Abstract
Fragile X syndrome (FXS), caused by loss of the Fragile X Mental Retardation 1 (FMR1) gene product (FMRP), is the most common heritable cause of intellectual disability and autism spectrum disorders. It has been long hypothesized that the phosphorylation of serine 500 (S500) in human FMRP controls its function as an RNA-binding translational repressor. To test this hypothesis in vivo, we employed neuronally targeted expression of three human FMR1 transgenes, including wild-type (hFMR1), dephosphomimetic (S500A-hFMR1) and phosphomimetic (S500D-hFMR1), in the Drosophila FXS disease model to investigate phosphorylation requirements. At the molecular level, dfmr1 null mutants exhibit elevated brain protein levels due to loss of translational repressor activity. This defect is rescued for an individual target protein and across the population of brain proteins by the phosphomimetic, whereas the dephosphomimetic phenocopies the null condition. At the cellular level, dfmr1 null synapse architecture exhibits increased area, branching and bouton number. The phosphomimetic fully rescues these synaptogenesis defects, whereas the dephosphomimetic provides no rescue. The presence of Futsch-positive (microtubule-associated protein 1B) supernumerary microtubule loops is elevated in dfmr1 null synapses. The human phosphomimetic restores normal Futsch loops, whereas the dephosphomimetic provides no activity. At the behavioral level, dfmr1 null mutants exhibit strongly impaired olfactory associative learning. The human phosphomimetic targeted only to the brain-learning center restores normal learning ability, whereas the dephosphomimetic provides absolutely no rescue. We conclude that human FMRP S500 phosphorylation is necessary for its in vivo function as a neuronal translational repressor and regulator of synaptic architecture, and for the manifestation of FMRP-dependent learning behavior.
INTRODUCTION
Fragile X syndrome (FXS) is the most common monogenic cause of intellectual disability and autism (1–5), with an estimated prevalence of ∼1:4000 males and ∼1:6000 females (6,7). The X-linked neurodevelopmental disorder is caused by the loss of fragile X mental retardation 1 (FMR1) gene function, most frequently via expansion of a CGG trinucleotide repeat (>200 repeats) in the 5′ untranslated region leading to subsequent DNA hypermethylation, transcriptional silencing and thus to the loss of the FMR gene product (FMRP) (8–10). FMRP has three well-defined RNA-binding domains, including KH1/2 domains (heterogeneous nuclear ribonucleoprotein K homology) (11) and RGG box (containing repeats of an Arg-Gly-Gly motif) (12). Consistent with its ability to bind mRNA, FMRP regulates transcript trafficking and functions as a negative regulator of translation (13–16). In Fmr1 null mice, rates of cerebral protein synthesis are increased (17), showing that FMRP acts as a negative regulator of translation in vivo. FMRP is phosphorylated on a specific serine (human S500; murine S499; Drosophila S406) that is N-terminal to the RGG box (18). Following phosphorylation of this residue, hierarchical phosphorylation occurs on two neighboring serines. In a phosphomimetic, the negative charge from the aspartic acid substitution at mouse S499 has been shown to be necessary and sufficient for FMRP function in vitro (18). This phosphorylation switch is widely hypothesized to control the activity of FMRP as a translational repressor modulating neuronal function and behavioral output.
Post-mortem analyses of FXS patient brains reveal abnormal synaptic architecture (19,20). The hallmark of the disease state is an increase in postsynaptic dendritic spines with immature morphology, and a decrease in spines with mature morphology. In particular, neocortical pyramidal cells in FXS patients exhibit significant elevation of long dendritic spines and fewer mature dendritic spines compared with control subjects (19,20). These changes in the synaptic architecture are thought to underlie the major behavioral symptoms of the FXS disease state, including cognitive dysfunction and learning disabilities (21–23). FXS has been extensively investigated in both vertebrate and invertebrate genetic model systems (24–26). Both Drosophila and mouse disease models exhibit the loss of translational control with elevated brain protein levels, synaptic architecture defects and deficits in learning abilities (15,27–33). A very recent study has shown that FMRP phosphorylation modulates miR-125a regulation of PSD-95 mRNA translation in vitro (31). Using the murine phosphomimetic S499D, this work showed reduced PSD-95 protein levels, while expression of the dephosphomimetic S499A had no effect, indicating the critical role of S499 in mediating the inhibition and mGluR-mediated activation of PSD-95 translation (31). Dephosphorylation of FMRP was suggested to be an essential step for dissociation of RNA-induced silencing complex from FMRP-bound mRNA, thus activating mRNA translation. We have shown previously that the introduction of human FMRP into the Drosophila FXS model rescues all defects (34), demonstrating functional conservation. This enables us to now pursue systematic structure–function analyses of human FMRP within the genetically malleable Drosophila system. Here, we investigate for the first time the in vivo requirements of S500 phosphorylation in human FMRP.
In this study, we generate dephosphomimetic and phosphomimetic transgenes (S500A-hFMR1 and S500D-hFMR1, respectively) transformed into the Drosophila FXS disease model (35). Both mutant transgenic conditions are compared with the dfmr1 null mutant alone (negative control) or containing the wild-type human FMR1 transgene (positive control), with expression targeted by GAL4 drivers specific to neurons and specific brain regions. Each human transgene is investigated in two independent transgenic lines in the dfmr1 null mutant background. A wide-ranging series of phenotypic tests at the molecular (29), cellular (27,36) and behavioral (30) levels were selected to survey functional requirements in the nervous system. The results show that the transgene mimicking constitutive phosphorylation of the serine 500 residue, S500D-hFMR1, has the ability to completely rescue a full range of FXS neuronal defects. Only S500D-hFMR1 is able to restore normal brain protein levels and synaptic architecture in dfmr1 null neurons. S500A-hFMR1 completely lacks this ability to compensate, mimicking the dfmr1 null condition. Moreover, S500D-hFMR1 successfully rescues learning performance back to wild-type levels in a Pavlovian olfactory learning assay. In contrast, S500A-hFMR1 is unable to rescue learning deficits and is just as impaired as the complete loss of FMRP condition. These results clearly indicate that the phosphorylation of a unique, site-specific serine (S500) within human FMRP is necessary for FMRP function in vivo.
RESULTS
Transgenic constructs with targeted pan-neuronal expression
Human, murine and Drosophila FMRP are all similarly phosphorylated on a specific, conserved serine residue N-terminal to the RGG box; human S500, murine S499 and Drosophila S406 (11,18). Phosphorylation of this key serine is proposed to switch FMRP from a resting state to an active state as a negative translational regulator (11,25,37). To test the hypothesis that human FMRP function is regulated via S500 phosphorylation, we engineered transgenic human cDNA constructs for wild-type hFMR1 (positive control), dephosphomimetic (S500A-hFMR1) and phosphomimetic (S500D-hFMR1). We then expressed each transgene with a neural-specific driver (elav-GAL4) in the Drosophila FXS model (dfmr1 null mutant). Several independent lines were made for each transgenic condition. The generation and testing of these transgenic animals is illustrated in Figure 1.
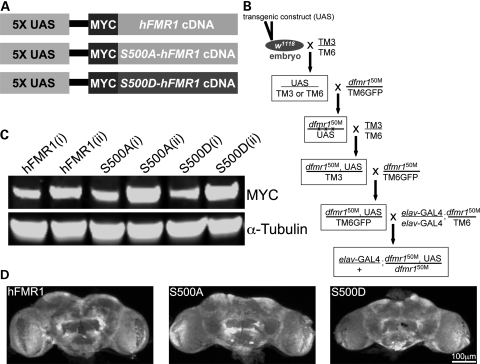
Figure 1.
Generation of transgenic constructs with targeted neuronal expression. (A) The three UAS transgenic constructs generated and tested in this study. The positive control is wild-type human FMR1 (hFMR1). The two mimetics are S500A-hFMR1 and S500D-hFMR1. All cDNA transgenic constructs are tagged with a MYC epitope in a pUAST (5X UAS) expression vector to follow protein expression. In all assays, two independent transgenic lines for each human transgenic construct have been analyzed. (B) The embryonic transformation and genetic crossing scheme to introduce each stably integrated UAS transgene into the dfmr1 null mutant background and then drive expression with the pan-neuronal GAL4 driver elav-GAL4. (C) Western blot analyses of transgenic protein expression for the hFMR1 line (control) and two independent lines of S500A-hFMR1 and S500D-hFMR1 (denoted as i/ii). Expression from brain extracts (1–2-day-old adult) was tested with anti-MYC against the epitope tag common to all four transgenes (A). Lines were selected for comparable low/high transgene expression. The loading control is α-tubulin. (D) Brain imaging for transgene expression in the hFMR1 line (control) and two mimetics (S500A-hFMR1 and S500D-hFMR1). Drosophila adult brains (1–2 days old) probed with anti-MYC to detect the transgene epitope tag. Comparable transgene expression occurs in all conditions.
All three cDNA constructs were sub-cloned downstream of the upstream activation sequence (UAS) promoter sequence (5X UAS; Fig. 1A). A MYC epitope tag was added at the amino terminus of each transgene to track transgenic protein expression. Each construct was microinjected into genetic background control w1118 embryos (Fig. 1B). Multiple stably integrated genomic lines for each transgene were isolated and self-perpetuating stocks generated. Third chromosome transformants were recombined onto the dfmr1 null (dfmr150M) background, and a stock was produced with TM6GFP serving to balance the recombinant chromosome (Fig. 1B). In order to assay neuronal phenotypes, all transgenic lines were crossed with a stock line homozygous for the pan-neuronal driver elav-GAL4 and heterozygous for the dfmr150M allele. The resulting experimental animals were homozygous null for dfmr1 harboring a single copy of the UAS transgene and a single copy of the elav-GAL4 driver (Fig. 1B). Two independent insertion lines for each human transgene were selected for full phenotype analyses, compared to w1118 with elav-GAL4 driver alone (wild-type control), the dfmr1 null with elav-GAL4 driver alone (negative control) or driving UAS-hFMR1 (positive control). Thus, eight genetic lines were compared in all subsequent experimental assays.
The expression of all transgenes was compared with a combination of brain western blots and brain immunocytochemistry imaging for the common MYC epitope tag (Fig. 1C and D). Endogenous Drosophila FMRP expression is ubiquitous in neurons and relatively uniform between neurons throughout the wild-type brain (34). We therefore selected elav-GAL4 as the best-described pan-neuronal driver mimicking this expression (26). Transgenic lines with low and high elav-GAL4 driven expression comparable with matched UAS-hFMR1-positive controls were selected (Fig. 1C), and two independent insertion lines with comparable expression for each transgene used for detailed analyses. Western blot analyses of brain protein extracts show comparable MYC epitope tag low/high expression levels across all selected transgenic genotypes (Fig. 1C). Anti-MYC labeling of brains from all three transgenic conditions showed comparable transgene expression levels and protein distribution across genotypes (Fig. 1D). Importantly, the UAS-hFMR1-positive control was indistinguishable from the two mimetic human transgenes in brain expression profile (Fig. 1D). Matched lines were thus selected to systematically test their ability to rescue a wide range of dfmr1 null mutant phenotypes.
Only S500D-hFMR1 restores brain protein translation levels
In both rodents and Drosophila, FMRP acts as a negative regulator of protein synthesis in neurons (35,38–40). Loss of FMRP-dependent translational regulation is believed to be the root cause of all FXS impairments. In the dfmr1 null mutant condition, specific FMRP targets such as Chickadee (homolog of actin-binding Profilin) as well as total brain protein levels are significantly elevated during key stages of synaptic development and refinement, particularly in the immature brain shortly following eclosion (29,34). We therefore first examined whether these fundamental molecular defects could be differentially rescued by the hFMR1 phosphomimetic versus dephosphomimetic proteins. The dfmr1 null mutant brain is unaltered in size and gross architecture compared with wild-type and genetic controls (34). Lysates from single Drosophila heads were analyzed at the developmental time window of 0–3 h post-eclosion (25°C) to compare Chickadee expression levels among genotypes. Total protein was extracted from developmentally staged heads at 0–7 h post-eclosion (25°C) to compare gross protein levels among genotypes. Eight independent genetic lines were analyzed in parallel; the wild-type control, dfmr1 null mutant (negative control), wild-type UAS-hFMR1 in the dfmr1 null background (positive control) and two independent lines for the phosphomimetic and dephosphomimetic transgenes.
In order to confirm that hFMRP is indeed phosphorylated in our transgenic animals, we first analyzed western blots for phospho-hFMRP expression in brain extracts (Fig. 2A). The phospho-specific antibody specifically detects phosphorylation of the targeted amino acid residue S500. Two independent wild-type UAS-hFMR1 lines showed robust phosphorylation at S500, revealing that human FMRP is phosphorylated normally in the Drosophila brain (Fig. 2A). Next, we examined two lines of both the phosphomimetic and dephosphomimetic transgenes. As expected, neither S500A nor S500D have detectable bands, denoting phosphorylation does not occur at these residues due to the introduced point mutations (Fig. 2A). A MYC antibody was used to compare protein-loading levels among the genotypes, confirming an equal comparison. We then turned our attention to a well-known FMRP target, Chickadee/Profilin, to assess the function of the mimetics at the level of a single protein. Chickadee protein levels are elevated in the dfmr1 null animals compared with w1118 control (Fig. 2B). Both hFMR1 and S500D-hFMR1 restore Chickadee protein levels to control brain levels. The dephosphomimetic, S500A-hFMR1, was unable to restore the level of protein expression and mimics the dfmr1 null condition (Fig. 2B). A tubulin antibody was used to compare protein-loading levels among the genotypes, confirming an equal comparison (Fig. 2B).
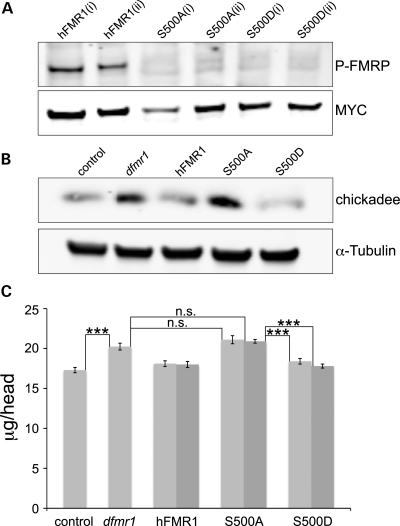
Figure 2.
S500D-hFMR1 rescues elevated brain protein levels in dfmr1 null. (A) Representative western blot of S500 phosphorylation state of wild-type hFMR1 and the two mimetic transgenes in the dfmr1 null (dfmr150M) Drosophila brain. Brain extracts (2 days old) were probed with anti-phospho-FMRP and anti-MYC to control for levels of protein. The two independent lines for each genotype are denoted as i/ii. (B) Representative western blot of Chickadee expression levels in wild-type control, dfmr1 null and the three human transgenic lines. Brain extracts (0–3 h post-eclosion) were probed with anti-Chickadee, with anti-α-tubulin used for protein-loading control. (C) Total brain protein was extracted from young adult (0–7 h post-eclosion) animals and quantified with a MicroBCA assay. The five genotypes compared are w1118 control, dfmr1 null (dfmr150M) and elav-GAL4 driving UAS-hFMR1 (positive control) and two independent lines each of UAS-S500A-hFMR1 and UAS-S500D-hFMR1 (light and dark gray bars) in the dfmr1 null background. Each bar shows the average protein (μg per head). Sample size: 10–20 pooled heads per sample, n= 8. Significance: ***P< 0.001.
We next quantitatively measured gross brain protein levels in all five genotypes. Null dfmr1 mutants with the elav-GAL4 driver alone (elav/+; dfmr150M/dfmr150M) have ∼20% higher brain protein levels compared with genetic controls (elav-GAL4/+) (Fig. 2C). Protein levels per head were 17.3 ± 0.33 μg in control compared with 20.2 ± 0.43 μg in the null mutant (P < 0.001, n= 8). The positive transgenic control, elav-GAL4 driven UAS-hFMR1 in the null mutant background, displayed protein levels of 18.0 ± 0.36 μg per head, showing rescue to control levels (not significantly different from wild-type, n= 8; Fig. 2C). Both independent UAS-hFMR1 lines (light and dark bars) restored brain protein levels indistinguishable from the wild-type control (18.1 ± 0.36 μg, 18.0 ± 0.38 μg; not significantly different from wild-type, n= 8). Both dephosphomimetic lines, UAS-S500A-hFMR1, exhibited elevated brain protein levels comparable with dfmr1 nulls, with no indication of rescue. The two independent lines showed levels of 21.1 ± 0.51 and 20.9 ± 0.23 μg per head, significantly increased from positive controls (P< 0.001, n= 8; Fig. 2C). In sharp contrast, both phosphomimetic lines, UAS-S500D-hFMR1, rescued brain protein expression back to control levels. The two independent lines showed levels of 18.4 ± 0.35 and 17.8 ± 0.25 μg, significantly different from the dfmr1 null (P< 0.001, n= 8; Fig. 2C).
These results demonstrate that only the phosphomimetic S500D can rescue the hallmark elevation of brain protein levels in the dfmr1 null back to the control condition. The dephosphomimetic S500A is unable to restore with a single known FMRP protein target or total brain protein levels to the control condition, which remain elevated comparable with the dfmr1 null. In contrast, by mimicking the negative charge of a phosphate group with an aspartic acid residue on S500, the phosphomimetic appears functionally active. By preventing S500 phosphorylation, the dephosphomimetic appears to provide no activity and thus resembles the null protein state. This is the first demonstration that S500 phosphorylation is necessary and sufficient in controlling the functional state of FMRP as a negative translational regulator in the in vivo brain.
Only S500D-hFMR1 restores neuromuscular junction synaptic architecture
In the Drosophila FXS model, phenotypes at the glutamatergic neuromuscular junction (NMJ) synapse are extremely well characterized (27,34–36). The size and accessibility of this synaptic arbor, combined with the wealth of synaptic markers and structural information, make this terminal particularly suited to a systematic investigation. Null dfmr1 mutants display synaptogenesis defects on several levels of synaptic architecture, including elevated synaptic area, increased synaptic branching and the formation of supernumerary synaptic boutons. Most strikingly, developmentally arrested satellite boutons accumulate in the absence of FMRP function (34,36), which represent an early stage of normal bouton maturation (41–43). To compare synaptic structure in transgenic animals, we co-labeled wandering third instar larval NMJs with presynaptic [horseradish peroxidase (HRP) membrane marker] and postsynaptic (DLG scaffold marker) antibody probes. We then quantified synaptic morphology in the wild-type control, dfmr1 null, elav-GAL4 (presynaptic) driven UAS-hFMR1-positive control and the phosphomimetic and dephosphomimetic transgenes in the dfmr1 null mutant background.
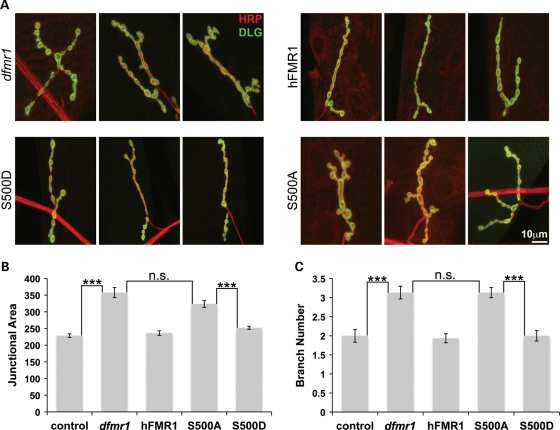
Labeling for anti-HRP delineates the innervating presynaptic neuron (red), and co-labeling with anti-DLG reveals the postsynaptic domain (green) of the target muscle (Fig. 3A). The positive transgenic control of elav-GAL4 driven UAS-hFMR1 fully rescued both the enlarged junctional area and increased synaptic branching that characterizes the dfmr1 null condition (Fig. 3B and C). To quantify synaptic area, the junction delimited by DLG expression was measured. The dfmr1 null mutation resulted in a significant increase in synaptic area (control, 228.4 ± 5.4 μm2; dfmr1 null, 357.6 ± 15.1 μm2; n ≥ 10, P< 0.001; Fig. 3B), while presynaptic wild-type human FMR1 expression in the null mutant background completely restored junctional area to control levels (236.3 ± 6.9 μm2; n≥ 10, not significantly different from wild-type; Fig. 3B). To quantify branching, HRP-labeled synaptic arbor projections with more than two boutons were counted. There was a significant increase in branching in the dfmr1 mutants (control, 2.0 ± 0.17; dfmr1 null 3.1 ± 0.16; n≥ 10, P< 0.001; Fig. 3C). Presynaptic hFMR1 expression completely restored synaptic branching from the elevated mutant levels (1.9 ± 0.11 branches; n≥ 10, P< 0.001). Strikingly, S500D-hFMR1 was equally able to restore synaptic junctional area and arbor branching to wild-type levels (252 ± 4.5 μm2 area, 2.0 ± 0.14 branches; n≥ 10, not significantly different from wild-type; Fig. 3B and C). In sharp contrast, the S500A-hFMR1 dephosphomimetic was unable to restore synaptic area in the null mutant (323.7 ± 10.3 μm2; n≥ 10; Fig. 3B). Similarly, S500A-hFMR1 failed to restore normal synaptic branch number in the mutant (3.1 ± 0.13 branches; n≥ 10; Fig. 3C). Thus, only the S500D-hFMR1 phosphomimetic has the ability to maintain gross synaptic architecture, and S500A-hFMR1 dephosphomimetic completely lacks this ability.
Figure 3.
S500D-hFMR1 rescues NMJ synapse architecture in dfmr1 null mutant. The wandering third instar NMJ synapse was co-labeled with presynaptic and postsynaptic markers and compared among the five genotypes: wild-type control, dfmr1 null and elav-GAL4 driven expression in the dfmr1 null background of hFMR1 (positive control) and two independent lines each of S500A-hFMR1 and S500D-hFMR1. (A) Representative images of the muscle 4 NMJ labeled for presynaptic HRP (red) and postsynaptic DLG (green). Three example synaptic arbors are shown for each of the five genotypes. Scale bar: 10 μm. Quantification of synapse junction area measured based on DLG domain expression (B) and the number of synaptic branches measured based on HRP labeling (C). The two independent lines for each human transgene were not significantly different in any case, and were therefore pooled for these comparisons. Sample size: n≥ 10 animals. Significance: ***P< 0.001.
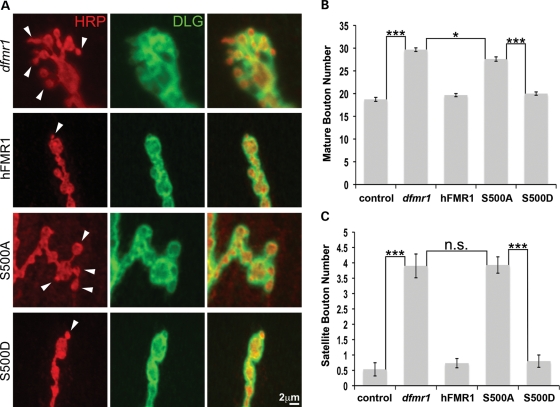
FMRP plays a key role in limiting synaptic bouton number and regulating the normal rate of bouton differentiation (34,36,44). To quantify mature type Ib bouton number, HRP/DLG co-labeled varicosities >2 μm in minimum diameter were counted within individual synaptic arbors (Fig. 4A). Null dfmr1 mutants exhibit a significantly increased number of synaptic boutons compared with controls (dfmr1, 29.7 ± 0.4; control, 18.7 ± 0.44; n≥ 10, P< 0.001; Fig. 4B). Presynaptic elav-GAL4 driven expression of the UAS-hFMR1-positive control rescued bouton number back to control levels (19.7 ± 0.35; n≥ 10, not significantly different from wild-type; Fig. 4B). Strikingly, S500D-hFMR1 was also able to completely rescue the supernumerary synaptic bouton number to the wild-type array (20.0 ± 0.37 boutons; n≥ 10, not significantly different from wild-type; Fig. 4B). Conversely, the S500A-hFMR1 had little or no impact on synaptic bouton number in the null mutant (27.6 ± 0.49; n≥ 10, P< 0.001; Fig. 4B). A key feature of the null mutant phenotype is the accumulation of satellite (immature) boutons (Fig. 4A; arrows). These boutons were elevated 8-fold in the dfmr1 null compared with genetic controls (dfmr1, 3.9 ± 0.4; control, 0.53 ± 0.22; n≥ 10, P< 0.001; Fig. 4C). The positive control hFMR1 was able to rescue satellite bouton number back to wild-type levels (0.73 ± 0.15; n≥ 10, not significantly different from wild-type; Fig. 4C). Similarly, S500D-hFMR1 was equally able to completely rescue satellite bouton number to the wild-type condition (0.8 ± 0.2 boutons; n≥ 10, not significantly different from wild-type; Fig. 4C). Conversely, S500A-hFMR1 failed to restore satellite bouton number in the null mutant (3.9 ± 0.27; n≥ 10, P< 0.001; Fig. 4C). Thus, only the S500D-hFMR1 phosphomimetic has the ability to regulate synaptogenesis and thus maintain fine synaptic architecture, whereas the S500A-hFMR1 dephosphomimetic completely lacks this ability and provides no activity beyond the null mutant condition.
Figure 4.
S500D-hFMR1 rescues synapse bouton differentiation in dfmr1 null. (A) Representative high-magnification images of synaptic boutons. Mature type 1b boutons defined as >2 μm in minimal diameter. Satellite boutons, representing an early stage in bouton differentiation, are <2 μm diameter and directly attached to a mature type 1b bouton (arrows). Developmentally arrested satellite boutons accumulate in the dfmr1 null mutant. Quantification of the number of mature boutons (B) and satellite boutons (C) per synaptic arbor in the five genotypes is shown. The two independent lines for each human transgene were not significantly different, and were therefore pooled for these comparisons. Sample size: n≥ 10 animals for each genotype. Significance: ***P< 0.001 for all comparisons.
Only S500D-hFMR1 restores Futsch/MAP1B synaptic cytoskeletal loops
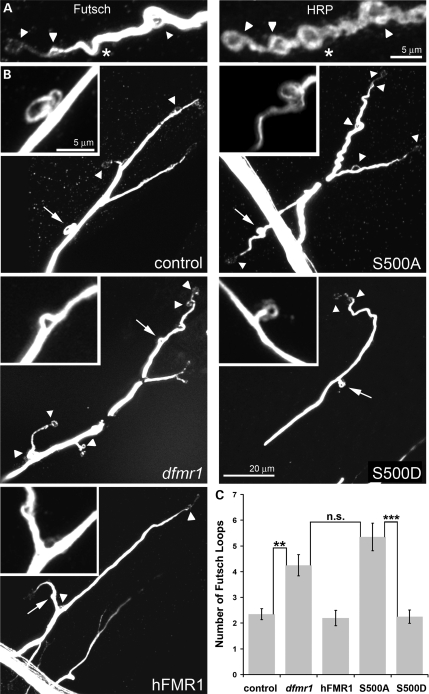
Synaptic architecture is highly dependent on the microtubule cytoskeleton, which is tightly regulated by the FMRP-target Futsch [microtubule-associated protein 1B (MAP1B) homolog] (45). Futsch/MAP1B is associated with the axonal nerve-terminal microtubule cytoskeleton and is necessary for the regulation of normal synaptic growth and bouton differentiation (43,46). Futsch/MAP1B translation is negatively regulated by FMRP via a direct mRNA-binding interaction (35), and excess Futsch-positive microtubule loops accumulate in the synaptic arbor in the dfmr1 null mutant condition (36). In order to quantify the number of Futsch loops per synaptic junction, wandering third instar larval synaptic arbors were co-labeled with anti-HRP, to outline the terminal boutons, and with anti-Futsch antibody to reveal protein levels and outline microtubule loops (Fig. 5A).
Figure 5.
S500D-hFMR1 rescues Futsch/MAP1B loops in dfmr1 null synapse. (A) Representative high-magnification images of Futsch loops located within NMJ synaptic boutons labeled with anti-HRP. Complete loops were quantified (arrows), but incomplete loops (asterisks) were not counted. (B) Representative images from the five genotypes are shown (loops denoted with an arrowhead) with a high-magnification inset of the Futsch loops (loop in inset denoted with an arrow). Scale bar for all low-magnification panels (20 μm) and all high-magnification insets (5 μm). (C) Quantification of the number of Futsch-positive loops per NMJ terminal. The two independent lines for each human transgene were not significantly different, and were therefore pooled for these comparisons. Sample size: n≥ 10 animals for each genotype. Significance: ***P< 0.001 or **P< 0.01.
Futsch/MAP1B cytoskeletal loops were compared and quantified in all five genotypes in parallel (Fig. 5). Only Futsch loops that made a completely enclosed circuit (arrowheads) were quantified, with partial-forming loops (asterisks) not included in the counts (Fig. 5A). Null dfmr1 mutants exhibit significantly increased Futsch synaptic loops compared with controls (dfmr1, 4.3 ± 0.41 loops; control, 2.4 ± 0.21 loops; n≥ 10, P< 0.01; Fig. 5B and C). Presynaptic elav-GAL4 driven expression of the UAS-hFMR1-positive control completely rescued Futsch loop number back to control levels (2.2 ± 0.3; n≥ 10, not significantly different from wild-type; Fig. 5B and C). S500D-hFMR1 was equally able to completely rescue synaptic loop number to wild-type levels (2.3 ± 0.26 loops; n≥ 10, not significantly different from wild-type; Fig. 5B and C). Conversely, S500A-hFMR1 was totally unable to restore synaptic Futsch loop number in the null mutant (5.35 ± 0.54; n≥ 10, P< 0.001; Fig. 5B and C). Thus, only the S500D-hFMR1 phosphomimetic has the ability to correctly regulate synaptic Futsch/MAP1B and maintain the synaptic Futsch loop refinement, whereas the S500A-hFMR1 dephosphomimetic completely lacks this ability and functionally resembles the complete absence of FMRP protein.
Only S500D-hFMR1 restores brain circuit synaptic architecture
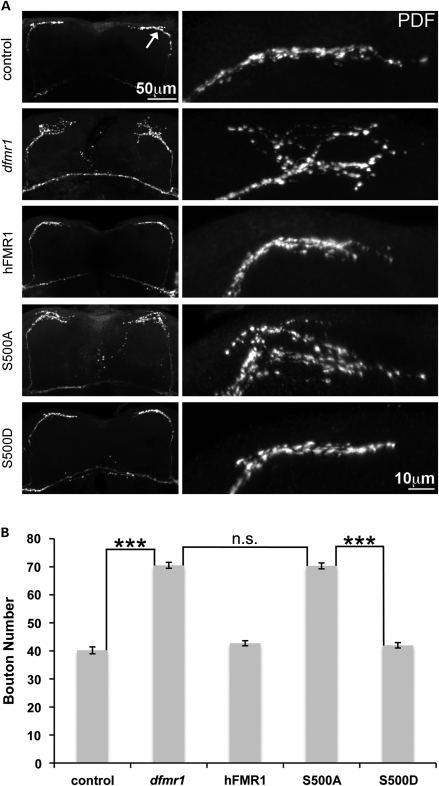
The hallmark defect in FXS patients and disease models is inappropriate synaptic connectivity in the central brain (47–50). In both mouse and Drosophila models, brain synapse architecture also appears immature or developmentally arrested. We therefore next examined synapse architecture in the central brain, based on well-established dfmr1 phenotypes. In Drosophila, a particularly well-defined system is the circadian clock circuitry, in which the small ventrolateral (sLNv) neurons drive pacemaker activity (51–53). Null dfmr1 mutants exhibit strikingly abnormal sLNv synaptic architecture with expanded terminals containing supernumerary synaptic boutons (26,54–56). These neurons express the neuropeptide pigment dispersing factor (PDF) and exhibit a characteristic branching pattern with axonal processes projecting dorsally to a defasiculation point in the protocerebrum and then synaptic processes extending medially (57,58). We used anti-PDF labeling on isolated brains to examine impacts on these phenotypes in the elav-GAL4 driven UAS-hFMR1-positive control and the phosphomimetic and dephosphomimetic transgenes in the dfmr1 null background.
Brains labeled with anti-PDF clearly display the dorsal sLNv projections into the protocerebrum (Fig. 6A). At the point of axonal defasiculation, the processes split into a localized array of fine termini comprised of small synaptic projections at the dorsal horn and into the protocerebrum. These projections are bilaterally symmetrical on the two sides of the brain (Fig. 6A). Null dfmr1 animals exhibited a highly significant (n≥ 10; P< 0.001) increase in the individually identifiable number of PDF-labeled boutons compared with controls (Fig. 6A and B). Wild-type terminals contained a mean of 40.2 ± 1.2 boutons compared with 70.5 ± 1.1 in the dfmr1 null. Thus, the mutant condition shows a ∼75% increase in PDF-positive synaptic boutons (Fig. 6B). Expression of wild-type hFMR1 completely rescued the synaptic overgrowth and excessive defasiculation characterizing the null mutant, and the terminals become clearly more restricted in extent and refined in number of synaptic boutons (Fig. 6B). In the positive control, there was 42.7 ± 1.0 boutons, a number indistinguishable from control and significantly (n≥ 10; P< 0.001) rescued compared with the dfmr1 null condition (Fig. 6B). Each mimetic transgene was next expressed to evaluate rescue of the sLNv synaptic arbor defect in dfmr1 nulls. S500D-hFMR1 was able to completely rescue the synaptic bouton number to the wild-type level (42.0 ± 1 boutons; n≥ 10, not significantly different from wild-type; Fig. 6B). Conversely, the S500A-hFMR1 transgenic condition failed to restore synaptic bouton number in the null mutant (70.3 ± 1.1 boutons; n≥ 10, P< 0.001; Fig. 6B). Thus, only the S500D-hFMR1 phosphomimetic has the ability to regulate synaptic architecture, whereas the S500A-hFMR1 dephosphomimetic completely lacks this ability and provides no discernible activity beyond the null mutant condition.
Figure 6.
S500D-hFMR1 rescues central brain synapse arbors in dfmr1 null. (A) Representative images of adult brain sLNv neurons labeled with anti-PDF. The low-magnification image on the left shows the bilaterally symmetrical sLNv projections, terminating in synaptic arbor projections (arrow) in the dorsal protocerebrum. The higher-magnification images show the left side (right panel) synaptic arbors. Note the PDF-positive punctae marking the synaptic boutons. Representative images shown from the five genotypes assayed: w1118 (control), dfmr150M null (dfmr1) and the null background with elav-GAL4 driven hFMR1, S500A-hFMR1 and S500D-hFMR1. Note the synaptic overgrowth characteristic of the null mutant, and the rescue of this overgrowth defect only by wild-type hFMR1 and S500D-hFMR1. (B) Quantification of the number of PDF-positive boutons per synaptic arbor in the five genotypes shown. Sample size: n≥ 10 animals for each genotype. Significance: ***P< 0.001 for all comparisons.
Only S500D-hFMR1 restores associative learning
To conclude our tests of FMRP requirements, we assayed a key behavioral output. The hallmark of FXS is cognitive dysfunction, including learning disabilities (21–23). Likewise, both the mouse and Drosophila FXS genetic models manifest clear learning impairments (30,55,59–63). In this study, we employed the best-characterized assay for associative learning in Drosophila, olfactory learning dependent on the mushroom body (MB) learning center in the central brain. Classical conditioning experiments for olfactory learning were employed (64,65). Animals were acclimated in a cylindrical shock tube and then exposed to one of two odors (3-octanol or 4-methylcyclohexanol). Following training trials pairing one odor with the aversive shock, the animals were then lowered into a T-maze choice point between the two odors. Wild-type controls have a high learning index (LI) with movement toward the un-paired odor, whereas dfmr1 null mutants display a highly significant decrease in olfactory learning (30). Here, we assay whether this learning defect can be rescued by the introduction of human FMRP, and then test the rescue abilities of the phosphomimetic and dephosphomimetic transgenes in this behavioral paradigm (Fig. 7).
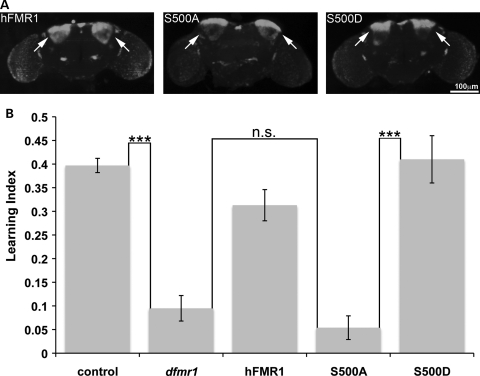
Figure 7.
Only S500D-hFMR1 restores olfactory learning in dfmr1 null. (A) Representative images of anti-hFMRP brain expression profiles with the MB-specific driver ok107-GAL4. Expression specifically targeted to the MB Kenyon cells (arrows). (B) Quantification of learning indices for each of the five genotypes is shown. Only S500D-hFMR1 restores learning to wild-type levels. Sample size: 75–100 animals per n; n= 10 for each genotype. Significance: ***P< 0.001.
Each human transgenic line was targeted specifically to the brain MB learning center with the ok107-GAL4 driver (Fig. 7A). The arrows indicate transgenic expression restricted to MB Kenyon cell bodies with high specificity. The intensity and distribution of hFMRP was indistinguishable between wild-type and the two mimetic conditions (Fig. 7A). In order to evaluate learning performance indices, 3-octanol and 4-methylcyclohexanol were employed as matched aversive odors. Null dfmr1 mutants exhibit a significantly decreased LI compared with controls (dfmr1, 0.10 ± 0.03; control, 0.40 ± 0.02; n≥ 10, P< 0.001; Fig. 7B). There was a 4-fold decrease in learning performance in the absence of FMRP protein. MB ok107-GAL4 driven expression of the wild-type hFMR1-positive control strongly rescued the null mutant LI back towards control levels (0.31 ± 0.03; n≥ 10, not significantly different from wild-type; Fig. 7B). Strikingly, S500D-hFMR1 was able to completely rescue learning to the wild-type level (0.41 ± 0.05; n≥ 10, not significantly different from wild-type; Fig. 7B). Conversely, S500A-hFMR1 was totally unable to restore LI in the null mutant (0.05 ± 0.03; n≥ 10, P< 0.001; Fig. 7B). Learning performance was reduced 8-fold when the FMRP protein was present but could not be phosphorylated at S500. Thus, only the S500D-hFMR1 phosphomimetic has the ability to rescue the severe learning deficit characterizing the dfmr1 null mutants. The S500A-hFMR1 dephosphomimetic exhibits little learning ability and is functionally equivalent to the complete absence of the FMRP protein.
DISCUSSION
FXS is caused solely by the loss of human FMRP. It has been widely hypothesized that the phosphorylation state of S500 acts as a ‘switch’ to transition human FMRP from an inactive to active state (18,37,66). This hypothesis predicts that human FMRP that cannot be phosphorylated will remain functionally inactive, equivalent to full protein loss, whereas a constitutively phosphorylated protein will be constantly active, but this has never been tested in vivo. To test this hypothesis, we expressed both a phosphomimetic (S500D-hFMR1) and a dephosphomimetic (S500A-hFMR1) in the well-characterized Drosophila FXS model [dfmr1 null mutant; (67,68)]. We then tested for functional in vivo rescue of a diverse range of null mutant phenotypes. Specifically, we assayed core molecular and cellular phenotypes in diverse circuits in the neuromusculature and brain, as well as the core behavioral defect of learning impairment. Our findings show that the phosphorylation of the S500 residue of human FMRP is necessary for protein function as a regulator of translation and modulator of synaptic connectivity, which, in turn, lays the foundation for normal behavioral output. The phosphomimetic, S500D-hFMR1, provides activity that restores normal function at all levels, to closely mimic the wild-type state. Since the phosphomimetic rescues the morphological defects seen in the dfmr1 null mutants, our data suggest that the excess growth may be due to elevated protein synthesis. In contrast, the dephosphomimetic, S500A-hFMR1, is incapable of providing any functional rescue and closely mimics dfmr1 null phenotypes at molecular, cellular and behavioral levels.
FMRP is an mRNA-binding protein best characterized as a negative regulator of translation, although it may also activate translation in some cases (14,35,40,69). FMRP is present in stalled polyribosomes and inhibits the translation of mRNA targets (18,39,40,70–73). In the absence of FMRP, both single FMRP-target protein (e.g. Chickadee/Profilin) and total protein levels are elevated in the Drosophila brain, particularly acutely during the late developmental stages of synaptogenesis and early-use synaptic refinement (29). The mouse FMR1 knockout similarly exhibits increased protein synthesis in the brain (17). Phosphorylation mechanisms regulate activity-dependent protein synthesis (74). Phosphorylated FMRP preferentially associates with stalled polyribosomes, whereas non-phosphorylated FMRP associates with actively translating polyribosomes (18). Phosphorylation likely confers a protein-binding site conformational change that modulates ribosomal association. Although the molecular mechanism by which FMRP stalls ribosomes has not been elucidated, it is likely to be dynamic, as it can be acutely reversed by RNA decoys in run-off assays (75). This reversibility would most likely be modulated by FMRP phosphorylation (18), but could also involve FMRP degradation (76). We have shown previously that human FMRP is just as effective as the native fly protein in restraining brain protein expression, although neither of the human paralogs (FXR1, FXR2) provides any activity (34). Using targeted neuronal expression, we show here that only the phosphomimetic (S500D-hFMR1) can restore the elevated single protein and total brain protein levels back to the wild-type condition in the Drosophila FXS model. Whereas S500D-hFMR1 is both necessary and sufficient for this inhibitory mechanism in neurons, S500A-hFMR1 is unable to provide any molecular function. This provides the first proof that S500 phosphorylation is an essential prerequisite for FMRP's function as a negative translational regulator in the in vivo brain.
The hallmark cellular defect in FXS patients, as well as both murine and Drosophila disease models, is the over-proliferation of synaptic connections, many of which appear to be immature (19,20,77). Although most research has focused on the elevated number of postsynaptic dendritic spines, apposing presynaptic bouton specializations accumulate in parallel. In the Drosophila FXS model, both presynaptic boutons and postsynaptic dendrites are over-grown and over-elaborated in the absence of FMRP, and we have demonstrated that this is a FMRP cell-autonomous requirement within neurons (28,36). Our previous studies of the well-characterized NMJ synaptic arbor have established a solely presynaptic requirement for FMRP in restraining terminal area, synaptic branching and synaptic bouton differentiation (36). Null dfmr1 synapses display increased terminal area, synaptic branching and supernumerary synaptic boutons (34,36). Our work here demonstrates that only the phosphomimetic (S500D-hFMR1) is able to curb growth and restore normal synaptic architecture in the dfmr1 null mutant. In sharp contrast, the dephosphomimetic (S500A-hFMR1) does not possess this ability to any detectable degree. Thus, phosphorylation is required for the FMRP function in regulating synapse architecture.
A defining feature of the overgrown synaptic connections arising in the absence of FMRP is that they appear structurally immature. For example, the dfmr1 null NMJ is characterized by the accumulation of mini/satellite boutons (34,36). These immature boutons represent a developmentally arrested state of an otherwise normal stage of bouton maturation (41,78–80). In the absence of FMRP, there is a ∼50% increase in the number of structurally mature boutons, but a striking 8–10-fold elevation in the abundance of these immature satellite boutons. Only the transgenic introduction of hFMR1 and S500D-hFMR1 can overcome this developmental arrest, restoring the normal number of mature synaptic boutons and eliminating the accumulation of developmentally arrested satellite boutons. Dephosphorylated S500A-hFMR1, in contrast, exhibits no restorative activity in synaptic bouton differentiation or in alleviating the synaptogenic arrest. Thus, phosphorylation of human FMRP is absolutely required for the protein to regulate synaptogenesis.
We first showed that FMRP acts to translationally repress Futsch/MAP1B, and that dfmr1 null synaptic structure defects are rescued by restoring normal Futsch expression levels (35). At the Drosophila NMJ, Futsch binds microtubule loops in a subset of developing synaptic boutons (46). These Futsch-positive microtubule structures are proposed to regulate synaptic growth and bouton differentiation (81,82). In dfmr1 null mutants, there is an increased number of Futsch-positive loops throughout the overgrown synaptic arbor, and these supernumerary structures are eliminated by presynaptic FMRP expression (36). This current study shows a doubling in the number of Futsch loops in the absence of FMRP, compared with wild-type control. Only the transgenic introduction of hFMR1 and S500D-hFMR1 can overcome this Futsch elevation, restoring the normal number of Futsch-positive loops in mutant synapses. Dephosphorylated S500A-hFMR1, in contrast, exhibits no restorative activity. Thus, phosphorylation of human FMRP is absolutely required for the regulation of Futsch/MAP1B during synaptogenesis.
In the Drosophila central brain, the clock circuit is particularly well characterized (58,83,84). Much attention has focused on the sLNv clock neurons, which secrete the neuropeptide PDF to regulate circadian rhythms (51–53). In dfmr1 null mutants, it has long been known that these neurons exhibit over-elaborated and over-extended synaptic arbors in the protocerebrum (26,39,54,85), a phenotype strikingly similar to the NMJ defect. Introduction of human FMRP can fully rescue this synaptic architecture defect. Moreover, only the phosphomimetic (S500D-hFMR1) is able to rescue the synaptic defect in the central brain. In contrast, the dephosphomimetic (S500A-hFMR1) has absolutely no effect on the null mutant phenotype. Thus, there is the same requirement for human FMRP phosphorylation in very distinctive neural circuits: in a peripheral motor circuit and in a central brain circuit. These results demonstrate for the first time the absolute requirement for FMRP phosphorylation to regulate synaptic connectivity in vivo.
The hallmark of FXS is cognitive dysfunction learning disabilities (21–23). Consistently, both the mouse and Drosophila FXS genetic models manifest clear learning impairments (30,55,59–63). A key brain center of learning in Drosophila is the MB and dfmr1 null mutants have defects in MB organization (β lobe midline crossing) and synaptic connectivity (30,86). Consistently, our previous work has shown that dfmr1 null mutants have significant defects in MB-dependent learning (30). Wild-type controls learn to move toward an odor not paired to electrical shock at a T-maze choice point, whereas dfmr1 nulls have strong deficits in this associative learning task (30). We show here that MB-targeted expression of human FMRP rescues this defect, and that only the phosphomimetic (S500D-hFMR1) maintains this function. In contrast, the dephosphomimetic (S500A-hFMR1) has absolutely no effect on the null mutant phenotype. These results show that the FMRP functional requirement in learning is conserved from man to fly, that this requirement occurs within the learning circuit in the central brain and that phosphorylation of human FMRP at S500 is an absolute prerequisite for function in behavioral learning output. The current model is that the FMRP–mRNA complex at the synapse exists in a phosphorylated translationally repressed state until a signal, e.g. mGluR activation, triggers FMRP dephosphorylation leading to a burst of local translation. Our data show that mRNAs are over-translated in the presence of an unphosphorylated form of FMRP (S500A-hFMRP), but that the phosphomimetic constitutively inhibits translation.
FMRP was first shown to be a translation repressor by in vitro studies using recombinant FMRP in reticulocytes and oocytes (14). Given our data showing FMRP phosphorylation is a prerequisite of this function, FMRP must have been introduced in a phosphorylated form or phosphorylated by native kinases present in these systems. It is not clear why S500 phosphorylation confers molecular function on FMRP, but it might modulate a protein-binding site that regulates ribosome association, or the ribosomal ‘stalling’ proposed to be caused by FMRP (75). Mouse FMRP is dynamically phosphorylated by ribosomal protein S6 kinase (S6K1) downstream of the mammalian target of rapamycin pathway, and dephosphorylated by the phosphatase PP2A (33,87). In murine hippocampal cultures, the non-phosphorylatable murine S499A-mFMR1 fails to associate with S6K1. In Drosophila, FMRP is phosphorylated in vitro by casein kinase II (88), although S6K1 might similarly be involved. Early work on mouse FMRP phosphomimetic and dephosphomimetic constructs (S499D and S499A, respectively) has strongly suggested that the phosphorylation state regulates translation repressor function (18). More recently, the loss of hippocampal S6K1 or introduction of S499A-FMRP has been shown to similarly elevate expression of SAPAP3 and PSD-95, both synaptic FMRP targets (31,33). The current study supports and expands on this work, showing a similar phosphorylation requirement for human FMRP in the broad context of the Drosophila FXS model. The new conclusion derived from this study is that synaptic mRNAs are similarly over-translated in the absence of FMRP or presence of unphosphorylated FMRP. Constitutively inhibiting translation with a phosphomimic closely resembles the wild-type state of dynamic regulation, suggesting that it is better to have less protein expression in the synapse than more.
It is quite surprising that the constitutive phosphorylation mimicry achieved by human FMRP S500D is quite adequate to recapitulate wild-type FMRP function in all molecular, cellular and behavioral assays pursued here. In vivo FMRP is dynamically phosphorylated and dephosphorylated—shuttling between a functional and non-functional form—in an activity-dependent mechanism (18,37,66). It was just recently shown that this reversibility is controlled by receptor-stimulated dephosphorylation of FMRP, which removes translational repression (31). Why then does the S500D transgene not produce gain-of-function phenotypes, or simply fail to function? Perhaps animals expressing the FMRP phosphomimetic develop an adaptive mechanism to manage the constitutive activation induced by the phosphorylation state of the transgenic protein. FMRP is acutely degraded upon synaptic stimulation (89), and so one possibility is that increased FMRP degradation after synaptic stimulation releases the critical subset of mRNAs from translation repression. Another possibility is that even though there is constitutively mimicked upregulation of the FMRP phosphorylated state, the phosphomimetic may not yield activation comparable with native phosphorylation, but rather more partial phosphorylation mimicry. Experimentally, while this is the best available mimic condition, it is not phosphorylation per se, but rather substitution of a phosphate group with a negatively charged aspartic acid residue. Thus, the phosphomimetic may enable partial function resembling an averaged state between the normal dynamic conformations of phosphorylation and dephosphorylation, thereby rescuing near the wild-type level. Of course, this explanation does not adequately address the need for a dynamic ‘switch’, which seems dispensable based on all the molecular, cellular and behavioral studies presented here. However, it is likely an oversimplification to assume that all FXS phenotypes result from constitutive excess protein synthesis. The loss of stimulus-induced translation in FXS may underlie other phenotypes not studied here, such as the ability of synapses to rapidly remodel structure and function in a manner dependent on the synthesis of new proteins.
MATERIALS AND METHODS
Drosophila stocks and genetics
All Drosophila stocks were maintained on the standard cornmeal/agar/molasses medium at 25°C in incubators with a 12 h light:dark cycle. The GAL4 driver lines elav-GAL4 (i) (P[GawB]elavC155); (ii) (P{w[+mC] = GAL4-elav.L}2/CyO) and ok107-GAL4 (P{GawB}ey [ok107]) were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN, USA). The control genotype was w1118 with a single copy of one of the two GAL4 driver lines: elav-GAL4/+ (all neuronal assays) and ok107-GAL4/+ (behavioral learning assays). The null mutant genotype was homozygous dfmr150M (35) with a single copy of the two GAL4 driver lines: elav-GAL4/+; dfmr150M and dfmr150M; ok107-GAL4/+. As described below, the three transgenic UAS constructs generated were UAS-MYC-hFMR1 (positive control), UAS-MYC-S500A-hFMR1 (dephosphomimetic) and UAS-MYC-S500D-hFMR1 (phosphomimetic). Third chromosome transformants were recombined with the dfmr150M allele by conventional genetic techniques.
Generation of UAS-hFMR1/S500A-hFMR1/S500D-hFMR1
An hFMR1 cDNA was subcloned into pBluescript II and used for the generation of both amino acid substitution constructs using the QuikChange site-directed mutagenesis kit (Stratagene, Cedar Creek, TX, USA). Primers used for S500A-hFMR1 (alanine at 500) substitution construct: 5′-GAAGCATCAAATGCTGCTGAAACAGAATCTGACCACAGAG AC-3′ and the reverse complement. Primers used for S500D-hFMR1 (aspartic acid at 500) substitution construct: 5′- GAAGCATCAAATGCTGATGAAACAGAATCTGACCAC AGAGAC-3′ and the reverse complement. Substituted cDNA fragments were double digested out of pBluescript II with EcoRI and Not1. Each double-digested mutation fragment was then ligated singly into similarly digested pUAS-T vectors to generate pUAS-S500A-hFMR1 and pUAS-S500D-hFMR1. A MYC tag was generated using the following oligo: 5′-AAGAATTCATGGAACAGAAACTGATTAGCGAAGAAGATCTGGAAT TCAA-3′ and the reverse complement. Oligos were boiled for 5min and allowed to cool to 25°C for 1 h. The product was digested with EcoRI and ligated into the similarly digested pUAS-T vectors already containing the substituted human cDNAs. The final plasmids were purified, sequenced and microinjected into w1118 embryos by Genetic Services, Inc. (Cambridge, MA, USA). Transformants with stably integrated cDNA inserts were identified and mapped to chromosome locations using standard genetic techniques. Multiple transgenic lines were isolated for pUAS-MYC-hFMR1, pUAS-MYC-S500A-hFMR1 and pUAS-MYC-S500D-hFMR1. In all assays, two independent inserts were assayed for each of the three human transgenic lines.
Western blot analyses
Western blots were performed as described previously (29,34). In brief, a pool of four to six heads was homogenized in 1X NuPage sample buffer (Invitrogen, Carlsbad, CA, USA) supplemented with 40 mm dithiothreitol. Debris was pelleted by centrifugation at 12 000 rpm at 25°C and samples boiled for 5 min. Extracts were loaded onto a 4–12% Bis–Tris gel, electrophoresed (1 h at 200 V) and transferred (1 h at 100 V) to nitrocellulose. Membranes were rinsed once with NanoPure water, blocked for 1 h in Odyssey Blocking Buffer (Li-Cor, Lincoln, NE, USA) and probed for 12–16 h at 4°C with primary antibodies. Antibodies used include: anti-phospho-hFMRP (1 μg/ml; ab48127, AbCam, Cambridge, MA, USA), anti-α-tubulin (1:400,000; B512, Sigma, St Louis, MO, USA), anti-MYC [1:15; 9E10, Drosophila Studies Hybridoma Bank (DSHB), Iowa City, IA, USA], anti-Chickadee (1:10; Chi1J, DSHB, Iowa City, IA, USA) and anti-MYC (1:1000; 71D10, Cell Signaling Technology, Danvers, MA, USA). Blots were washed with THAM/NaCl/NP-40 buffer and then probed for 1 h at 25°C with secondary antibodies. Antibodies used include: Alexa Fluor 680-conjugated goat anti-mouse (1:10,000) and Alexa Fluor 680-conjugated goat anti-rabbit (1:10,000) (Invitrogen-Molecular Probes, Carlsbad, CA, USA). Blots were imaged using the Odyssey Infrared Imaging System (Li-Cor). Raw integrated intensities were calculated, with levels normalized to α-tubulin.
Protein extraction and assay
Brain protein concentrations were determined as described previously (29,34). In brief, adult Drosophila heads (0–7 h old) were rapidly frozen in liquid nitrogen and then stored at −80°C. Protein was extracted from 10 to 20 pooled heads by homogenizing in 8 m urea, 1% sodium dodecyl sulfate (SDS) supplemented with 1× Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN, USA). The homogenate was incubated at 60°C for 1 h. Protein concentrations were determined using a MicroBCA assay (Pierce, Rockford, IL, USA). All concentrations are reported as micrograms of protein per head.
Immunocytochemistry
Adult brains and third instar larvae were dissected and fixed for immunolabeling as described previously (29,34). In brief, all tissues were fixed for 40 min with 4% paraformaldehyde in phosphate buffered saline (PBS) (pH 7.4). Preparations were then rinsed with PBS, blocked and permeabilized with 0.2% Triton X-100 in PBS (PBST) containing 1% bovine serum albumin (BSA) for 1 h at 25°C. Primary and secondary antibodies were diluted in PBST/BSA and incubated 12–16 h at 4°C and 2 h at 25°C, respectively. Primary antibodies used: anti-dFMRP (1:500; 6A15, mouse, Sigma), anti-PDF (1:5; C7 mouse, DSHB), anti-hFMRP (1:200; mouse, Chemicon, Temecula, CA, USA), anti-Discs Large (DLG) (1:200; 4F3, mouse, DSHB), anti-HRP (1:250; rabbit, Sigma), anti-Futsch (1:200; 22C10, mouse, DSHB) and anti-MYC (1:15; 9E10, mouse, DSHB) (1:500; 71D10, rabbit, Cell Signaling Technology). Secondary antibodies used were Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:250; Invitrogen-Molecular Probes). Preparations were mounted in FluoroMount G (EMS, Hatfield, PA, USA). Fluorescent images were collected using an upright Zeiss LSM 510 META laser-scanning confocal microscope. Images are presented as maximum z-projections.
NMJ analyses
The wandering third instar larval NMJ was quantified for structural features as described previously (34,36). In brief, the muscle 4 NMJ of abdominal segment 3 (A3) was used for all quantification. All fluorescent images were collected using a Zeiss confocal microscope. Intensity values were determined for both left and right A3 hemi-segments, and then averaged for each animal (n= 1). Synapse area was measured as the maximal cross-sectional area in a maximum projection of each collected z-stack. A synaptic branch was defined as an axonal projection with at least two synaptic boutons. Synaptic bouton classes defined included (i) type Ib (>2 μm diameter) and (ii) mini/satellite (≤2 μm diameter and directly attached to a type Ib bouton). Each class is reported as number per terminal. ImageJ (http://rsbweb.nih.gov/ij/) was used for automated regional outline and area calculation.
Brain circuit analyses
Brains from staged adult animals (zeitgeber time 2–4 h; ZT 2–4) were dissected in standard saline and then fixed for 40 min with 4% paraformaldehyde in PBS, pH 7.4 (26,34). Dissected brains were blocked and permeablized with 0.2% Triton X-100 in PBS (PBST) supplemented with 1% BSA for 1 h at 25°C. The sLNv clock neurons were labeled with anti-PDF antibody staining with Alexa Fluor secondary (1:250; Invitrogen-Molecular Probes). Primary and secondary antibodies were diluted in PBST with 0.2% BSA and incubated overnight at 4°C and 2 h at 25°C, respectively. All fluorescent images were collected using a Zeiss confocal microscope. The total number of PDF-positive synaptic boutons (>1 μm diameter) was counted for each sLNv terminal projection on the right and left hemispheres of the brain, and then averaged for each animal (n= 1).
Pavlovian olfactory learning
For classical conditioning, Drosophila were raised at 25°C in a 12:12 light/dark cycle with lights on at 3:00 AM and lights off at 3:00 PM. To avoid variation due to circadian modulation (90), all flies were tested at ZT 14–16. Flies 2 to 4 days post-eclosion were used in all assays. Training and testing were carried out in a dark box kept between 22 and 23°C and humidified to 85–95% humidity. The experiments were performed in dim red light provided by a darkroom safelight equipped with a filter that limited wavelengths to >600 nm (Kodak 1A or GBX-2, Rochester, NY, USA). Light intensity was adjusted with a rheostat to a final intensity of 0.5 μE m−2 s−1. Classical conditioning procedures were similar to those used in previous studies (30,64,65). Seventy-five to 100 flies were loaded into a cylindrical ‘shock tube’ and acclimated for 2 min. The flies were then exposed for 1 min to one of two odors diluted in mineral oil: 10−3 3-octanol (OCT) or 1.5 × 10−3 4-methylcyclohexanol (MCH)—carried by an air current of 500 ml/min. The concentrations used were experimentally determined to be equally aversive to the flies in a T-maze. During exposure to the odor serving as the conditioned stimulus (CS+), flies were subjected to a series of 10 shocks (2.5 s, 80 V DC) given every 5 s via a copper grid that covered the inner surface of the tube. Air was then administered for 50 s to flush the tube of residual odor, and the second odor (the control stimulus or CS−) was presented without shock. The chamber was again flushed with air for 1 min and the flies were gently tapped into a central compartment where they were acclimated for 2 min. The central compartment was lowered to the T-maze where the flies were exposed to two converging currents of odorant, one from each arm of the maze, and given 2 min to choose between the CS+ and the CS− odors. Flies were then trapped in the arms of the maze, anesthetized with CO2 and counted. In each experiment, two groups of flies of identical age and genotype were trained and tested, one in which the OCT was used as the CS+ and one in which the MCH was the CS+. A LI was calculated by taking the number of flies who had chosen the arm with the un-shocked odor and subtracting by the number of flies who had preferred the arm with the shocked odor, and then dividing by the total number of flies within the two arms. To control for any residual odor bias, the LI for each experiment was the average of the two consecutive trials, one in which MCH was paired with a shock and the second in which OCT was paired with the shock.
Statistics
All statistical analyses were performed using GraphPad InStat 3 (GraphPad Software, San Diego, CA, USA). Unpaired, non-parametric Tukey–Kramer multiple comparisons tests were used to compare means and were applied in parallel to all control, dfmr1 null and transgenic construct lines in the dfmr1 null background. Significance levels in figures are represented as P< 0.05 (*), P< 0.01 (**) and P< 0.001 (***). All error bars represent standard error of the mean (SEM) for independent trials.
Conflict of Interest statement. None declared.
FUNDING
This work was fully supported by the National Institutes of Health (NIH) grant MH084989 to K.B.
ACKNOWLEDGMENTS
We thank Dr Cheryl Gatto for many discussions and her critical input on this manuscript. We are particularly grateful for the Bloomington Drosophila Stock Center and the University of Iowa Developmental Studies Hybridoma Bank for Drosophila strains and antibodies, respectively.
REFERENCES
- 1.Clifford S., Dissanayake C., Bui Q.M., Huggins R., Taylor A.K., Loesch D.Z. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J. Autism Dev. Disord. 2007;37:738–747. doi: 10.1007/s10803-006-0205-z. doi:10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 2.Fisch G.S., Simensen R.J., Schroer R.J. Longitudinal changes in cognitive and adaptive behavior scores in children and adolescents with the fragile X mutation or autism. J. Autism Dev. Disord. 2002;32:107–114. doi: 10.1023/a:1014888505185. doi:10.1023/A:1014888505185. [DOI] [PubMed] [Google Scholar]
- 3.Rogers S.J., Wehner D.E., Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J. Dev. Behav. Pediatr. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. doi:10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Hagerman R.J., Ono M.Y., Hagerman P.J. Recent advances in fragile X: a model for autism and neurodegeneration. Curr. Opin. Psychiatry. 2005;18:490–496. doi: 10.1097/01.yco.0000179485.39520.b0. doi:10.1097/01.yco.0000179485.39520.b0. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D., Pichard N., Tordjman S., Baumann C., Burglen L., Excoffier E., Lazar G., Mazet P., Pinquier C., Verloes A., et al. Specific genetic disorders and autism: clinical contribution towards their identification. J. Autism Dev. Disord. 2005;35:103–116. doi: 10.1007/s10803-004-1038-2. doi:10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- 6.Penagarikano O., Mulle J.G., Warren S.T. The pathophysiology of fragile x syndrome. Annu. Rev. Genomics Hum. Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. doi:10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 7.Koukoui S.D., Chaudhuri A. Neuroanatomical, molecular genetic, and behavioral correlates of fragile X syndrome. Brain Res. Rev. 2007;53:27–38. doi: 10.1016/j.brainresrev.2006.06.001. doi:10.1016/j.brainresrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Heitz D., Devys D., Imbert G., Kretz C., Mandel J.L. Inheritance of the fragile X syndrome: size of the fragile X premutation is a major determinant of the transition to full mutation. J. Med. Genet. 1992;29:794–801. doi: 10.1136/jmg.29.11.794. doi:10.1136/jmg.29.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pieretti M., Zhang F.P., Fu Y.H., Warren S.T., Oostra B.A., Caskey C.T., Nelson D.L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. doi:10.1016/0092-8674(91)90125-I. [DOI] [PubMed] [Google Scholar]
- 10.Oberle I., Rousseau F., Heitz D., Kretz C., Devys D., Hanauer A., Boue J., Bertheas M.F., Mandel J.L. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. doi:10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- 11.Siomi H., Siomi M.C., Nussbaum R.L., Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74:291–298. doi: 10.1016/0092-8674(93)90420-u. doi:10.1016/0092-8674(93)90420-U. [DOI] [PubMed] [Google Scholar]
- 12.Darnell J.C., Jensen K.B., Jin P., Brown V., Warren S.T., Darnell R.B. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. doi:10.1016/S0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 13.Mazroui R., Huot M.E., Tremblay S., Filion C., Labelle Y., Khandjian E.W. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet. 2002;11:3007–3017. doi: 10.1093/hmg/11.24.3007. doi:10.1093/hmg/11.24.3007. [DOI] [PubMed] [Google Scholar]
- 14.Laggerbauer B., Ostareck D., Keidel E.M., Ostareck-Lederer A., Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum. Mol. Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. doi:10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- 15.Dictenberg J.B., Swanger S.A., Antar L.N., Singer R.H., Bassell G.J. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev. Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. doi:10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estes P.S., O'Shea M., Clasen S., Zarnescu D.C. Fragile X protein controls the efficacy of mRNA transport in Drosophila neurons. Mol. Cell. Neurosci. 2008;39:170–179. doi: 10.1016/j.mcn.2008.06.012. doi:10.1016/j.mcn.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Qin M., Kang J., Burlin T.V., Jiang C., Smith C.B. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J. Neurosci. 2005;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. doi:10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceman S., O'Donnell W.T., Reed M., Patton S., Pohl J., Warren S.T. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum. Mol. Genet. 2003;12:3295–3305. doi: 10.1093/hmg/ddg350. doi:10.1093/hmg/ddg350. [DOI] [PubMed] [Google Scholar]
- 19.Irwin S.A., Patel B., Idupulapati M., Harris J.B., Crisostomo R.A., Larsen B.P., Kooy F., Willems P.J., Cras P., Kozlowski P.B., et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am. J. Med. Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. doi:10.1002/1096-8628(20010115)98:2<161::AID-AJMG1025>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Irwin S.A., Galvez R., Greenough W.T. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb. Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. doi:10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 21.Rousseau F., Heitz D., Tarleton J., MacPherson J., Malmgren H., Dahl N., Barnicoat A., Mathew C., Mornet E., Tejada I., et al. A multicenter study on genotype-phenotype correlations in the fragile X syndrome, using direct diagnosis with probe StB12.3: the first 2,253 cases. Am. J. Hum. Genet. 1994;55:225–237. [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher A., Hallahan B. Fragile X-associated disorders: a clinical overview. J. Neurol. 2011 doi: 10.1007/s00415-011-6161-3. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Mercaldo V., Descalzi G., Zhuo M. Fragile X mental retardation protein in learning-related synaptic plasticity. Mol. Cells. 2009;28:501–507. doi: 10.1007/s10059-009-0193-x. doi:10.1007/s10059-009-0193-x. [DOI] [PubMed] [Google Scholar]
- 24.Bhogal B., Jongens T.A. Fragile X syndrome and model organisms: identifying potential routes of therapeutic intervention. Dis. Model. Mech. 2010;3:693–700. doi: 10.1242/dmm.002006. doi:10.1242/dmm.002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassell G.J., Warren S.T. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. doi:10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatto C.L., Broadie K. Temporal requirements of the fragile x mental retardation protein in modulating circadian clock circuit synaptic architecture. Front. Neural Circuits. 2009;3:1–12. doi: 10.3389/neuro.04.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan L., Woodruff E., 3rd, Liang P., Broadie K. Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol. Cell. Neurosci. 2008;37:747–760. doi: 10.1016/j.mcn.2008.01.003. doi:10.1016/j.mcn.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan L., Zhang Y.Q., Woodruff E., Broadie K. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr. Biol. 2004;14:1863–1870. doi: 10.1016/j.cub.2004.09.085. doi:10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 29.Tessier C.R., Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. doi:10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolduc F.V., Bell K., Cox H., Broadie K.S., Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat. Neurosci. 2008;11:1143–1145. doi: 10.1038/nn.2175. doi:10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muddashetty R.S., Nalavadi V.C., Gross C., Yao X., Xing L., Laur O., Warren S.T., Bassell G.J. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol. Cell. 2011;42:673–688. doi: 10.1016/j.molcel.2011.05.006. doi:10.1016/j.molcel.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamoto M., Nalavadi V., Epstein M.P., Narayanan U., Bassell G.J., Warren S.T. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc. Natl Acad. Sci. USA. 2007;104:15537–15542. doi: 10.1073/pnas.0707484104. doi:10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayanan U., Nalavadi V., Nakamoto M., Thomas G., Ceman S., Bassell G.J., Warren S.T. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. doi:10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coffee R.L., Jr, Tessier C.R., Woodruff E.A., 3rd, Broadie K. Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P. Dis. Model. Mech. 2010;3:471–485. doi: 10.1242/dmm.004598. doi:10.1242/dmm.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y.Q., Bailey A.M., Matthies H.J., Renden R.B., Smith M.A., Speese S.D., Rubin G.M., Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. doi:10.1016/S0092-8674(01)00589-X. [DOI] [PubMed] [Google Scholar]
- 36.Gatto C.L., Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development. 2008;135:2637–2648. doi: 10.1242/dev.022244. doi:10.1242/dev.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeiffer B.E., Huber K.M. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J. Neurosci. 2007;27:3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. doi:10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu R., Wang H., Liang Z., Ku L., O'Donnell W.T., Li W., Warren S.T., Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc. Natl Acad. Sci. USA. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. doi:10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeve S.P., Bassetto L., Genova G.K., Kleyner Y., Leyssen M., Jackson F.R., Hassan B.A. The Drosophila fragile X mental retardation protein controls actin dynamics by directly regulating profilin in the brain. Curr. Biol. 2005;15:1156–1163. doi: 10.1016/j.cub.2005.05.050. doi:10.1016/j.cub.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 40.Schutt J., Falley K., Richter D., Kreienkamp H.J., Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J. Biol. Chem. 2009;284:25479–25487. doi: 10.1074/jbc.M109.042663. doi:10.1074/jbc.M109.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beumer K.J., Rohrbough J., Prokop A., Broadie K. A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development. 1999;126:5833–5846. doi: 10.1242/dev.126.24.5833. [DOI] [PubMed] [Google Scholar]
- 42.Gorczyca D., Ashley J., Speese S., Gherbesi N., Thomas U., Gundelfinger E., Gramates L.S., Budnik V. Postsynaptic membrane addition depends on the Discs-Large-interacting t-SNARE Gtaxin. J. Neurosci. 2007;27:1033–1044. doi: 10.1523/JNEUROSCI.3160-06.2007. doi:10.1523/JNEUROSCI.3160-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz-Canada C., Ashley J., Moeckel-Cole S., Drier E., Yin J., Budnik V. New synaptic bouton formation is disrupted by misregulation of microtubule stability in aPKC mutants. Neuron. 2004;42:567–580. doi: 10.1016/s0896-6273(04)00255-7. doi:10.1016/S0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siller S.S., Broadie K. Neural circuit architecture defects in a Drosophila model of Fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis. Model. Mech. 2011;4:673–685. doi: 10.1242/dmm.008045. doi:10.1242/dmm.008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hummel T., Krukkert K., Roos J., Davis G., Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–370. doi: 10.1016/s0896-6273(00)81169-1. doi:10.1016/S0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- 46.Roos J., Hummel T., Ng N., Klambt C., Davis G.W. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. doi:10.1016/S0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 47.Braun K., Segal M. FMRP involvement in formation of synapses among cultured hippocampal neurons. Cereb. Cortex. 2000;10:1045–1052. doi: 10.1093/cercor/10.10.1045. doi:10.1093/cercor/10.10.1045. [DOI] [PubMed] [Google Scholar]
- 48.Bureau I., Shepherd G.M., Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J. Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. doi:10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Comery T.A., Harris J.B., Willems P.J., Oostra B.A., Irwin S.A., Weiler I.J., Greenough W.T. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl Acad. Sci. USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. doi:10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanson J.E., Madison D.V. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J. Neurosci. 2007;27:4014–4018. doi: 10.1523/JNEUROSCI.4717-06.2007. doi:10.1523/JNEUROSCI.4717-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grima B., Chelot E., Xia R., Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. doi:10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 52.Renn S.C., Park J.H., Rosbash M., Hall J.C., Taghert P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. doi:10.1016/S0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 53.Stoleru D., Peng Y., Agosto J., Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. doi:10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 54.Morales J., Hiesinger P.R., Schroeder A.J., Kume K., Verstreken P., Jackson F.R., Nelson D.L., Hassan B.A. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–972. doi: 10.1016/s0896-6273(02)00731-6. doi:10.1016/S0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 55.Dockendorff T.C., Su H.S., McBride S.M., Yang Z., Choi C.H., Siwicki K.K., Sehgal A., Jongens T.A. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34:973–984. doi: 10.1016/s0896-6273(02)00724-9. doi:10.1016/S0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 56.Sofola O., Sundram V., Ng F., Kleyner Y., Morales J., Botas J., Jackson F.R., Nelson D.L. The Drosophila FMRP and LARK RNA-binding proteins function together to regulate eye development and circadian behavior. J. Neurosci. 2008;28:10200–10205. doi: 10.1523/JNEUROSCI.2786-08.2008. doi:10.1523/JNEUROSCI.2786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Helfrich-Forster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 1995;92:612–616. doi: 10.1073/pnas.92.2.612. doi:10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helfrich-Forster C. Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 2005;4:65–76. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- 59.Chang S., Bray S.M., Li Z., Zarnescu D.C., He C., Jin P., Warren S.T. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat. Chem. Biol. 2008;4:256–263. doi: 10.1038/nchembio.78. doi:10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- 60.McBride S.M., Choi C.H., Wang Y., Liebelt D., Braunstein E., Ferreiro D., Sehgal A., Siwicki K.K., Dockendorff T.C., Nguyen H.T., et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. doi:10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 61.Larson J., Kim D., Patel R.C., Floreani C. Olfactory discrimination learning in mice lacking the fragile X mental retardation protein. Neurobiol. Learn. Mem. 2008;90:90–102. doi: 10.1016/j.nlm.2008.01.002. doi:10.1016/j.nlm.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacLeod L.S., Kogan C.S., Collin C.A., Berry-Kravis E., Messier C., Gandhi R. A comparative study of the performance of individuals with fragile X syndrome and Fmr1 knockout mice on Hebb-Williams mazes. Genes Brain Behav. 2010;9:53–64. doi: 10.1111/j.1601-183X.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 63.Krueger D.D., Osterweil E.K., Chen S.P., Tye L.D., Bear M.F. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc. Natl Acad. Sci. USA. 2011;108:2587–2592. doi: 10.1073/pnas.1013855108. doi:10.1073/pnas.1013855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tully T., Quinn W.G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. A. 1985;157:263–277. doi: 10.1007/BF01350033. doi:10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 65.Tully T., Preat T., Boynton S.C., Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. doi:10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 66.Cheever A., Ceman S. Phosphorylation of FMRP inhibits association with Dicer. RNA. 2009;15:362–366. doi: 10.1261/rna.1500809. doi:10.1261/rna.1500809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gatto C.L., Broadie K. The fragile X mental retardation protein in circadian rhythmicity and memory consolidation. Mol. Neurobiol. 2009;39:107–129. doi: 10.1007/s12035-009-8057-0. doi:10.1007/s12035-009-8057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tessier C.R., Broadie K. Activity-dependent modulation of neural circuit synaptic connectivity. Front. Mol. Neurosci. 2009;2:1–13. doi: 10.3389/neuro.02.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zalfa F., Giorgi M., Primerano B., Moro A., Di Penta A., Reis S., Oostra B., Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. doi:10.1016/S0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 70.Napoli I., Mercaldo V., Boyl P.P., Eleuteri B., Zalfa F., De Rubeis S., Di Marino D., Mohr E., Massimi M., Falconi M., et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. doi:10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y., Xu S., Xia L., Wang J., Wen S., Jin P., Chen D. The bantam microRNA is associated with drosophila fragile X mental retardation protein and regulates the fate of germline stem cells. PLoS Genet. 2009;5:e1000444. doi: 10.1371/journal.pgen.1000444. doi:10.1371/journal.pgen.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khandjian E.W., Huot M.E., Tremblay S., Davidovic L., Mazroui R., Bardoni B. Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles. Proc. Natl Acad. Sci. USA. 2004;101:13357–13362. doi: 10.1073/pnas.0405398101. doi:10.1073/pnas.0405398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stefani G., Fraser C.E., Darnell J.C., Darnell R.B. Fragile X mental retardation protein is associated with translating polyribosomes in neuronal cells. J. Neurosci. 2004;24:7272–7276. doi: 10.1523/JNEUROSCI.2306-04.2004. doi:10.1523/JNEUROSCI.2306-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Routtenberg A., Rekart J.L. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. doi:10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Darnell J.C., Van Driesche S.J., Zhang C., Hung K.Y., Mele A., Fraser C.E., Stone E.F., Chen C., Fak J.J., Chi S.W., et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. doi:10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou L., Antion M.D., Hu D., Spencer C.M., Paylor R., Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. doi:10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Grossman A.W., Elisseou N.M., McKinney B.C., Greenough W.T. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006;1084:158–164. doi: 10.1016/j.brainres.2006.02.044. doi:10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 78.Ashley J., Packard M., Ataman B., Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J. Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. doi:10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickman D.K., Lu Z., Meinertzhagen I.A., Schwarz T.L. Altered synaptic development and active zone spacing in endocytosis mutants. Curr. Biol. 2006;16:591–598. doi: 10.1016/j.cub.2006.02.058. doi:10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 80.Torroja L., Packard M., Gorczyca M., White K., Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J. Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanaka E.M., Kirschner M.W. Microtubule behavior in the growth cones of living neurons during axon elongation. J. Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. doi:10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dent E.W., Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J. Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang D.C. Neural circuits underlying circadian behavior in Drosophila melanogaster. Behav. Process. 2006;71:211–225. doi: 10.1016/j.beproc.2005.12.008. doi:10.1016/j.beproc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 84.Nitabach M.N., Taghert P.H. Organization of the Drosophila circadian control circuit. Curr. Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. doi:10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 85.Sekine T., Yamaguchi T., Hamano K., Siomi H., Saez L., Ishida N., Shimoda M. Circadian phenotypes of Drosophila fragile x mutants in alternative genetic backgrounds. Zoolog. Sci. 2008;25:561–571. doi: 10.2108/zsj.25.561. doi:10.2108/zsj.25.561. [DOI] [PubMed] [Google Scholar]
- 86.Michel C.I., Kraft R., Restifo L.L. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J. Neurosci. 2004;24:5798–5809. doi: 10.1523/JNEUROSCI.1102-04.2004. doi:10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Narayanan U., Nalavadi V., Nakamoto M., Pallas D.C., Ceman S., Bassell G.J., Warren S.T. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J. Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. doi:10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siomi M.C., Higashijima K., Ishizuka A., Siomi H. Casein kinase II phosphorylates the fragile X mental retardation protein and modulates its biological properties. Mol. Cell Biol. 2002;22:8438–8447. doi: 10.1128/MCB.22.24.8438-8447.2002. doi:10.1128/MCB.22.24.8438-8447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gabel L.A., Won S., Kawai H., McKinney M., Tartakoff A.M., Fallon J.R. Visual experience regulates transient expression and dendritic localization of fragile X mental retardation protein. J. Neurosci. 2004;24:10579–10583. doi: 10.1523/JNEUROSCI.2185-04.2004. doi:10.1523/JNEUROSCI.2185-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lyons L.C., Roman G. Circadian modulation of short-term memory in Drosophila. Learn Mem. 2009;16:19–27. doi: 10.1101/lm.1146009. doi:10.1101/lm.1146009. [DOI] [PMC free article] [PubMed] [Google Scholar]