of the core dyskerin-Nhp2-Nop10 trimer and the loading of Naf1. X-DC-associated dyskerin mutations were shown to affect the interaction between dyskerin and Shq1, and the resultant decrease in steady-state availability of the mutant dyskerins for pre-ribonucleoprotein (RNP) assembly was postulated as a molecular mechanism for the decrease in telomerase RNP (33).
Forced expression of two telomerase components, telomerase reverse transcriptase (TERT) and TER, in two selected X-DC patient cell lines from each of the two clusters of X-DC mutations (▵L37 and A386T dyskerin), corrects for growth deficiency phenotypes (29). Molecular characterizations reveal that telomere elongation and maintenance functions are restored by the activities of exogenous telomerase components, without first correcting for dyskerin protein sequence and function. Cell lines with restored telomere maintenance capacity do not exhibit any ribosomal dysfunction (29). Hence, the current disease model of X-DC stipulates that dyskerin mutations impact telomere biology by affecting the steady-state accumulation of TER (28,34). Supplying X-DC cells with an over-abundance of recombinant TER overcomes the deficient TER accumulation caused by dyskerin protein mutations, supporting the use of recombinant telomerase expression as a viable clinical strategy.
Beyond promoting TER accumulation, dyskerin remains in the active telomerase holoenzyme complex. Conceivably, some dyskerin variants could also change the catalytic activity of the assembled ribonucleoprotein enzyme. This has important implications for clinical therapy: in addition to increasing the expression of wild-type (WT) TER to compensate for reduced accumulations, one would also need to compensate for an altered dyskerin sequence and a lower specific activity of telomerase. Studies of mouse models engineered to express specific X-DC-associated dyskerin variants suggest that not all dyskerin variants have the same impact on TER and telomerase function. Testing patient cells with common isoforms, and those with clinically severe isoforms, of X-DC-associated dyskerin variants is essential to establish the generality of prior conclusions.
RESULTS
Expression of telomerase components restores telomerase activity and telomere maintenance in X-DC patient fibroblasts
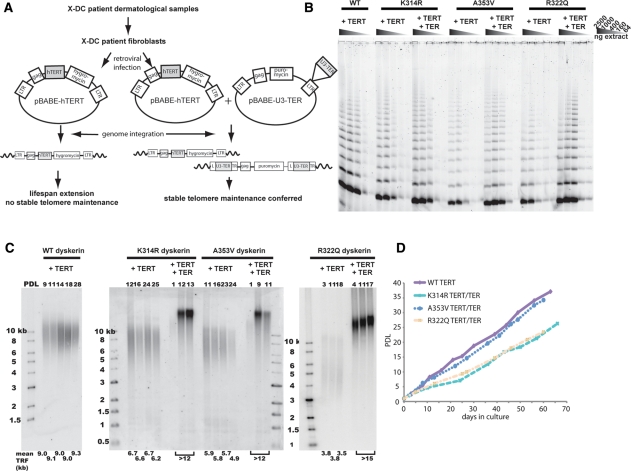
Stable expression of TERT, mediated by retroviral or other gene transfer methods, confers replicative immortality and stable telomere length maintenance in human fibroblasts (34–36). We previously showed that in contrast to immortalization of WT human fibroblasts, restoring stable telomere maintenance in X-DC cells requires the recombinant expression of TER in addition to TERT (29). In this study, we used the same telomerase component expression scheme (Fig. 1A) to restore stable telomere length maintenance in three new X-DC patient fibroblast lines encoding for K314R, R322Q and A353V dyskerin. All three mutations are in the carboxy-terminal cluster of mutations within the conserved PUA RNA-binding domain. Notably, both K314R and A353V dyskerin are reported to associate with the HHS variant of DC, in addition to X-DC (31).
Figure 1.
Construction and characterization of telomerase-rescued X-DC fibroblasts. (A) Schematic of retroviral infection. (B) Whole cell extracts from each X-DC cell line, expressing TERT alone, or both TERT and TER, were normalized according to protein concentration and assayed for telomerase activity using TRAP. Comparison of TRAP activities in X-DC-TERT cells without the co-expression of recombinant TER with WT cells expressing TERT showed that telomerase activity was reduced more than 2.5-fold. Telomerase activities of the TERT and TER expressing X-DC cells were all similar or higher than the levels observed in WT cells expressing only TERT. (C) Terminal restriction fragment analysis. X-DC cells, expressing TERT alone or both TERT and TER were grown in continuous culture and telomere length profiles were obtained using Southern blot analysis. In contrast to WT cells, X-DC cells expressing TERT alone exhibited a proliferation-dependent loss of telomere repeats. Stable telomere maintenance was restored in X-DC cells expressing both TERT and TER. (D) Growth characteristics of telomerase-rescued X-DC cells. All TERT/TER expressing X-DC cells were grown in continuous culture for many population doublings beyond what is shown. There were no observable changes in morphology or other growth characteristics.
We confirmed the restoration of telomerase activity with telomere repeat amplification protocol (TRAP) activity assays (Fig. 1B). X-DC cells expressing TERT and TER exhibited TRAP activity profiles similar to, or slightly higher than, WT dyskerin control cells receiving TERT alone. For cells forced to express TERT alone, X-DC cells' telomerase activity was at least 2.5-fold lower than control cells expressing WT dyskerin. The three new X-DC cell lines showed similar reductions in reconstituted telomerase activity, following the forced expression of TERT, in agreement with prior established X-DC models (29) and primary patient materials (28), which showed ∼2–5-fold reductions in TER accumulation and TRAP activity.
Following the co-expression of TERT and TER in X-DC cells, increased telomerase activity was accompanied by telomere length maintenance. This was not the case in X-DC cells expressing TERT alone (Fig. 1C). Increases in telomere restriction fragment (TRF) length occurred soon after retroviral infection and persisted over many population doublings. Analysis of the morphology and proliferation pattern of these ‘telomerase-rescued’ X-DC cells did not reveal any substantial growth defects or loss of viability compared with WT cells expressing TERT alone (Fig. 1D). Dyskerin protein levels in X-DC cells and WT cells were similar following retrovirus-directed TERT and TER expression (Supplementary Material, Fig. S2). X-DC-associated dyskerin mutations, including the A353V mutation tested here, were recently reported to affect the pre-RNP assembly of dyskerin with its biogenesis factor Shq1 (33). Increased association (with A353V, T49M and M350I) and decreased association (with T66A and M350T) between dyskerin variants and Shq1 were both suggested to reduce the availability of dyskerin for pre-RNP assembly with other core H/ACA proteins, thus reducing the steady-state levels of TER–H/ACA RNP complexes. Our data showed that these pre-RNP assembly deficiencies, at least in the case of A353V dyskerin, could be overcome by the recombinant over-expression of TER. We also demonstrated that recombinant TER over-expression could be a viable option in the clinical treatment of X-DC, as recombinant TER expression restored efficient telomere length maintenance in X-DC cells, comparable with the levels observed in WT controls.
Steady-state TER levels are dependent on the DKC1 genotype
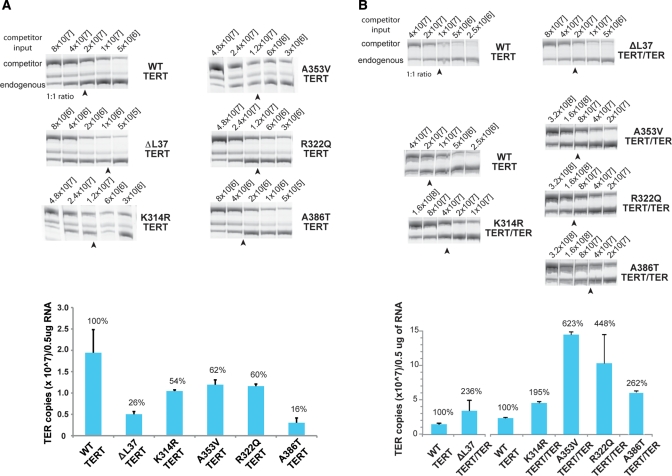
Short telomeres have a strong correlation with clinically significant telomere biology disorders (26,37). Current models of X-DC pathology posit that the maintenance of telomeres by telomerase is limited by steady-state TER accumulations (38,39). We have shown that various X-DC dyskerin isoforms differently affect steady-state TER accumulations (28,29). To broaden this observation with the new panel of X-DC cells, we used a previously described competitive reverse transcription polymerase chain reaction (RT–PCR) assay to quantify endogenous TER copy numbers.
In agreement with our prior data, WT control fibroblasts showed the largest pool of steady-state TER (28,29). Expression of mutant dyskerin isoforms variably impacted steady-state TER levels, with ▵L37 and A386T producing significantly lower TER levels than other isoforms. Steady-state TER levels were reduced to 26 and 16% of those in WT cells (Fig. 2A). The three PUA domain mutants (K314R, R322Q and A353V) showed moderate reductions in steady-state TER levels: 54, 62 and 60% of WT cells respectively. Notably, we found steady-state TER levels to be compromised for all X-DC isoforms examined, confirming the generality of the observation that X-DC disease etiology is strongly associated with reduced TER steady-state accumulation.
Figure 2.
TER steady-state levels are significantly different in X-DC isoforms. (A) Steady-state TER levels were measured in X-DCTERT cells using a previously published competitive RT–PCR assay (see text). Input-tagged competitor RNA copy numbers are indicated for each series, and the densitometry quantification data are shown in the bar graph, expressed as a percentage of levels in WT cells expressing TERT alone. All X-DC isoforms exhibited lower steady-state accumulations of TER, compared with WT cells. In agreement with previously determined TER levels, ▵L37 and A386T dyskerin mutations resulted in ∼5-fold lower steady-state accumulations of TER. The three new X-DC cell lines with mutations within the PUA domain (K314R, R322Q and A353V) each exhibit close to 2-fold reduction in TER steady-state levels. (B) Steady-state TER levels in X-DC cells expressing recombinant TERT and TER. Forced expression of telomerase components significantly increased TER accumulation beyond WT control levels. The arrows indicate the approximate competitor inputs where 1:1 ratios of competitor to endogenous TERT RT–PCR signals were obtained. For the quantification experiment, each RNA sample was measured first with a 10-fold serial dilution range of input competitors, then repeated with a suitable 2-fold serial dilution range for final copy number calculation. Each quantification experiment was repeated at least two times. Error bars represent standard error. TER quantification experiments in (B) for ▵L37 TERT/TER cells were performed using a different competitor RNA stock than the other quantification experiments shown.
Using the same competitive RT–PCR protocol, we verified that forced TER expression in X-DC cells leads to increased TER accumulation, higher than levels in WT control cells (Fig. 2B). These data confirm that the forced expression of TER, under a heterogeneous promoter, can overcome defective in vivo steady-state accumulations conferred by X-DC dyskerin mutations. Interestingly, the steady-state levels of TER, under transcriptional regulation by the housekeeping U3 (C/D-box snoRNA) promoter, exhibited marked variations in steady-state TER accumulations. We inferred that recombinant TER steady-state accumulation was most strongly influenced by the chromatin neighborhood through stochastic retroviral integration, rather than dyskerin functional status.
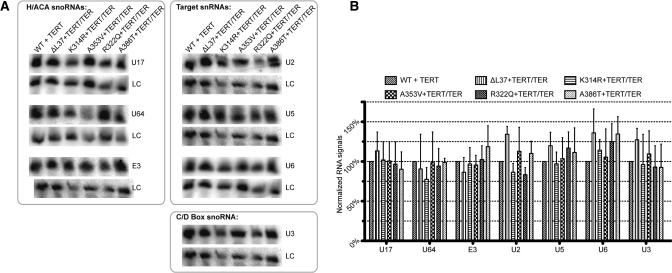
Levels of selected H/ACA RNAs, or their targets, are not affected by DKC-1 mutations
We had previously reported that the steady-state accumulation of other H/ACA small nuclear RNAs were not affected by X-DC-associated dyskerin variants. We tested the steady-state accumulation of these RNAs in Q31E and T66A mutant dyskerins which occur in the N-terminal mutational hotspot (28). In contrast, a recent report posited that X-DC-associated dyskerin mutations affect the pre-RNP assembly of the H/ACA complex, inferring that the stability of all H/ACA RNPs, and not just the telomerase RNP, could be compromised (33). Using the newly constructed X-DC cell models, we performed Northern blot analysis of steady-state levels of representative H/ACA RNAs, including the U17(E1), E3 and U64 RNAs. We also examined the accumulation of two pseudouridinylase modification targets: the small nuclear RNA U2 (targeted by the H/ACA-scaRNAs ACA26, ACA35, ACA45, U92, U93 and U100) and U5 (targeted by the H/ACA-scaRNAs ACA57, U85, U87, U88, U89 and U93; Fig. 3A). The steady-state accumulations of C/D-box snoRNA U3, as well as the signal recognition particle RNA (7SL), were used as controls. Consistent with our previous data using peripheral mononuclear blood cells from X-DC patients, we did not observe any statistically significant differences in small RNA accumulations in X-DC cells with an N-terminal (▵L37) mutation, nor in X-DC models harboring the C-terminal PUA domain (K314R, R322Q, A353V and A386T) dyskerin mutations (Fig. 3B). In parallel, we examined the steady-state levels of 28S and 18S ribosomal RNAs from our X-DC cell model collection. Together, the 28S and 18S rRNAs contain the largest sum of pseudouridine modification target sites, and ∼10% of the total uridine residues were pseudouridine modified. We did not find any significant differences between WT and X-DC cells in 28S and 18S ribosomal RNA accumulations (Supplementary Material, Fig. S3). We conclude that PUA domain dyskerin mutations do not generally compromise steady-state accumulation of H/ACA RNAs, and thereby affect the in vivo stability of their modification targets (U2 and U5 RNAs, as well as 18S and 28S rRNAs). Conceivably, in vivo half-lives of H/ACA RNAs, other than TER, may be minimally affected by the loss in pre-RNP assembly, and our assays to measure steady-state levels of these RNAs are not able to reveal these perturbations. This is not a comprehensive test for all available H/ACA-containing small RNAs and therefore we cannot rule out that some specific small RNAs could be affected by X-DC-associated dyskerin mutations in a manner analogous to decreased steady-state accumulation of TER. Further, systematic testing using assays more sensitive than Northern blots may confirm whether such small RNA species exist.
Figure 3.
Selected H/ACA RNAs and pseudourinylase modification targets are not affected by X-DC-associated DKC-1 mutations. (A) Northern blotting analysis of steady-state levels of selected small RNAs in X-DC cells stably expressing recombinant TERT and TER. (B) Quantification of the steady-state accumulation of small RNAs normalized to the signal recognition particle (7SL) RNA. RNA measurements by Northern analysis were repeated at least three times and error bars represent standard deviations.
The specific catalytic activity of telomerase is unaffected by DKC-1 mutations
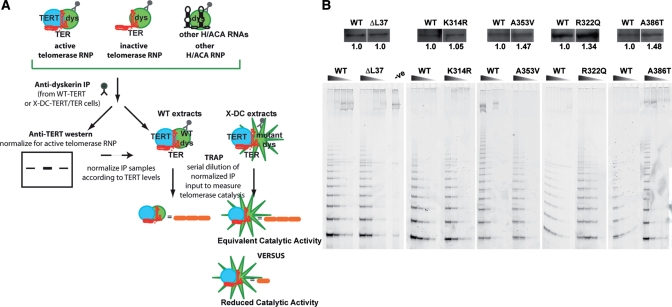
Biochemical purifications of telomerase holoenzyme from human cells show that dyskerin remains in stable association with core catalytic components of the telomerase holoenzyme throughout its in vivo lifespan (40). Although we have shown that the over-expression of telomerase components can overcome deficient telomere synthesis in X-DC, it is not known whether the presence of mutant dyskerin in the assembled telomerase holoenzyme can compromise its catalytic activity.
To measure the specific catalytic activity of the telomerase holoenzyme, we performed a series of immunoprecipitation experiments with anti-dyskerin antibodies from whole cell extracts prepared from X-DC fibroblasts stably expressing telomerase components. Anti-dyskerin immunoprecipitates are comprised of a mixture of TER complexes, which may (catalytically active RNP) or may not (catalytically inactive RNP) contain a TERT molecule, in addition to other dyskerin-containing nucleolar ribonucleoprotein complexes. To ensure that we are measuring equal number of active RNPs, we standardized our telomerase activity measurements with TERT signals. TERT protein levels were determined with immunoblotting analysis for each anti-dyskerin immunoprecipitate sample. Quantified TERT protein levels were then used to normalize the anti-dyskerin immunoprecipitate input for the determination of telomerase activity by TRAP. This normalization procedure allowed us to measure the relative activity of purified telomerase holoenzyme complexes that contain both dyskerin and TERT in a manner that is directly proportional to the number of holoenzyme units that were presented in the immunoprecipitated samples (Fig. 4A).
Figure 4.
Telomerase catalytic activity is not affected by X-DC-associated dyskerin mutations. (A) Schematic and rationale of the immunoprecipitation and normalization procedure. Each TRAP activity profile was determined with a calculated input anti-dyskerin immunoprecipitate, normalized for TERT content, shown immediately above the TRAP products. (B) Representative immunoprecipitation-western-TRAP experiment. There were no observable differences between telomerase holoenzyme activities isolated from WT versus X-DC isoforms. TRAP activity profiles of a 5-fold dilution series are shown. Each IP-western-TRAP experiment was repeated at least three times with similar results. We also performed TRAP assays with the same immunoprecipitates in a 2.5-fold dilution series, and similar results were obtained.
Using endogenously assembled telomerase holoenzyme isolated from X-DC fibroblasts ensured that each complex contained the mutant form of dyskerin protein. By comparing TRAP activity profiles of immunoprecipitates isolated from X-DC and WT cells, we assessed the activity of holoenzyme complexes containing mutant versus WT dyskerins. Our quantitative immunoprecipitation-western-TRAP data revealed that telomere repeat addition activity per holoenzyme unit did not differ between X-DC isoforms and control WT dyskerin (Fig. 4B). We also tested the activity profiles of recombinant WT and X-DC dyskerin-containing telomerase complexes assembled in a heterologous system (293 HEK cells). Again, we did not detect any substantial differences in specific telomerase catalytic activity between WT or X-DC dyskerin-containing telomerase holoenzymes, confirming our data obtained from primary human cells (Supplementary Material, Fig. S4). The presence of X-DC-associated (mutant) dyskerin isoforms did not adversely affect telomere repeat catalysis by the assembled telomerase holoenzyme, indicating that the reduced telomerase activity in X-DC is due to a lower steady-state TER accumulation rather than enzymatic defects in telomere repeat synthesis.
DISCUSSION
We previously demonstrated that telomere-length-maintenance defects in ΔL37 and A386T X-linked DC patient fibroblasts arise solely from reduced accumulation of TER. Stable expression of TER transgene phenotypically rescues the telomere-length-maintenance defects in these two X-DC cell models. In this study, we examined and characterized an extended panel of X-DC patient fibroblasts. The A353V dyskerin mutation is the most common genetic lesion associated with X-DC, and together with K314R encodes for protein isoforms that are associated with the severe HHS variant of DC. Our study shows that X-DC disease etiology strongly associates with limited TER steady-state accumulation. We also report the first biochemical measurement of the specific activity of telomerase holoenzyme purified from different X-DC patient cells. Our data indicate that the specific activity of the telomerase holoenzyme is not affected by coding sequence changes in its obligate cofactor dyskerin, but is due to the reduction in TER accumulation.
Patients with X-DC have an early age of onset of bone marrow failure, very short telomeres and numerous other medical complications. Conceivably, restricted telomere maintenance brought on by reduced steady-state TER levels could be corrected by clinical interventions before disease onset (39). Recently, two studies examining the characteristics of telomere maintenance capacity with induced pluripotent stem cells (iPSC) derived from DC samples. The first study reported the significant gain in mean telomere length of X-DC cells upon iPSC conversion, ascribed to the up-regulation of TERC. This gain in telomere length in iPSC, however, is not believed to be clinically relevant as TRF loss following re-differentiation of these iPSC populations will be substantial (41). In contrast, a second study using the iPSC derived from the same X-DC sample recapitulates the accelerated telomere attrition phenotypes (42), even without re-differentiation attempts. Conclusions from both studies argued that the development of clinical therapies using X-DC iPSC is contingent on being able to restore optimal telomerase activity to these cells. The lack of dyskerin mutation effects on telomerase catalysis and the consistent decrease in steady-state levels of TER in X-DC should encourage the development of parallel clinical therapies to boost TER levels, particularly in ex vivo transplant materials, for disorders of telomere biology such as X-DC and specific forms of autosomal dominant DC.
MATERIALS AND METHODS
Construction of X-DC cell lines
Patients with X-DC were participants in the National Cancer Institute's IRB approved Inherited Bone Marrow Failure Syndromes (IBMFS) study (http://marrowfailure.cancer.gov, protocol number 02-C-0052, NCT00056121) (43). The IBMFS study collects detailed clinical information and laboratory studies of patients with DC and other IBMFS. Skin biopsies were obtained from patients with K314R, R322Q and A353V dyskerin mutations with informed consent from each patient or their parent. Identity-blinded primary fibroblast lines were created using standard protocols. Retroviral vector-mediated expressions of TERT and TER were performed as previously described (29,44) (Fig. 1A). Frozen aliquots of established K314R (PDL3), R322Q (PDL2) and A353V (PDL3) fibroblasts were thawed, allowed to recover in culture until fibroblasts were settled and then infected with the respective viruses directly. Following infection, cells were selected with the appropriate antibiotic (hygromycin or puromycin) and grown in mass culture. The first passage following antibiotic selection was set as PDL1.
X-DC cells with ▵L37 and A386T dyskerins stably expressing telomerase components (TERT alone or TERT with U3-TER) were obtained with permission from the Laboratory of Kathleen Collins in Berkeley (29).
Whole cell lysate and telomerase activity assay
Cells were harvested and whole cell extracts were isolated with previously described protocols (29,44). Protein concentrations were determined using the Bradford protein assay (BioRad). Telomerase activity was measured with the TRAP, with slight modifications, as described. In brief, whole cell extracts, in a 5-fold dilution series, or other serial dilution series as stated, were incubated in 1× TPCR buffer, 50 µm dNTP and 0.1 μg M2 primer (5′-AATCCGTCGAGCAGAGTT-3′) for 1 h at 30°C, followed by 3 min at 95°C. The products were then amplified by PCR, with the addition of 1× TPCR buffer, 0.1 μg Cy5-fluorophor-labeled CX3 reverse primer (CY5-5′-CCGCCCCTAACCCTAACCT-3′) and 1.25 U Pfu polymerase enzyme. The reactions were subjected to 27 cycles of PCR (30 s at 94°C, 30 s at 50°C and 90 s at 72°C). TRAP products were resolved on non-denaturing polyacrylamide gel electrophoresis (PAGE), and the fluorescent images were obtained with a Typhoon Imager, fitted with the appropriate laser and filters (GE Healthcare Life Sciences).
Genomic DNA isolation and terminal restriction fragment analysis
Genomic DNA, from continuous cultures of X-DC cells, was collected using standard protocols (45). After quantification by optical density (OD), genomic DNA was digested overnight with RsaI and HinfI endonucleases. Digested samples were phenol:chloroform:isoamyl alcohol purified and sodium acetate/ethanol precipitated. Picogreen fluorescent dye was used to quantify digested genomic DNA, and 1 μg of digested DNA was resolved by agarose gel electrophoresis, followed by in-gel hybridization with radioactively end-labeled telomeric repeat DNA oligo (TTAGGG)3. Southern blot images were acquired using storage phosphor screen and a Typhoon Imager (GE Healthcare Life Sciences).
Signal quantification was performed using the ImageQuant (v. 5.2) software. TRF length was determined as a weighted average. The entire gel image was divided into horizontal segments corresponding to the size markers. To obtain the TRF score for each sample, the OD of each horizontal segment was multiplied by the length corresponding to the respective segment, summed, and then divided by the sum of the OD of all segments (i.e. TRF = Σ(ODi*Li)/ΣOdi, where ODi is optical density at interval i, and Li is average length at interval i). TRF lengths are reported as kilobase, to one decimal place.
Anti-dyskerin immunoprecipitation
Whole cell lysates from different X-DC cells were immunoprecipitated with an anti-dyskerin (Santa Cruz Biotechnology Inc.) antibody, according to the published protocols (6,46). The immunoprecipitates were divided into equal aliquots and stored in IP wash buffer. One aliquot of the anti-dyskerin immunoprecipitates was assayed for TERT protein levels, using standard immunoblotting analysis. Following quantification of TERT protein levels by densitometry, equivalent amounts of immunoprecipitate, normalized to the TERT content from each IP, were subjected to TRAP to measure corresponding telomerase activities.
Protein measurement by western blot
Equal amounts of anti-dyskerin immunoprecipitates, or 50 µg whole cell extracts, were resolved in non-continuous Tris-glycine sodium dodecyl sulphate gels. Following polyvinylidene fluoride (PVDF), the resolved protein samples were transferred to PVDF membranes (GE HealthCare Life Sciences). PVDF membranes were incubated with hTERT primary antibodies (Epitomics Inc.), or the dyskerin polyclonal antibodies and control beta-actin monoclonal antibodies. Protein signals were detected using chemiluminescence, with the ECL western blotting kit (GE Healthcare Life Sciences). Densitometry and the ImageJ software (NIH) quantified protein signal.
Small RNA quantification by Northern blot
Total RNA from telomerase-rescued X-DC fibroblasts was isolated with TriZol reagent according to the manufacturer's protocol. Following quantification of RNA samples with spectrophotometry, isolated RNA samples were run on 1% denaturing 3-(N-morpholino)propanesulfonic acid gel and stained with SybrGreen II RNA stain (Molecular Probes). The 28S and 18S rRNA ratio was measured for each collected RNA sample. Image collection and densitometry were done according to the manufacturer's protocol using the Typhoon Imager and the ImageQuant software.
For Northern analysis, 20 µg of total RNA was separated by polyacrylamide gel electrophoresis on denaturing 5% polyacrylamide-urea gel. Size-separated RNAs were transferred onto the Hybond N+ nylon membrane. Northern hybridization signals, following a probe with end-labeled oligomers against various small RNAs, were quantified using a storage phosphor screen and the Typhoon imager. Densitometry and image processing were performed with the ImageQuant software.
TER copy number measurement by competitive RT–PCR
TER was quantified with slight modifications to a previously published protocol (28): the forward primer was labeled with a CY5 fluorophor tag to allow for fluorescent imaging of the amplified products. Following RT–PCR and product resolution with non-denaturing PAGE, fluorescent images were obtained by scanning with a Typhoon imager fitted with the appropriate filters. Using the ImageQuant software for signal quantification, a rough estimate of the total TER copy number was determined and a second RT–PCR was performed using sample-specific ranges of TER competitors in a 2-fold dilution series.
The RT–PCR products were resolved by non-denaturing PAGE, stained with SybrGreen I, according to the manufacturer's protocol (Molecular Probes) and imaged with a Typhoon Imager. Densitometry readings were quantified with ImageQuant software.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
FUNDING
This work was supported by the Canadian Institutes of Health Research operating grant (MOP-81094) and the Michael Smith Foundation for Health Research. J.M.Y.W. is a Tier 2 Canada Research Chair and a Michael Smith Foundation for Health Research Scholar. This work was also supported in part by the intramural research program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Kathleen Collins for sharing reagents and cell lines. We also thank Lisa Leathwood, Westat, Inc (NIH contracts N02-CP-11019, N02-CP-65504 and N02-CP-65501) for excellent study management. Rafael Zhao provide technical help with data analysis and we thanked him for this contribution.
REFERENCES
- 1.Dokal I. Dyskeratosis congenita: an inherited bone marrow failure syndrome. Br. J. Haematol. 1996;92:775–779. doi: 10.1046/j.1365-2141.1996.355881.x. doi:10.1046/j.1365-2141.1996.355881.x. [DOI] [PubMed] [Google Scholar]
- 2.Dokal I. Dyskeratosis congenita in all its forms. Br. J. Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. doi:10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 3.Sirinavin C., Trowbridge A.A. Dyskeratosis congenita: clinical features and genetic aspects. J. Med. Genet. 1975;12:339–354. doi: 10.1136/jmg.12.4.339. doi:10.1136/jmg.12.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrone A., Walne A., Dokal I. Dyskeratosis congenita: telomerase, telomeres and anticipation. Curr. Opin. Genet. Dev. 2005;15:249–257. doi: 10.1016/j.gde.2005.04.004. doi:10.1016/j.gde.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Alter B.P., Giri N., Savage S.A., Rosenberg P.S. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. doi:10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell J.R., Wood E., Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. doi:10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 7.Vulliamy T., Marrone A., Goldman F., Dearlove A., Bessler M., Mason P.J., Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. doi:10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 8.Vulliamy T., Beswick R., Kirwan M., Marrone A., Digweed M., Walne A., Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc. Natl Acad. Sci. USA. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. doi:10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walne A.J., Vulliamy T., Marrone A., Beswick R., Kirwan M., Masunari Y., Al-Qurashi F.H., Aljurf M., Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum. Mol. Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. doi:10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage S.A., Giri N., Baerlocher G.M., Orr N., Lansdorp P.M., Alter B.P. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am. J. Hum. Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. doi:10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong F., Savage S.A., Shkreli M., Giri N., Jessop L., Myers T., Chen R., Alter B.P., Artandi S.E. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 2011;25:11–16. doi: 10.1101/gad.2006411. doi:10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaghmai R., Kimyai-Asadi A., Rostamiani K., Heiss N.S., Poustka A., Eyaid W., Bodurtha J., Nousari H.C., Hamosh A., Metzenberg A. Overlap of dyskeratosis congenita with the Hoyeraal-Hreidarsson syndrome. J. Pediatr. 2000;136:390–393. doi: 10.1067/mpd.2000.104295. doi:10.1067/mpd.2000.104295. [DOI] [PubMed] [Google Scholar]
- 13.Knight S.W., Heiss N.S., Vulliamy T.J., Greschner S., Stavrides G., Pai G.S., Lestringant G., Varma N., Mason P.J., Dokal I., et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am. J. Hum. Genet. 1999;65:50–58. doi: 10.1086/302446. doi:10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiss N.S., Knight S.W., Vulliamy T.J., Klauck S.M., Wiemann S., Mason P.J., Poustka A., Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. doi:10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 15.Cossu F., Vulliamy T.J., Marrone A., Badiali M., Cao A., Dokal I. A novel DKC1 mutation, severe combined immunodeficiency (T+B-NK- SCID) and bone marrow transplantation in an infant with Hoyeraal-Hreidarsson syndrome. Br. J. Haematol. 2002;119:765–768. doi: 10.1046/j.1365-2141.2002.03822.x. doi:10.1046/j.1365-2141.2002.03822.x. [DOI] [PubMed] [Google Scholar]
- 16.Knight S.W., Heiss N.S., Vulliamy T.J., Aalfs C.M., McMahon C., Richmond P., Jones A., Hennekam R.C., Poustka A., Mason P.J., et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br. J. Haematol. 1999;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. doi:10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- 17.Pearson T., Curtis F., Al-Eyadhy A., Al-Tamemi S., Mazer B., Dror Y., Abish S., Bale S., Compton J., Ray R., et al. An intronic mutation in DKC1 in an infant with Hoyeraal-Hreidarsson syndrome. Am. J. Med. Genet. A. 2008;146A:2159–2161. doi: 10.1002/ajmg.a.32412. doi:10.1002/ajmg.a.32412. [DOI] [PubMed] [Google Scholar]
- 18.Meier U.T. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. doi:10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zucchini C., Strippoli P., Biolchi A., Solmi R., Lenzi L., D'Addabbo P., Carinci P., Valvassori L. The human TruB family of pseudouridine synthase genes, including the Dyskeratosis Congenita 1 gene and the novel member TRUB1. Int. J. Mol. Med. 2003;11:697–704. [PubMed] [Google Scholar]
- 20.He J., Navarrete S., Jasinski M., Vulliamy T., Dokal I., Bessler M., Mason P.J. Targeted disruption of Dkc1, the gene mutated in X-linked dyskeratosis congenita, causes embryonic lethality in mice. Oncogene. 2002;21:7740–7744. doi: 10.1038/sj.onc.1205969. doi:10.1038/sj.onc.1205969. [DOI] [PubMed] [Google Scholar]
- 21.Kiss A.M., Jady B.E., Bertrand E., Kiss T. Human box H/ACA pseudouridylation guide RNA machinery. Mol. Cell Biol. 2004;24:5797–5807. doi: 10.1128/MCB.24.13.5797-5807.2004. doi:10.1128/MCB.24.13.5797-5807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell J.R., Cheng J., Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol. Cell Biol. 1999;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terns M., Terns R. Noncoding RNAs of the H/ACA family. Cold Spring Harb. Symp. Quant. Biol. 2006;71:395–405. doi: 10.1101/sqb.2006.71.034. doi:10.1101/sqb.2006.71.034. [DOI] [PubMed] [Google Scholar]
- 24.Ye K. H/ACA guide RNAs, proteins and complexes. Curr. Opin. Struct. Biol. 2007;17:287–292. doi: 10.1016/j.sbi.2007.05.012. doi:10.1016/j.sbi.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Kim N.K., Theimer C.A., Mitchell J.R., Collins K., Feigon J. Effect of pseudouridylation on the structure and activity of the catalytically essential P6.1 hairpin in human telomerase RNA. Nucleic Acids Res. 2010;38:6746–6756. doi: 10.1093/nar/gkq525. doi:10.1093/nar/gkq525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alter B.P., Baerlocher G.M., Savage S.A., Chanock S.J., Weksler B.B., Willner J.P., Peters J.A., Giri N., Lansdorp P.M. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. doi:10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savage S.A., Dokal I., Armanios M., Aubert G., Cowen E.W., Domingo D.L., Giri N., Greene M.H., Orchard P.J., Tolar J., et al. Dyskeratosis congenita: the first NIH clinical research workshop. Pediatr. Blood Cancer. 2009;53:520–523. doi: 10.1002/pbc.22061. doi:10.1002/pbc.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong J.M., Kyasa M.J., Hutchins L., Collins K. Telomerase RNA deficiency in peripheral blood mononuclear cells in X-linked dyskeratosis congenita. Hum. Genet. 2004;115:448–455. doi: 10.1007/s00439-004-1178-7. [DOI] [PubMed] [Google Scholar]
- 29.Wong J.M.Y., Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. doi:10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrone A., Mason P.J. Dyskeratosis congenita. Cell Mol. Life Sci. 2003;60:507–517. doi: 10.1007/s000180300042. doi:10.1007/s000180300042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Podlevsky J.D., Bley C.J., Omana R.V., Qi X., Chen J.J. The telomerase database. Nucleic Acids Res. 2008;36:D339–D343. doi: 10.1093/nar/gkm700. doi:10.1093/nar/gkm700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grozdanov P.N., Roy S., Kittur N., Meier U.T. SHQ1 is required prior to NAF1 for assembly of H/ACA small nucleolar and telomerase RNPs. RNA. 2009;15:1188–1197. doi: 10.1261/rna.1532109. doi:10.1261/rna.1532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grozdanov P.N., Fernandez-Fuentes N., Fiser A., Meier U.T. Pathogenic NAP57 mutations decrease ribonucleoprotein assembly in dyskeratosis congenita. Hum. Mol. Genet. 2009;18:4546–4551. doi: 10.1093/hmg/ddp416. doi:10.1093/hmg/ddp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong J.M., Collins K. Telomere maintenance and disease. Lancet. 2003;362:983–988. doi: 10.1016/S0140-6736(03)14369-3. doi:10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- 35.Bodnar A.G., Ouellette M., Frolkis M., Holt S.E., Chiu C.P., Morin G.B., Harley C.B., Shay J.W., Lichtsteiner S., Wright W.E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. doi:10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 36.Fleisig H.B., Wong J.M. Telomerase as a clinical target: current strategies and potential applications. Exp. Gerontol. 2007;42:102–112. doi: 10.1016/j.exger.2006.05.011. doi:10.1016/j.exger.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Armanios M. Syndromes of telomere shortening. Annu. Rev. Genomics Hum. Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. doi:10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat. Rev. Mol. Cell. Biol. 2006;7:484–494. doi: 10.1038/nrm1961. doi:10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trudeau M., Wong J. Genetic variations in telomere maintenance, with implications on tissue renewal capacity and chronic disease pathologies. Curr. Pharmacogenomics Person Med. 2010;8:7–24. doi: 10.2174/1875692111008010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen S.B., Graham M.E., Lovrecz G.O., Bache N., Robinson P.J., Reddel R.R. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. doi:10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal S., Loh Y.H., McLoughlin E.M., Huang J., Park I.H., Miller J.D., Huo H., Okuka M., Dos Reis R.M., Loewer S., et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature. 2010;464:292–296. doi: 10.1038/nature08792. doi:10.1038/nature08792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batista L.F., Pech M.F., Zhong F.L., Nguyen H.N., Xie K.T., Zaug A.J., Crary S.M., Choi J., Sebastiano V., Cherry A., et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature. 2011;474:399–402. doi: 10.1038/nature10084. doi:10.1038/nature10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alter B.P., Giri N., Savage S.A., Peters J.A., Loud J.T., Leathwood L., Carr A.G., Greene M.H., Rosenberg P.S. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br. J. Haematol. 2010;150:179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong J.M., Kusdra L., Collins K. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat. Cell Biol. 2002;4:731–736. doi: 10.1038/ncb846. doi:10.1038/ncb846. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 1989. [Google Scholar]
- 46.Mitchell J.R., Collins K. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase in vivo and in vitro. Mol. Cell. 2000;6:361–371. doi: 10.1016/s1097-2765(00)00036-8. doi:10.1016/S1097-2765(00)00036-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.