Abstract
Storage of lipid in ectopic depots outside of abdominal visceral and subcutaneous stores, including within the pericardium and liver, has been associated with obesity, insulin resistance, and cardiovascular risk. We sought to determine whether anatomically distinct ectopic depots were physiologically correlated and site-specific effects upon cardiovascular function could be identified. Obese subjects (n = 28) with metabolic syndrome but without known atherosclerotic disease and healthy controls (n = 18) underwent magnetic resonance imaging (MRI) and proton MR spectroscopy (MRS) to quantify pericardial and periaortic lipid volumes, cardiac function, aortic compliance, and intrahepatic lipid content. Fasting plasma lipoproteins, glucose, insulin, and free-fatty acids were measured. Pericardial and intrahepatic (P < 0.01) and periaortic (P < 0.05) lipid volumes were increased in obese subjects vs. controls and were strongly and positively correlated (P ≤ 0.01) but independent of BMI (P = NS) among obese subjects. Intrahepatic lipid was associated with insulin resistance (P < 0.01) and triglycerides (P < 0.05), whereas pericardial and periaortic lipid were not (P = NS). Periaortic and pericardial lipid positively correlated to free-fatty acids (P ≤ 0.01) and negatively correlated to high-density lipoprotein (HDL) cholesterol (P < 0.05). Pericardial lipid negatively correlated to cardiac output (P = 0.03) and stroke volume (P = 0.01) but not to left ventricular ejection fraction (P = 0.46). None of the ectopic depots correlated to aortic compliance. In conclusion, ectopic storage of lipid in anatomically distinct depots appeared tightly correlated but independent of body size. Site-specific functional abnormalities were observed for pericardial but not periaortic lipid. These findings underscore the utility of MRI to assess individual differences in ectopic lipid that are not predictable from BMI.
INTRODUCTION
The prevalence of obesity, defined as a BMI ≥30 kg/m2, is rising rapidly in the United States. Recent estimates suggest that ~30.5% of the adult population meets this criterion (1). Obesity is also frequently associated with the development of a systemic metabolic derangement typified by insulin resistance and dyslipidemia, the coexistence of which with obesity and hypertension is termed the metabolic syndrome (2). Given the preponderance of atherosclerotic risk factors, it is not surprising that patients with metabolic syndrome are at higher risk for the development of complications from vascular diseases (3).
Although adipose tissue volume in subcutaneous and visceral abdominal locations is increased in obesity, recent data suggests that lipid is also stored in ectopic depots (i.e., outside of these recognized locations). These include deposits within organs such as the liver, skeletal muscle, and heart, as well as within the pericardium (pericardial lipid) or around blood vessels, such as the aorta (4). Although it is presently unclear how or whether lipid storage in these depots is similarly regulated, the role these depots play in the development of insulin resistance and metabolic syndrome is the subject of significant ongoing scientific scrutiny (4).
In addition to a predisposition to vascular disease, there are also specific structural and functional cardiac abnormalities that often occur in the setting of excessive weight, so termed the cardiomyopathy of obesity. The mechanism(s) through which alterations in cardiac structure and function occur are not well understood; however, recent evidence suggests that the phenotype may be related, in part, to myocardial triglyceride accumulation (5). Evidence is mounting that pericardial lipid, which surrounds the heart, may also play a significant role in the development of both cardiac and vascular disease (6,7). As adipose tissue is highly vascular, it has been hypothesized that mediators of lipid metabolism and inflammation may be supplied by the pericardial adipose tissue surrounding the heart, thereby directly affecting cardiac and coronary arterial function in a site-specific manner (8). Indeed, recent data from population cohorts have suggested that pericardial lipid volume is strongly associated with coronary atherosclerosis as measured by coronary calcification (6,7).
Although investigators have assessed the metabolic consequences of pericardial lipid accumulation in obesity in terms of abnormalities of metabolic indexes and the development of metabolic syndrome (9), relatively few have examined the association between cardiac structure/function and pericardial lipid volume in a site-specific manner (10,11). Furthermore, there have been no published reports evaluating vascular function with perivascular lipid volume. Using the three-dimensional technique of cardiovascular magnetic resonance imaging (MRI) and proton MR spectroscopy (MRS), we sought to determine whether the volume of lipid stored in ectopic depots was correlated and how each related to plasma indexes of insulin resistance and lipid levels. We hypothesized that site-specific abnormalities of organ function, for example cardiac function and pericardial lipid volume, would be observed.
METHODS AND PROCEDURES
Subjects
Obese subjects with metabolic syndrome (n = 28, BMI = 36.7 ± 4.0 kg/m2) but no known atherosclerotic vascular disease were recruited from the Nutrition and Weight Management Center of Boston Medical Center following granting of informed consent as approved by the institutional review board of Boston University Medical Center. Healthy controls (n = 18, BMI = 22.9 ± 2.8 kg/m2) were recruited from the general population and followed similar consent procedures. To qualify for inclusion, obese subjects must have, or be under medical treatment for, at least three of the following criteria derived from the revised Adult Treatment Panel III criteria for metabolic syndrome (12): (i) waist circumference ≥102 cm or 40 inches for men and ≥88 cm or 35 inches for women; (ii) high-density lipoprotein (HDL) ≤40 mg/dl for men and ≤50 mg/dl for women; (iii) triglyceride >150 mg/dl; (iv) fasting glucose ≥100 mg/dl; (v) systolic blood pressure ≥130 mm Hg and/or a diastolic blood pressure ≥85 mm Hg. Excluded subjects had a history of atherosclerotic vascular disease including stroke, myocardial infarction, heart failure, prior medical or surgical revascularization procedure, angina, or peripheral arterial disease. In addition, subjects with diabetes complicated by neuropathy or taking insulin for >1 year were excluded. Finally, given the limitations of the MRI scanner, subjects in excess of 300 pounds or with an abdominal circumference >140 cm, claustrophobia, or non-MRI compatible metallic implants were excluded.
Data acquisition
Subjects arrived at the test facility in a fasting state (no food or liquid other than water after midnight on the prior night). Anthropometric data including height, weight, and waist circumference, as well as blood pressure were measured. A blood sample was collected by venipuncture and analyzed for metabolic indexes including: glucose, insulin, lipoproteins including total cholesterol, HDL cholesterol, low-density lipoprotein cholesterol, triglycerides, and free-fatty acids.
Subjects then underwent MRI using a Philips Intera 3.0 Tesla system (Philips Medical Systems, Andover, MA). Data were acquired using a six-channel cardiac surface coil and synchronized to the cardiac cycle by continuous electrocardiographic-monitoring. Scan time for the entire session was ~45 min. Pericardial lipid volume was calculated from axial T1-weighted black blood images acquired in 4-mm contiguous slices. Scan parameters were: repetition time = 2 cardiac cycles, echo time = 13 ms, turbo factor = 13, number of excitations = 2, sense factor 2.5, matrix 256 × 256 reconstructed to 512 × 512, and in-plane resolution of 1.1 × 1.1 mm. Cardiac structural and functional data were collected using a steady-state free precession cine sequence optimized to 3.0T. Data were collected from contiguous 10-mm slices from the apex to the atrioventricular grooves of the heart without space gap in sequential 8–10 s breath holds. Scan parameters were: repetition time = 3.0 ms, echo time = 1.6 ms, number of excitations = 1, turbo factor = 14, sense factor 1.5, matrix 256, in-plane resolution = 2.5 × 2.8 mm, with 26–28 heart phases acquired per slice. Blood flow information was collected by velocity encoded MRI at the level of the bifurcation of the pulmonary artery from the ascending and descending aortic slices visible in this plane. Scan parameters were: repetition time = 3.0 ms, echo time = 1.6 ms, number of excitations = 1, turbo factor = 3, sense factor 1.5, matrix 128 reconstructed to 256, inplane resolution = 2.5 × 2.8 mm, with 40 cardiac phases acquired per each scan. Blood pressure was measured immediately before MRI scanning to permit calculation of aortic compliance from the velocity encoded images.
Intrahepatic lipid was determined by MRS using the point resolved spectroscopy technique. Selected elements of the cardiac surface coil that overlay the liver were chosen for data collection. A 9 cm3 voxel was localized within the right lobe of the liver and shimmed to optimize field homogeneity. A total of 32 spectral acquisitions were then performed without breath-holding using repetition time = 2,000 ms and echo time = 40 ms without water suppression.
Data analysis
MRI data were analyzed for pericardial lipid volume, cardiac structure and function, and vascular compliance. Pericardial lipid volume was calculated using an algorithm developed by two authors (N.H. and H.J.) using the MatLab software development environment (The MathWorks, Natick, MA). Images were first corrected for pixel intensity inhomogeneity and normalized to the pixel intensity of lipid (13). Pericardial lipid volume was quantified via segmentation using manual contour tracing on a slice by slice basis. The superior border of the image processing volume was set at the bifurcation of the pulmonary artery, and the inferior border was defined as the most caudal slice where cardiac muscle was identified. The anterior and posterior boarders were defined by the chest wall and descending thoracic aorta. Measurements were performed by two independent observers. A similar technique was applied to measure periaortic lipid using identical slices as for the pericardial lipid measurement to define regions of interest around the descending thoracic aorta. Intrareader reliability for pericardial lipid volumes was excellent with intraclass correlation coefficient values of 0.99. Bland and Altman plots displayed no difference in the reliability of quantification for different lipid volume levels.
Cardiac structural and functional data including myocardial mass, left ventricular (LV) end-diastolic volume, LV ejection fraction, LV stroke volume, and cardiac output were calculated using a dedicated workstation (Philips ViewForum, Andover, MA). Analysis was performed on the contiguous short axis slices by the Simpson’s technique. Flow information from the aorta, including LV stroke volume and cardiac output, was also analyzed using the Philips ViewForum workstation, QFlow package. Aortic compliance was calculated by dividing the change in aortic volume in systole and diastole from an axial slice in which the ascending and descending thoracic aorta was visible (taken at the bifurcation of the pulmonary artery) by the systolic and diastolic blood pressure difference.
Pericardial lipid was normalized to left ventricular mass (in ml/g) to control for variation in heart size, whereas periaortic lipid was normalized to aortic length (in ml/mm) to control for variation in scan field of view.
MR spectra were exported and postprocessed using LCModel (S. Provencher) and fitting was performed between −2 and 9 p.p.m. using the basis set supplied by the program. Spectral resonance peaks corresponding to lipid were integrated and divided into the integral of the peak area corresponding to water, yielding a percentage.
Blood samples were centrifuged and processed for analysis by the Clinical Laboratory of Boston Medical Center. Insulin resistance was calculated using the homeostatic model assessment (HOMA) calculated using the formula: HOMAIR = (fasting insulin (μU/ml) × fasting glucose (mg/dl))/405 (14).
Statistical analysis
Statistical analysis was performed using SPSS 11 (Systat Software, Chicago, IL) and SAS 9.1 (SAS Institute, Cary, NC). Associations between continuous variables were assessed using Pearson’s correlation. Comparisons between groups were performed using the pooled two-sample t-test for continuous characteristics and by Fisher’s exact test for categorical characteristics. The nonparametric Wilcoxon test was used to check whether the t-test results were affected by the assumption of the data following a Gaussian (normal) distribution (no differences in significance were observed). Specific comparison for HOMAIR was by the nonparametric Mann–Whitney U test. Intrareader reliability for the pericardial and periaortic lipid measures were determined by use of the intraclass correlation coefficient (15). The method of Bland and Altman was used to graphically evaluate whether reliability differed by level of lipid volume (16). Data are presented as mean ± s.d. Probability values of P < 0.05 were considered significant.
RESULTS
Subject characteristics
Clinical and metabolic features of the obese subjects and controls are summarized in Table 1. As intended, obese subjects had increased BMI (P < 0.01), waist circumference (P < 0.01), systolic blood pressure (P < 0.01), and diastolic blood pressure (P < 0.01) compared to controls. There were no differences between obese and control subjects in plasma lipid levels including total cholesterol (P = 0.45) and low-density lipoprotein (P = 0.94); however, obese subjects demonstrated lower HDL (P < 0.01) and increased fasting triglycerides (P < 0.01); and there was a trend toward increased free-fatty acids (P = 0.07). Among obese subjects, 23% were taking oral hypoglycemic agents (all metformin), 26% were taking lipid-lowering agents (of those 75% were statins), and 50% were taking antihypertensive medications. Only one obese subject and none of the control subjects were taking oral contraceptives. As predicted, obese subjects had greater insulin resistance as determined by HOMAIR vs. controls (P < 0.01).
Table 1. Clinical and metabolic features of subjects and controls.
| Subjects (n = 28) |
Controls (n = 18) |
P | |
|---|---|---|---|
| Age (years) | 44 ± 10 | 37 ± 13 | 0.04 |
| Female (%) | 92.9 | 77.8 | 0.14 |
| BMI (kg/m2) | 36.7 ± 4.0 | 22.9 ± 2.8 | <0.01 |
| Body surface area (m2) | 2.06 ± 0.21 | 1.72 ± 0.12 | <0.01 |
| Waist circumference (cm) | 109 ± 11 | 76 ± 8 | <0.01 |
| Systolic blood pressure (mm Hg) | 132 ± 11 | 110 ± 12 | <0.01 |
| Diastolic blood pressure (mm Hg) | 84 ± 7 | 71 ± 8 | <0.01 |
| Total cholesterol (mg/dl) | 174 ± 32 | 182 ± 33 | 0.45 |
| Free-fatty acids (mmol/l) | 0.53 ± 0.21 | 0.40 ± 0.22 | 0.07 |
| Fasting glucose (mg/dl) | 103 ± 29 | 89 ± 6 | 0.05 |
| High-density lipoprotein (mg/dl) | 48 ± 12 | 66 ± 15 | <0.01 |
| Low-density lipoprotein (mg/dl) | 103 ± 29 | 102 ± 26 | 0.94 |
| Triglycerides (mg/dl) | 116 ± 60 | 66 ± 28 | <0.01 |
| HOMAIR | 3.7 ± 3.6 | 0.8 ± 0.6 | <0.01 |
HOMAIR, homeostasis model assessment of insulin resistance.
Quantification and metabolic correlation of ectopic lipid depots in obese subjects
Figure 1 illustrates representative MR images that demonstrate the wide variation observed in ectopic fat volume, in this example pericardial lipid, among obese subjects. The subject with BMI of 43 kg/m2 had a significantly smaller volume of pericardial fat than the subject with a BMI of 34 kg/m2, as revealed in the segment illustrated. The mean volume of lipid stored in the three ectopic depots measured, as well as their normalized values, can be found in Table 2. There was increased pericardial lipid volume among obese subjects as compared to controls (P < 0.01), and this relationship was maintained when normalized to heart size (in ml/g of myocardial mass, P < 0.01). There were no statistically significant differences observed in absolute periaortic lipid volume (in ml), however, after normalization to aortic length there was a significant difference (P = 0.03). Obese subjects had greater intrahepatic lipid (P < 0.01), as predicted. Correlation coefficients comparing ectopic depot volumes and metabolic parameters for the obese subject group are illustrated in Table 3. Ectopic lipid depots were strongly and positively correlated (pericardial vs. periaortic (P ≤ 0.01), pericardial vs. intrahepatic (P ≤ 0.01)) (Figure 2). Pericardial and periaortic lipid also negatively correlated with HDL cholesterol and positively correlated to free-fatty acids. Intrahepatic lipid was strongly correlated to HOMAIR and triglyceride levels. However, none of the ectopic depots correlated with BMI.
Figure 1.
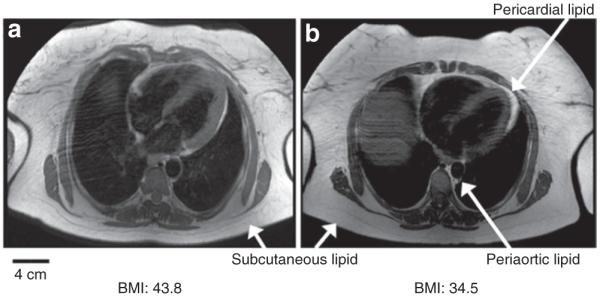
Differing pericardial lipid volume among obese subjects. Axial T1-weighted MR images obtained at the level of the heart from two subjects with differing BMI are illustrated. Both show a pronounced subcutaneous adipose layer (arrows). The smaller depots that are the focus of this study (pericardial lipid and periaortic lipid) are identified by their prominent bright signals. In the two examples shown, (a) illustrates a subject with a greater BMI but less pericardial lipid compared to the subject in b, as assessed by the area in this slice as well as the total volume of pericardial lipid. These images suggest that pericardial lipid is not correlated with BMI in obese subjects. MR, magnetic resonance.
Table 2. Ectopic lipid depot volumes among obese and control subjects.
| Subjects (n = 28) |
Controls (n = 18) |
P | |
|---|---|---|---|
| Pericardial lipid volume (ml) | 161 ± 88 | 85 ± 66 | <0.01 |
| Pericardial lipid volume/left ventricular wall mass (ml/g) |
2.0 ± 1.1 | 1.1 ± 0.7 | <0.01 |
| Periaortic lipid volume (ml) | 12.1 ± 6.7 | 9.4 ± 5.2 | 0.14 |
| Periaortic lipid volume/aortic length (ml/mm) |
0.12 ± 0.07 | 0.08 ± 0.05 | 0.03 |
| Intrahepatic lipid (%) | 9.5 ± 10.5 | 1.0 ± 1.0 | <0.01a |
Satterthwaite (unequal variance) t-test employed because of large discrepancy in variance between subjects and controls.
Table 3. Correlation coefficients between ectopic lipid depots and metabolic parameters.
| Pericardial | Periaortic | Intrahepatic | |
|---|---|---|---|
| Pericardial | – | 0.57** | 0.55** |
| Periaortic | 0.57** | – | 0.23 |
| Intrahepatic | 0.63** | 0.23 | – |
| BMI | −0.34 | −0.19 | −0.29 |
| Fasting glucose | 0.21 | 0.18 | 0.41 |
| Free-fatty acids | 0.55** | 0.52** | 0.20 |
| Triglyceride | 0.36 | 0.24 | 0.46* |
| HDL cholesterol | −0.42** | −0.37* | −0.12 |
| HOMAIR | 0.01 | 0.08 | 0.72** |
HDL, high-density lipoprotein; HOMAIR, homeostasis model assessment of insulin resistance.
P value significant at <0.05.
P value significant at ≤0.01.
Figure 2.
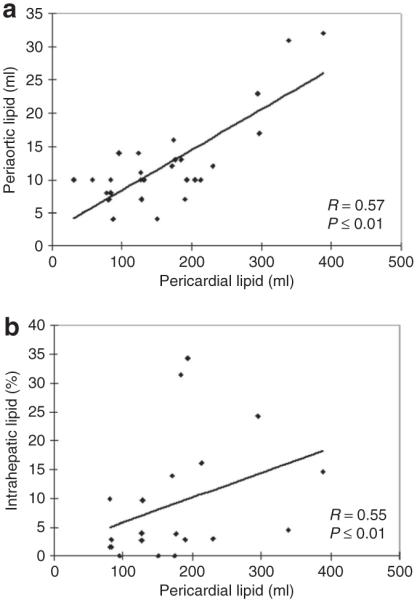
Correlation between ecotopic lipid depots. Scatter plots of ectopic lipid volumes are presented showing strong correlations between (a) pericardial vs. periaortic and (b) pericardial vs. intrahepatic lipid depots for obese subjects.
Ectopic lipid and site-specific effects upon cardiovascular function
Cardiac structural and functional parameters as determined by MRI are shown in Table 4. Cardiac output was greater in obese subjects compared to controls (P < 0.01), but this difference did not persist when cardiac output was indexed to body surface area (cardiac index in l/min/m2, P = 0.10). LV mass, both corrected and uncorrected to body surface area, and LV end-diastolic volume were similar between the two groups, whereas LV end-diastolic volume indexed to body surface area was lower in the obese subjects (P = 0.01). LV ejection fraction was higher in obese subjects vs. controls (P < 0.01) but still within the range of normal, whereas LV stroke volume was similar between the two groups (P = 0.16). Ascending and descending aortic compliance was decreased in obese subjects as compared to controls (P < 0.01).
Table 4. Cardiac MRI determined structural and functional parameters.
| Subjects (n = 28) |
Controls (n = 18) |
P | |
|---|---|---|---|
| Left ventricular mass (g) | 88 ± 25 | 81 ± 24 | 0.38 |
| Left ventricular mass/ body surface area (g/m2) |
42 ± 9 | 47 ± 12 | 0.13 |
| Left ventricular end-diastolic volume (ml) |
138 ± 38 | 135 ± 30 | 0.81 |
| Left ventricular end-diastolic volume/body surface area (ml/m2) |
66 ± 14 | 78 ± 15 | 0.01 |
| Left ventricular ejection fraction (%) | 67 ± 6 | 61 ± 6 | <0.01 |
| Left ventricular stroke volume (ml) | 91 ± 23 | 82 ± 17 | 0.16 |
| Cardiac output (l/min) | 6.3 ± 1.3 | 4.8 ± 1.0 | <0.01 |
| Ascending aortic compliance (ml/mm Hg) |
1.9 ± 0.8 | 2.8 ± 1.3 | 0.01 |
| Descending aortic compliance (ml/mm Hg) |
1.0 ± 0.5 | 1.5 ± 0.6 | 0.01 |
MRI, magnetic resonance imaging.
In site-specific functional analysis, pericardial lipid (in ml/g) inversely correlated to cardiac output (P = 0.01) and stroke volume (P = 0.01), but did not correlate with ejection fraction (P = 0.46) among obese subjects (Figure 3). Conversely, periaortic lipid did not correlate to descending aortic compliance (P = 0.40) or mean arterial blood pressure (P = 0.88). Periaortic lipid did correlate to left ventricular stroke volume (P = 0.03). Finally, intrahepatic lipid did not correlate to aortic compliance (P = 0.11) or stroke volume (P = 0.18), but did correlate to mean arterial blood pressure (P = 0.05). Among control subjects, no correlation was identified between the various ectopic lipid depots, nor was a significant association identified between pericardial lipid and cardiac output or stroke volume as was detected among the obese subjects.
Figure 3.
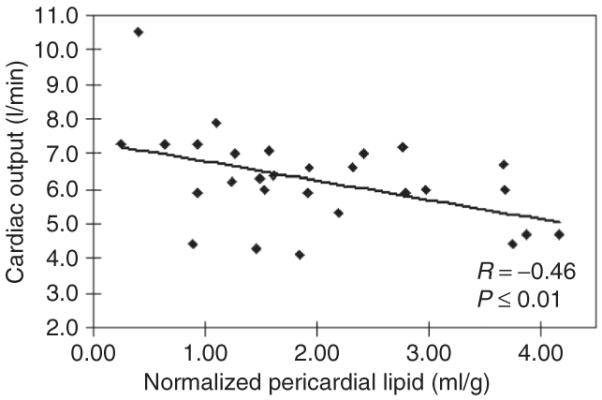
Site-specific effect of pericardial lipid and cardiac function. A scatter plot of pericardial lipid normalized to left ventricular mass (in ml/g) is presented vs. cardiac output for obese subjects. Cardiac output values presented are all within the normal range.
DISCUSSION
Ectopic lipid accumulation, within and around solid organs but outside of normally recognized abdominal visceral and subcutaneous depots, has emerged as a widely recognized feature of obesity and insulin resistant states. The vast majority of published studies have focused on a single-ectopic depot as it relates to metabolic risk factors or disease. For example, the quantity of pericardial lipid volume is rapidly developing as an important indicator that can stratify vascular and metabolic risk. In asymptomatic individuals without metabolic syndrome, pericardial lipid associates with fasting glucose levels and predicts the development of metabolic syndrome(9,17), as well as associates with coronary atherosclerosis (6,7,18-20). In addition, reduction in pericardial lipid volume correlates to improvement in insulin resistance in obese individuals (11). We sought to determine whether the volume of lipid stored in different ectopic depots was correlated among obese subjects with metabolic syndrome, and whether these depots had a site-specific correlation with organ function. In this study, using MRI and MRS, we identified a strong correlation between the three ectopic depots studied (pericardial, periaortic, and intrahepatic), and further show that among obese subjects, as the volume of pericardial adipose increases, cardiac function decreases through a mechanism that does not appear to relate to global systolic function. We did not detect a similar site-specific correlation of periaortic lipid upon aortic compliance or blood pressure. Finally, we observed variable associations between ectopic lipid depots and plasma lipids and HOMAIR, suggesting a nonuniform mechanism of lipid storage.
Pericardial lipid volume can be measured with various imaging modalities including echocardiography (21), computed tomography (7,22), and MRI (23). Periaortic adipose tissue is more difficult to quantify and has not been widely reported, with the exception of a recent publication using computed tomography (24). Computed tomography and MRI have advantages over echocardiography as they are three-dimensional volumetric techniques thus offering higher reproducibility as compared to measuring linear pericardial lipid thickness, which can be arbitrary thus introducing uncertainty. For this study, we used MRI because it affords high spatial- and temporal-resolution data sets without the penalties of high-ionizing radiation dose that comparably informative computed tomography protocols would require. For pericardial lipid volume quantification, we used manual contour tracing segmentation, which is widely accepted as the accurate gold standard for image-based volumetric assessment of continuous and well delineated tissue distributions such as pericardial adipose and solid structures such as the heart walls. MRS has emerged as the definitive noninvasive method by which hepatic lipid can be quantified (25).
Previous studies have demonstrated an association between pericardial lipid volume and cardiac function, but their conclusions were limited by sample size (n = 4 obese subjects in Kankaanpää et al. (10)), or a nonvolumetric technique (echocardiography in Iacobellis et al. (11) and Malavazos et al. (26)). Our study is the largest to date that demonstrates an association between pericardial lipid and LV functional and structural parameters using the more precise MRI pericardial lipid volume quantification technique. Although the association between pericardial adipose (7) and visceral and subcutaneous adipose has been described, to the authors’ knowledge, this the first study that identifies a correlation between anatomically distinct ectopic lipid depots. The precision offered by MRI may permit more subtle associations to be determined, and it may also afford greater sensitivity to changes with intervention, such as weight loss, as has been reported echocardiographically (11,27).
In this study, we assessed the significance of pericardial lipid that was normalized to heart size rather than unadjusted pericardial lipid volume. We chose to do so as we wanted to eliminate heart size as a confounding factor that might contribute to lipid volume. In this way, we could compare across body size groups for relationships between pericardial lipid and metabolic and cardiac functional parameters. We did not observe a relationship between pericardial lipid volume and BMI, as was reported previously (17) using population-based datasets with a large dispersion of BMI, possibly because our BMI range was by design skewed toward the obese.
The mechanisms by which pericardial lipid may affect cardiac function are unclear. Although some studies have suggested that pericardial lipid is merely a marker for visceral abdominal lipid stores, one might presume that a unifying feature of these two lipid depots could be a covariance observed in systemic metabolic parameters including insulin resistance and/or lipids. Although we did observe a strong correlation between the quantity of ectopic lipid around the heart and the descending thoracic aorta, we did not observe a relationship between these depots and insulin resistance. This finding may suggest that local rather than systemic factors could be responsible for the observed site-specific correlation with cardiac function. One possibility is that pericardial lipid itself exerts a restrictive pressure on myocardial expansion in diastole, and thereby slightly reduces cardiac diastolic filling, and thus affects cardiac output. Another possibility is that pericardial lipid elaborates signaling molecules that affect cardiac function via direct venous drainage, and in this way affects diastolic function (5,8,28). Future work utilizing sensitive measures of myocardial lipid accumulation by proton MRS, as well as concurrent measurement of pericardial lipid volume in subjects before and after weight loss, might better address this question. We are presently repeating these measurements following 1 year of weight loss to identify differences in pericardial lipid volume, cardiac function, and measures of lipid metabolism and insulin resistance.
A limitation of this study which uses MRI for the measurement of ectopic lipid is body size. We were unable to image subjects in excess of 300 lb or 140 cm of waist circumference due to the weight limit of the motorized table and size of the magnet bore. For this reason, our data were undoubtedly skewed toward shorter stature (to still permit increased BMI) and female gender, perhaps limiting its generalizability. We did not assess more precise measures of regional cardiac dysfunction, as reported by Sironi et al. (29), or diastolic function. The clinical heterogeneity of our obese sample, particularly in terms of glucose control as evidenced by the large s.d. in HOMAIR and intrahepatic lipid, may have biased our determination of significant associations. In addition, a sizeable proportion of our obese subjects were taking lipid lowering and/or hypoglycemic agents, thereby introducing heterogeneity into the characterization of insulin resistance and lipid levels i++n this group, as well as likely affecting ectopic lipid accumulation. Due to the small sample size of 28 subjects, we were unable to control for these potential effects. Finally, we observed similar LV mass between obese subjects and controls which was slightly unexpected but perhaps owing to the small sample size.
In this study of obese subjects with metabolic syndrome, we demonstrated that the amount of lipid stored in ectopic depots is directly correlated and that pericardial lipid in particular appeared correlated to cardiac function in a site-specific manner that appeared independent of insulin resistance and BMI. These findings suggest differing mechanisms and significance of ectopic lipid storage among obese vs. lean persons, as significant variation was observed despite a rather uniform BMI classification.
ACKNOWLEDGMENTS
We greatly appreciate the statistical advice provided by Dr L. Adrienne Cupples. This work was supported by a grant from the National Institutes of Health 5P50HL083801 to J.A.H.
Footnotes
The first two authors contributed equally to this work.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Poirier P, Giles TD, Bray GA, et al. American Heart Association Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Després JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 4.Lettner A, Roden M. Ectopic fat and insulin resistance. Curr Diab Rep. 2008;8:185–191. doi: 10.1007/s11892-008-0032-z. [DOI] [PubMed] [Google Scholar]
- 5.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 6.Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 8.Kremen J, Dolinkova M, Krajickova J, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620–4627. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 9.Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 2008;16:887–892. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 10.Kankaanpää M, Lehto HR, Pärkkä JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–4695. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- 11.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008;16:1693–1697. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 12.Expert panel on detection, evaluation, and treatment of high blood cholesterol In Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Salvado O, Hillenbrand C, Zhang S, Wilson DL. Method to correct intensity inhomogeneity in MR images for atherosclerosis characterization. IEEE Trans Med Imaging. 2006;25:539–552. doi: 10.1109/TMI.2006.871418. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 16.Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8476:307–310. [PubMed] [Google Scholar]
- 17.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 18.Ahn SG, Lim HS, Joe DY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94:e7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- 19.Jeong JW, Jeong MH, Yun KH, et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71:536–539. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 20.Gorter PM, van Lindert AS, de Vos AM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 22.Dey D, Suzuki Y, Suzuki S, et al. Automated quantitation of pericardiac fat from noncontrast CT. Invest Radiol. 2008;43:145–153. doi: 10.1097/RLI.0b013e31815a054a. [DOI] [PubMed] [Google Scholar]
- 23.Flüchter S, Haghi D, Dinter D, et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity (Silver Spring) 2007;15:870–878. doi: 10.1038/oby.2007.591. [DOI] [PubMed] [Google Scholar]
- 24.Schlett CL, Massaro JM, Lehman SJ, et al. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int J Obes (Lond) 2009;33:226–232. doi: 10.1038/ijo.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 26.Malavazos AE, Ermetici F, Coman C, et al. Influence of epicardial adipose tissue and adipocytokine levels on cardiac abnormalities in visceral obesity. Int J Cardiol. 2007;121:132–134. doi: 10.1016/j.ijcard.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 27.Willens HJ, Byers P, Chirinos JA, et al. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol. 2007;99:1242–1245. doi: 10.1016/j.amjcard.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 28.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sironi AM, Pingitore A, Ghione S, et al. Early hypertension is associated with reduced regional cardiac function, insulin resistance, epicardial, and visceral fat. Hypertension. 2008;51:282–288. doi: 10.1161/HYPERTENSIONAHA.107.098640. [DOI] [PubMed] [Google Scholar]


