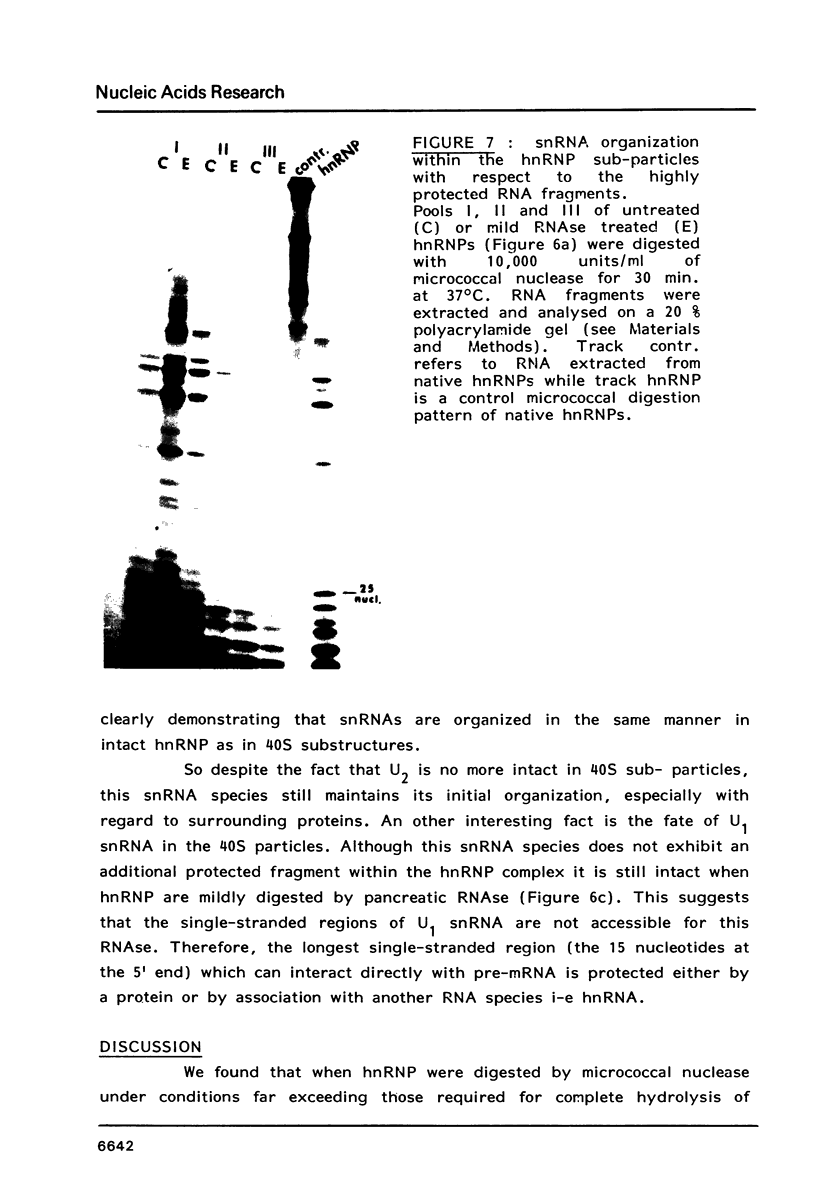
Abstract
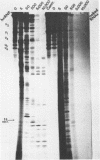
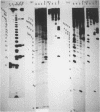
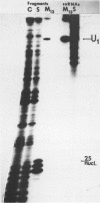
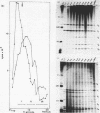
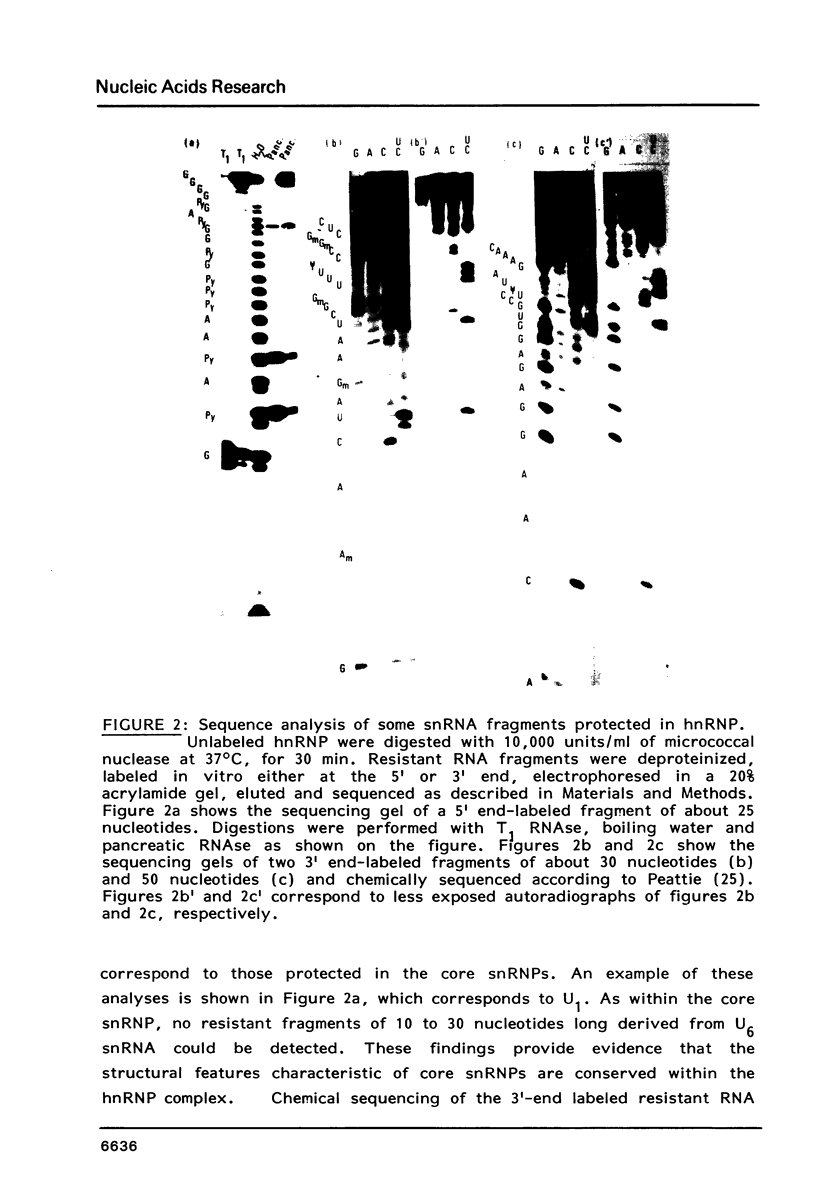
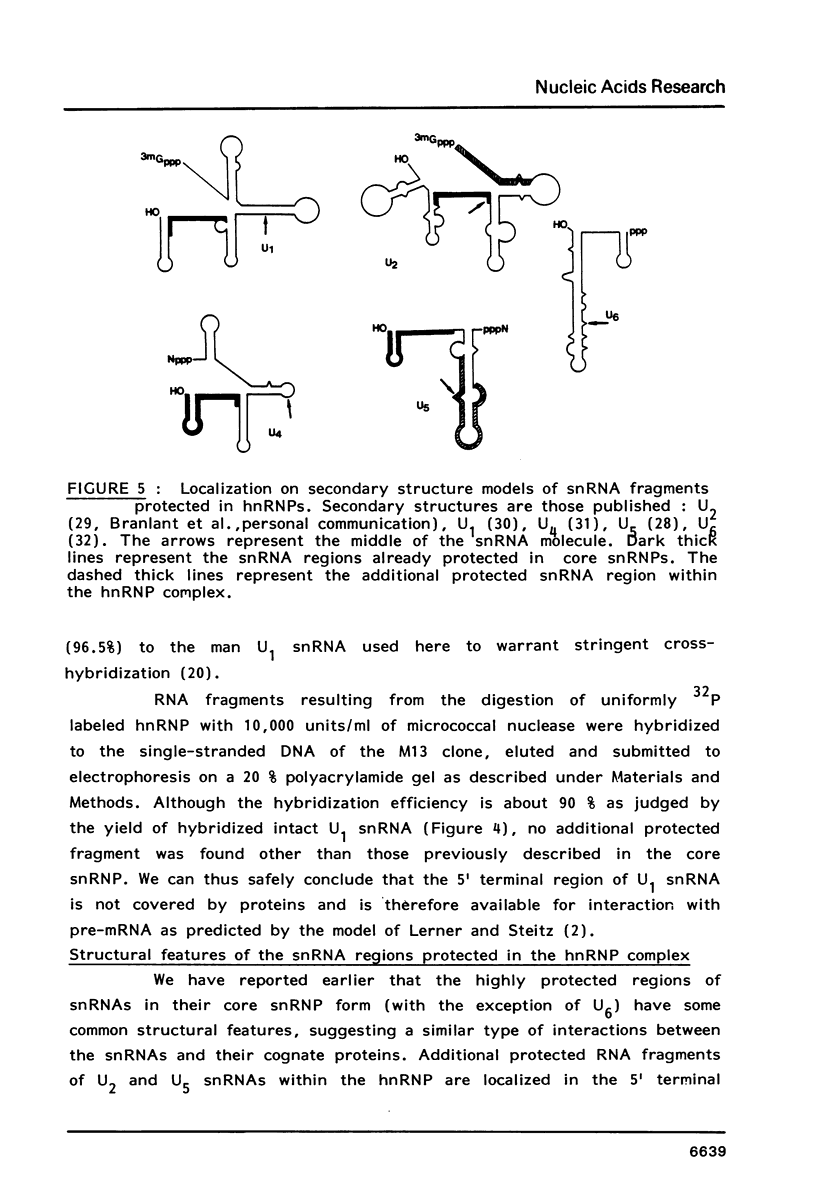
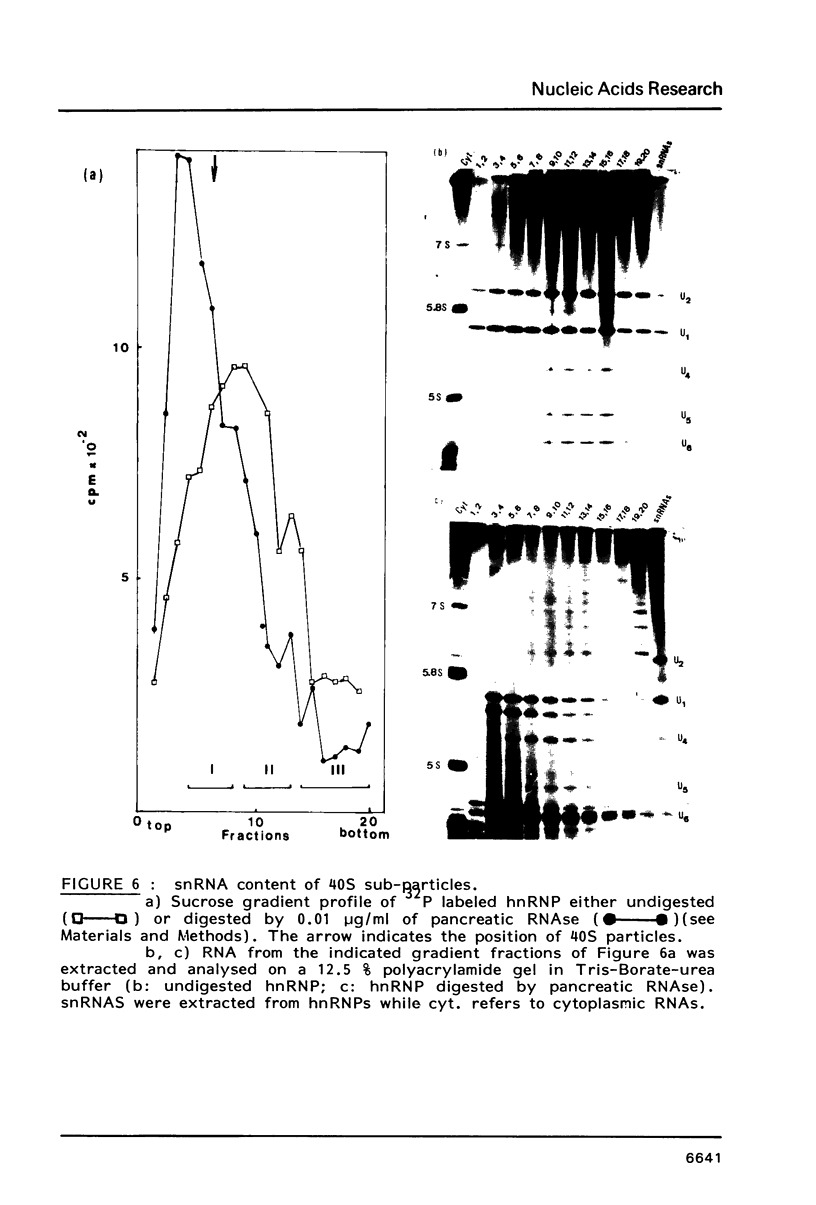
We have shown previously (Liautard et al., 1982, J. Mol. Biol., 162, 623-643) that digestion with micrococcal nuclease under drastic conditions of a pure U1 snRNP, as well as a mixture containing U2, U1, U4, U5 and U6 snRNPs, gives rise to resistant RNA fragments derived from all but U6 snRNAs. As an attempt to elucidate the way in which snRNPs are attached to their native structure, the same approach was applied to hnRNP which are known to contain snRNP (Guimont-Ducamp et al., 1977, Biochimie, 59, 755-758). Micrococcal nuclease digestion of hnRNPs yielded a population of 15-50 nucleotides long resistant fragments of snRNAs. Sequence analyses showed that all fragments previously identified in core snRNPs were also present. Only U2 and U5 snRNAs were further protected as a result of their association with the hnRNP complex (from the cap to nucleotide 32 for U2 and from nucleotide 22 to nucleotide 70 for U5). No additional protected fragment derived from U1, U4 and U6 snRNAs was found. This finding confirms that the 5' terminal region of U1 snRNP remains available for base-pairing interaction with the premessenger RNA, as predicted by the model of Lerner et al. (Nature, 1980, 283, 220-224).
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assens C., Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Highly purified snRNPs retain antigenic determinants towards anti Sm antibodies. Biochem Biophys Res Commun. 1982 Jun 15;106(3):953–960. doi: 10.1016/0006-291x(82)91803-4. [DOI] [PubMed] [Google Scholar]
- Blanchard J. M., Brunel C., Jeanteur P. Characterisation of an endogenous protein kinase activity in ribonucleoprotein structures containing heterogenous nuclear RNA in HeLa cell nuclei. Eur J Biochem. 1977 Sep 15;79(1):117–131. doi: 10.1111/j.1432-1033.1977.tb11790.x. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Beccari E., Luo Z. X., Amaldi F. Xenopus laevis ribosomal protein genes: isolation of recombinant cDNA clones and study of the genomic organization. Nucleic Acids Res. 1981 Mar 11;9(5):1069–1086. doi: 10.1093/nar/9.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Gallinaro H., Lazar E., Jacob M. The conformation of chicken, rat and human U1A RNAs in solution. Nucleic Acids Res. 1981 Feb 25;9(4):841–858. doi: 10.1093/nar/9.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Haendler B., Jacob M. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1982;1(10):1259–1265. doi: 10.1002/j.1460-2075.1982.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Ebel J. P. Structural study of ribosomal 23 S RNA from Escherichia coli. FEBS Lett. 1979 Nov 1;107(1):177–181. doi: 10.1016/0014-5793(79)80490-1. [DOI] [PubMed] [Google Scholar]
- Brunel C., Widada J. S., Lelay M. N., Jeanteur P., Liautard J. P. Purification and characterization of a simple ribonucleoprotein particle containing small nucleoplasmic RNAs (snRNP) as a subset of RNP containing heterogenous nuclear RNA (hnRNP) from HeLa cells. Nucleic Acids Res. 1981 Feb 25;9(4):815–830. doi: 10.1093/nar/9.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet J. P., Meyer L. M., Pederson T. Small nuclear RNA U2 is base-paired to heterogeneous nuclear RNA. Science. 1982 Jul 30;217(4558):456–458. doi: 10.1126/science.6178162. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Base-pairing interactions between small nuclear RNAs and nuclear RNA precursors as revealed by psoralen cross-linking in vivo. Cell. 1981 Nov;26(3 Pt 1):363–370. doi: 10.1016/0092-8674(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Deimel B., Louis C. H., Sekeris C. E. The presence of small molecular weight RNAs in nuclear ribonucleoprotein particles carrying HnRNA. FEBS Lett. 1977 Jan 15;73(1):80–84. [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. An evaluation of small nuclear RNA in hnRNP. FEBS Lett. 1979 Aug 1;104(1):176–182. doi: 10.1016/0014-5793(79)81110-2. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. The status of small nuclear RNA in the ribonucleoprotein fibrils containing heterogeneous nuclear RNA. Biochim Biophys Acta. 1981 Jan 29;652(1):109–120. doi: 10.1016/0005-2787(81)90214-8. [DOI] [PubMed] [Google Scholar]
- Guimont-Ducamp C., Sri-Widada J., Jeanteur P. Occurrence of small molecular weight RNAs in Hela nuclear ribonucleoprotein particles containing HnRNA. Biochimie. 1977;59(8-9):755–758. doi: 10.1016/s0300-9084(77)80259-9. [DOI] [PubMed] [Google Scholar]
- Guimont-Ducamp C., Sri-Widada J., Jeanteur P. Occurrence of small molecular weight RNAs in Hela nuclear ribonucleoprotein particles containing HnRNA. Biochimie. 1977;59(8-9):755–758. doi: 10.1016/s0300-9084(77)80259-9. [DOI] [PubMed] [Google Scholar]
- Harada F., Kato N., Nishimura S. The nucleotide sequence of nuclear 4.8S RNA of mouse cells. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1332–1340. doi: 10.1016/0006-291x(80)91620-4. [DOI] [PubMed] [Google Scholar]
- Hinterberger M., Pettersson I., Steitz J. A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J Biol Chem. 1983 Feb 25;258(4):2604–2613. [PubMed] [Google Scholar]
- Kinlaw C. S., Robberson B. L., Berget S. M. Fractionation and characterization of human small nuclear ribonucleoproteins containing U1 and U2 RNAs. J Biol Chem. 1983 Jun 10;258(11):7181–7189. [PubMed] [Google Scholar]
- Krol A., Branlant C., Lazar E., Gallinaro H., Jacob M. Primary and secondary structures of chicken, rat and man nuclear U4 RNAs. Homologies with U1 and U5 RNAs. Nucleic Acids Res. 1981 Jun 25;9(12):2699–2716. doi: 10.1093/nar/9.12.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Gallinaro H., Lazar E., Jacob M., Branlant C. The nuclear 5S RNAs from chicken, rat and man. U5 RNAs are encoded by multiple genes. Nucleic Acids Res. 1981 Feb 25;9(4):769–787. doi: 10.1093/nar/9.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar E., Jacob M., Krol A., Branlant C. Accessibility of U1 RNA to base pairing with a single-stranded DNA fragment mimicking the intron extremities at the splice junction. Nucleic Acids Res. 1982 Feb 25;10(4):1193–1201. doi: 10.1093/nar/10.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelay M. N., Brunel C., Jeanteur P. Peptide mapping of in vivo and in vitro phosphorylated sites of proteins from HeLa hnRNP. FEBS Lett. 1978 Jun 1;90(1):54–56. doi: 10.1016/0014-5793(78)80296-8. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Northemann W., Scheurlen M., Gross V., Heinrich P. C. Circular dichroism of ribonucleoprotein complexes from rat liver nuclei. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1130–1137. doi: 10.1016/0006-291x(77)90973-1. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Itoh M., Okada N., Miyata T. Novel models for RNA splicing that involve a small nuclear RNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4471–4474. doi: 10.1073/pnas.78.7.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Lelay-Taha M. N., Sri-Widada J., Brunel C., Liautard J. P., Jeanteur P. Mouse DNA sequences complementary to small nuclear RNA U1. Nucleic Acids Res. 1982 Aug 11;10(15):4627–4640. doi: 10.1093/nar/10.15.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Henning D., Epstein P., Busch H. Primary and secondary structure of U2 snRNA. Nucleic Acids Res. 1981 Nov 11;9(21):5645–5658. doi: 10.1093/nar/9.21.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri-Widada J., Assens C., Liautard J. P., Jeanteur P., Brunel C. Isolation of a pure U1 snRNP from HeLa cells. Biochem Biophys Res Commun. 1982 Jan 29;104(2):457–462. doi: 10.1016/0006-291x(82)90659-3. [DOI] [PubMed] [Google Scholar]
- Sri-Widada J., Liautard J. P., Assens C., Brunel C. Primary structure identification of snRNAs present in highly purified snRNPs from HeLa cells. Mol Biol Rep. 1981 Nov 30;8(1):29–36. doi: 10.1007/BF00798382. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Gallinaro-Matringe H., Gattoni R., Jacob M. Complexity of the structure of particles containing heterogeneous nuclear RNA as demonstrated by ribonuclease treatment. Eur J Biochem. 1977 Apr 15;74(3):589–602. doi: 10.1111/j.1432-1033.1977.tb11428.x. [DOI] [PubMed] [Google Scholar]
- Zieve G., Penman S. Subnuclear particles containing a small nuclear RNA and heterogeneous nuclear RNA. J Mol Biol. 1981 Jan 25;145(3):501–523. doi: 10.1016/0022-2836(81)90542-8. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]